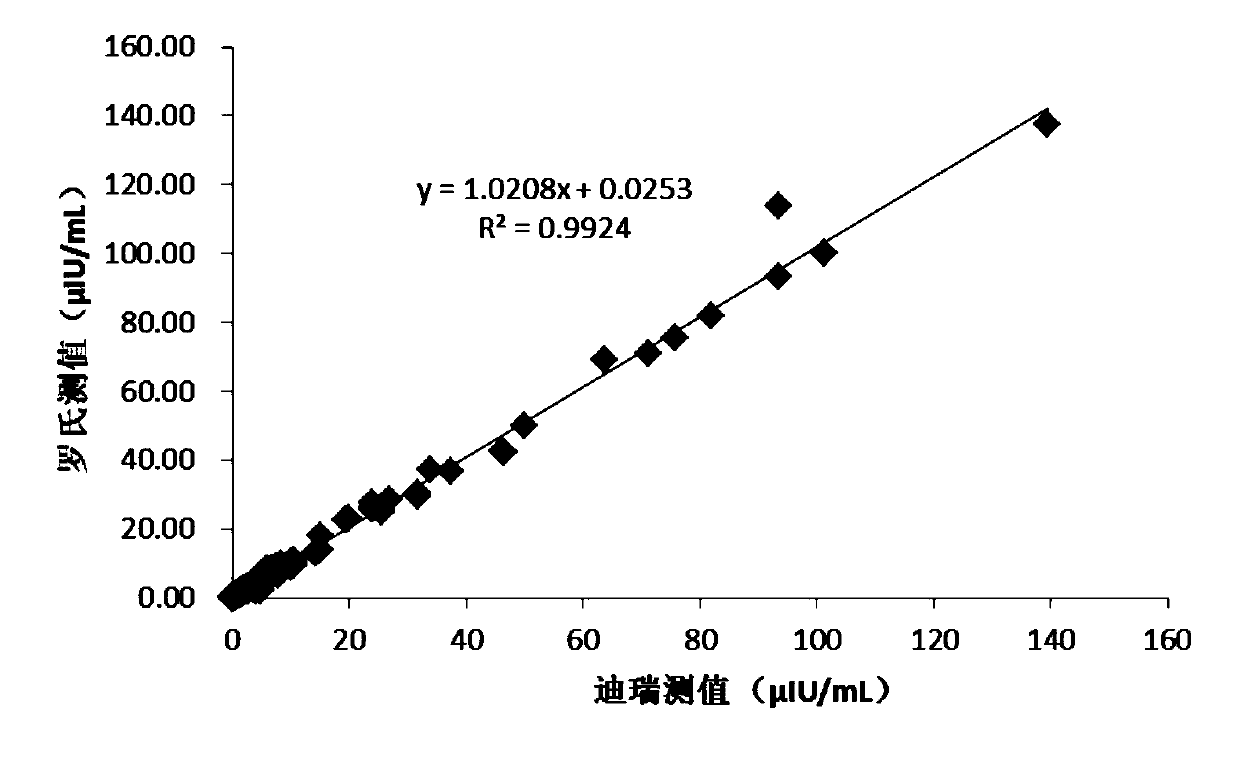
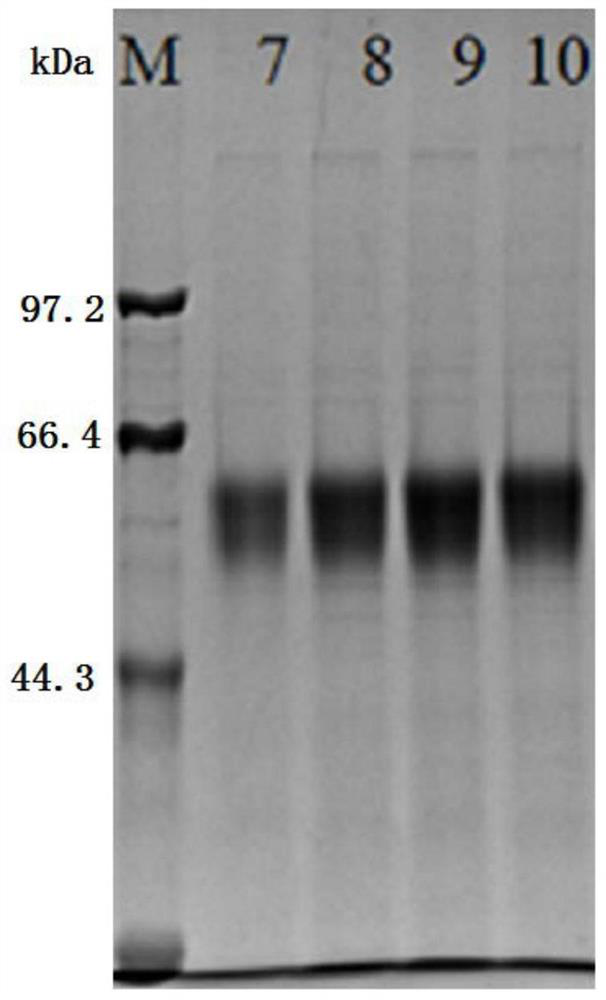
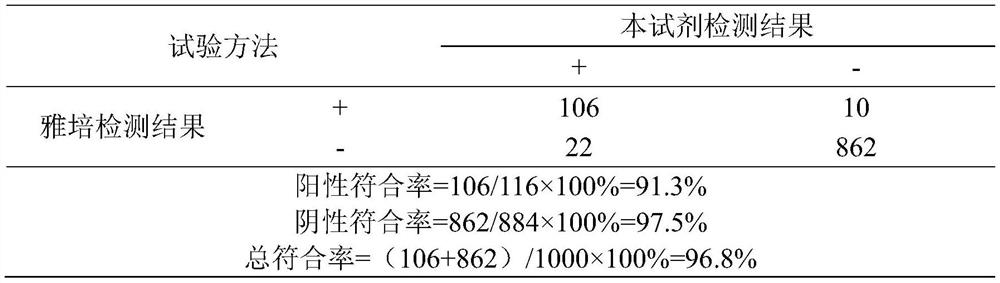
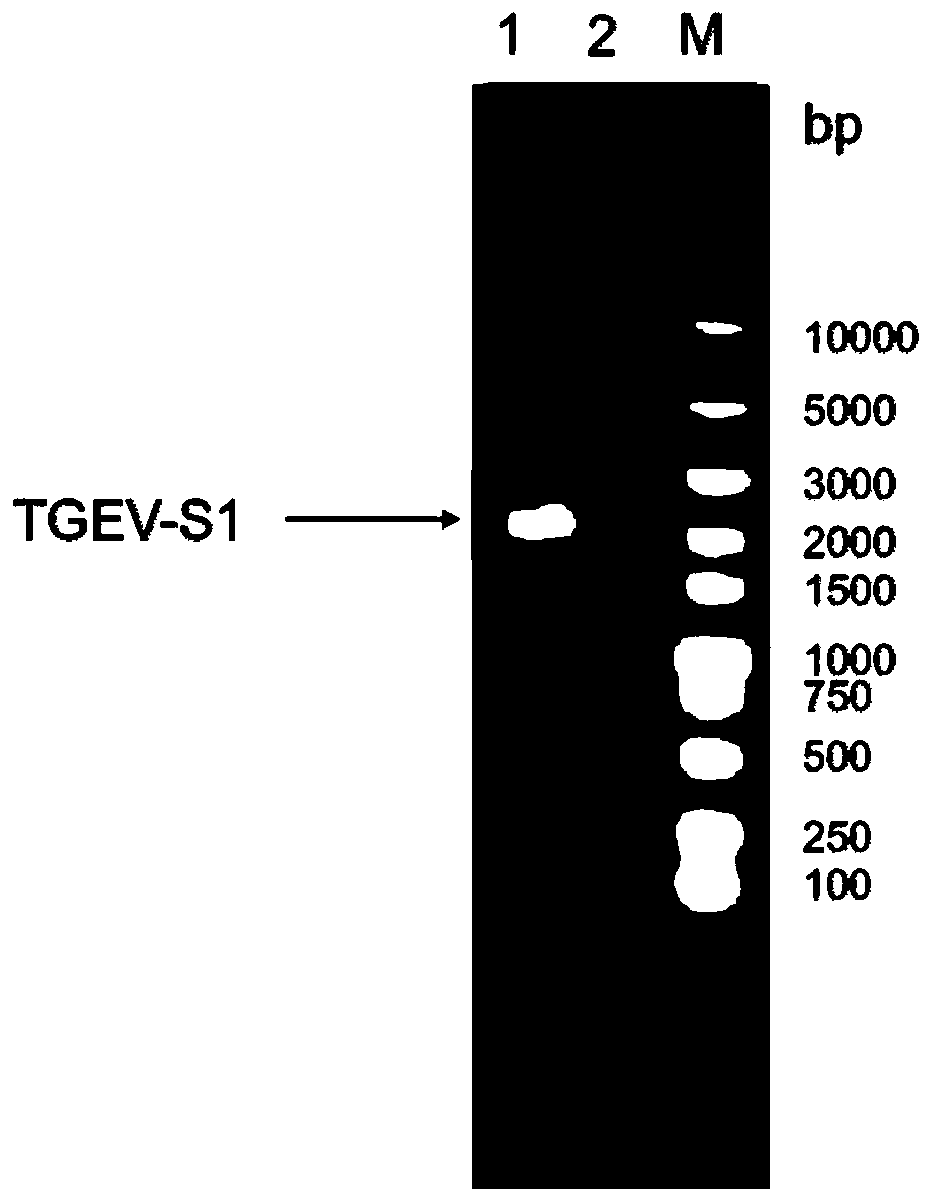
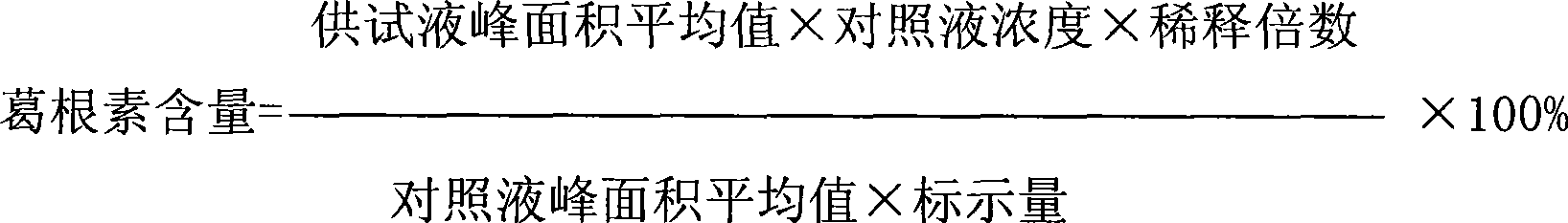Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49results about How to "Batch-to-batch stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
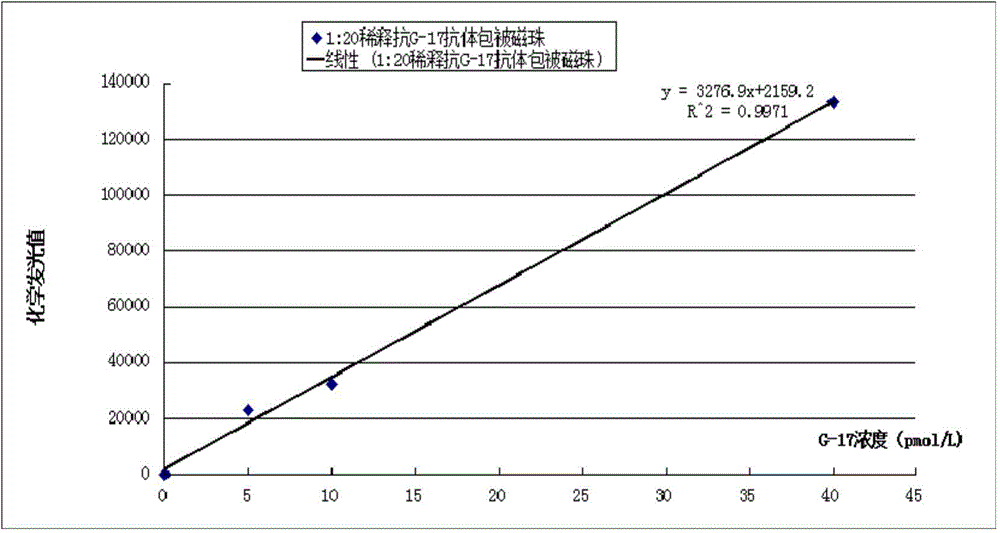
Gastrin-17 enzymatic chemiluminescence immunoassay kit
