Humanized cell factor hair agent and preparing method thereof
A cytokine and human-derived technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of inability to decrease biological activity, easy degradation of drugs, and difficulty in reaching effective concentrations, etc. problems, to achieve the effect of reducing adverse reactions, increasing retention volume and retention time, and applying safe and non-irritating effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Extraction of Active Cytokines in Cell Culture Supernatant
[0021] Use the ammonium sulfate fractional precipitation method to concentrate and extract active cytokines: collect the cell culture supernatant, add ammonium sulfate until the ammonium sulfate saturation reaches 30%, wait for the dissolution to be completed, let it stand for 30 minutes, then centrifuge at 20°C, 3000r / min for 30 minutes, and separate Clear and precipitate. The precipitate was suspended with PBS, ammonium sulfate was added to the supernatant until the ammonium sulfate saturation reached 50%, and centrifuged again. Repeat once until the saturation of ammonium sulfate reaches 70%, collect the precipitate three times, use PBS as the dialysate and W3500 dialysis bag for 3 hours to desalt, replace the dialysate every 1 hour, and use a magnetic stirrer to accelerate the desalination, collect the liquid in the dialysis bag, Freeze-dry at -70°C for 24 hours to obtain protein freeze-dried po...
Embodiment 2
[0022] The preparation of embodiment 2 human-derived cytokine hair growth preparation liposome dosage form
[0023] Weigh 1 g of egg yolk lecithin and 0.5 g of cholesterol, and dissolve them with 67 ml of chloroform and 33 ml of methanol. The solvent was evaporated on a RE-52 rotary evaporator in a water bath at 48°C and 120 r / min until a yellow-green milky lipid film was formed. Weigh 0.75 g of the dry protein powder described in Example 1, dissolve it with 12.5 ml of PBS, add it to the lipid film, add 100 ml of ether, and mix well. After oscillating and emulsifying for 10 minutes on a KQ-400KDE high-power ultrasonic cleaner at 20°C and 160W, use a RE-52 rotary evaporator to remove the solvent in a water bath at 38°C and 120r / min until curd / tofu-like coagulation Gel formed, suspended with 17.5ml PBS. Let stand for 1h. On the CP-750 ultrasonic breaker, ice bath, 150W, intermittent ultrasonic emulsification 100 times (ultrasonic 2s, intermittent 9s), to obtain a milky white ...
Embodiment 3
[0024] Morphological identification of embodiment 3 human-derived cytokine hair growth preparation liposome dosage form
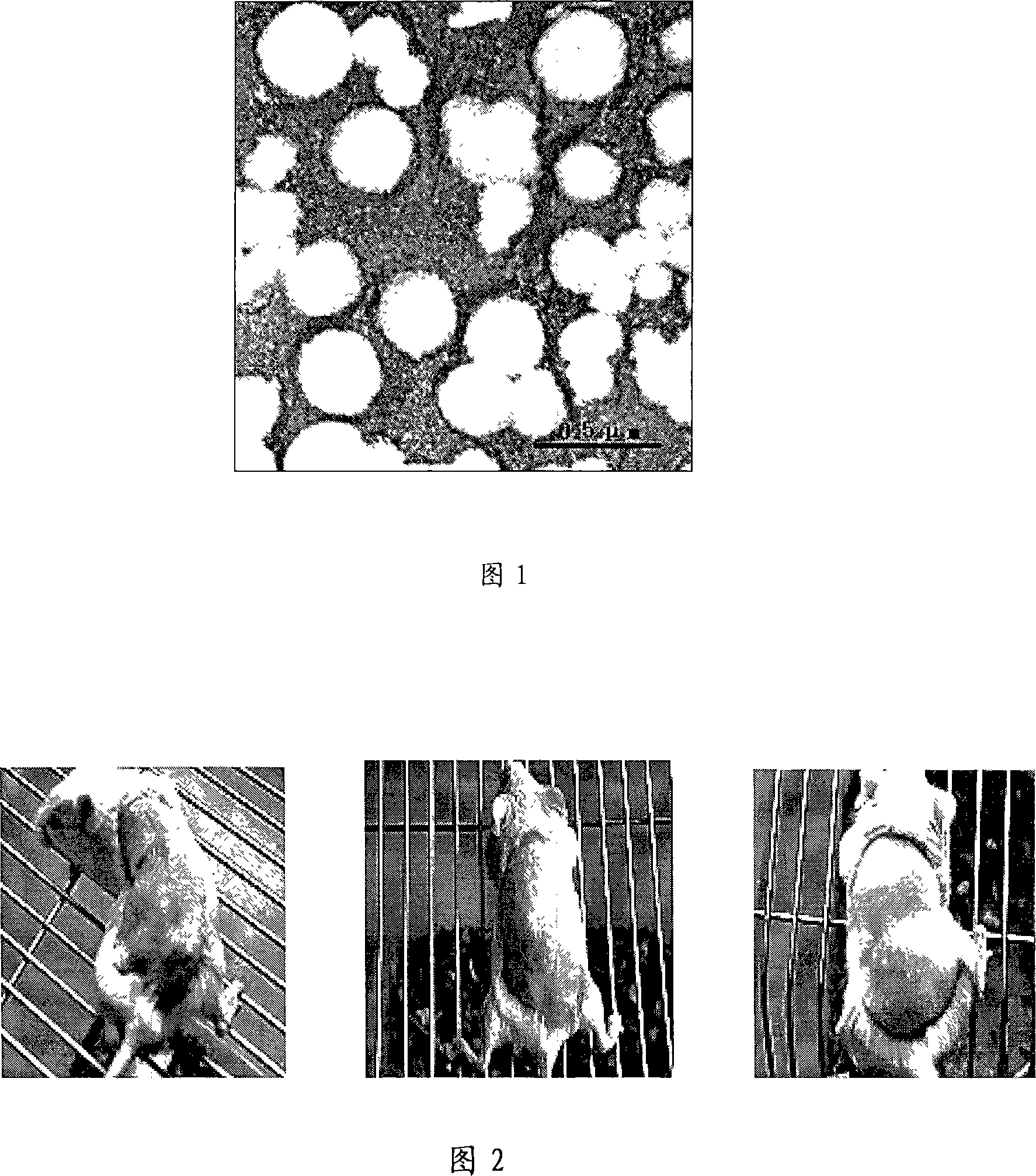
[0025] Adjust the ultrasonic frequency and time as needed to control the particle size and shape of liposomes, and use transmission electron microscopy to identify: 2% phosphotungstic acid staining, copper grid drying, observe liposome morphology under a transmission electron microscope after 15 minutes, take pictures and measure its diameter. The average diameter of the prepared liposomes is 140.8nm (59.3-296.3nm), and the morphology is shown in Figure 1. The results show that the liposomes prepared by this process are relatively uniform in size, and the membrane edges are smooth and wrinkle-free.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com