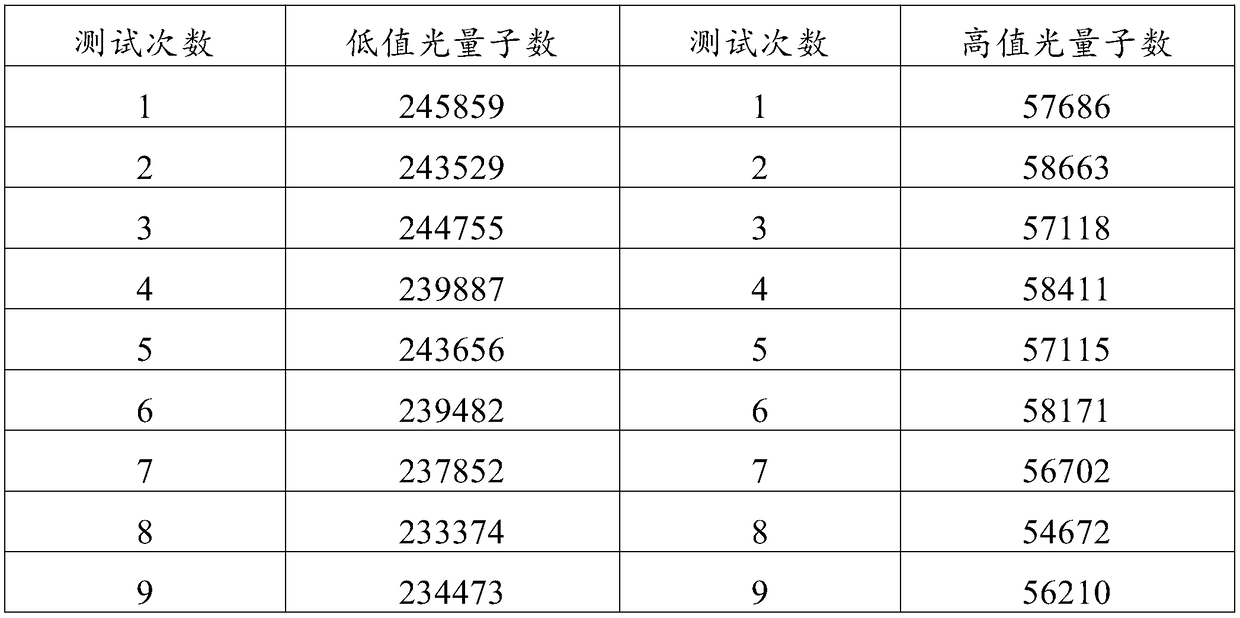
InactiveCN104914251ALittle variance between production batchesHigh affinityDisease diagnosisBiological testingMicrosphereImmune complex deposition
The invention discloses a gastrin-17 enzymatic chemiluminescence immunoassay kit and belongs to the technical field of chemiluminescence immunoassay analysis. The kit comprises an enzyme label liquid, a gastrin-17 standard, gastrin-17 monoclonal antibody-coated immunomagnetic beads, a sample diluent, a chemiluminescent substrate liquid and a washing liquid. The principle of the gastrin-17 enzymatic chemiluminescence immunoassay kit comprises that a gastrin-17 monoclonal antibody is connected to the surface of a magnetic bead so that a solid phase agent is obtained, and through capture of gastrin-17 in a sample and use of an enzyme-labeled anti-gastrin-17 monoclonal antibody, a solid phase-antibody-antigen-enzyme-labeled antibody sandwiched immune complex is formed. Through combination of a chemiluminescence technology and an immunomagnetic bead technology, the prepared kit has the advantages of high sensitivity, good specificity, wide linearity range and good stability and can satisfy clinical requirements on stomach function detection.
Owner:BIOHIT BIOTECH HEFEI +1
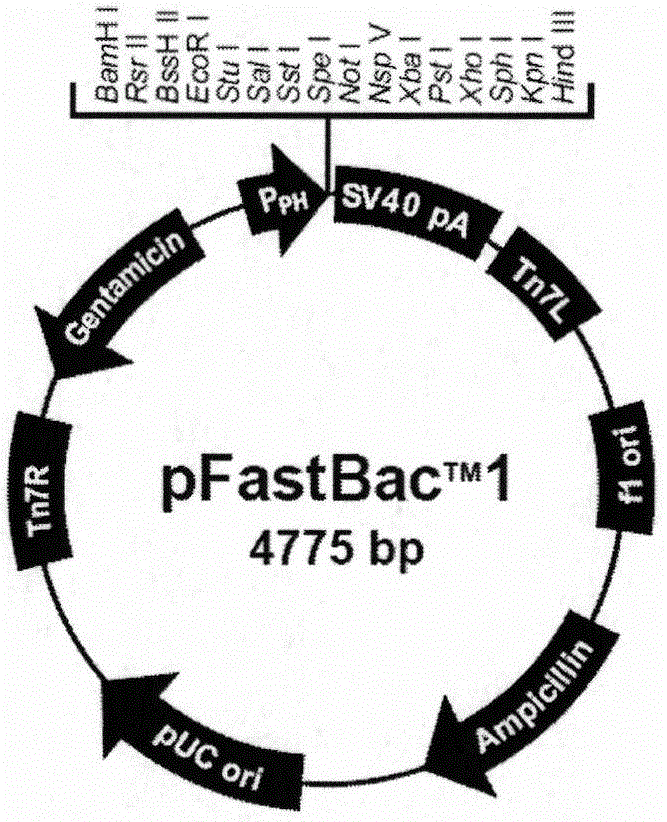
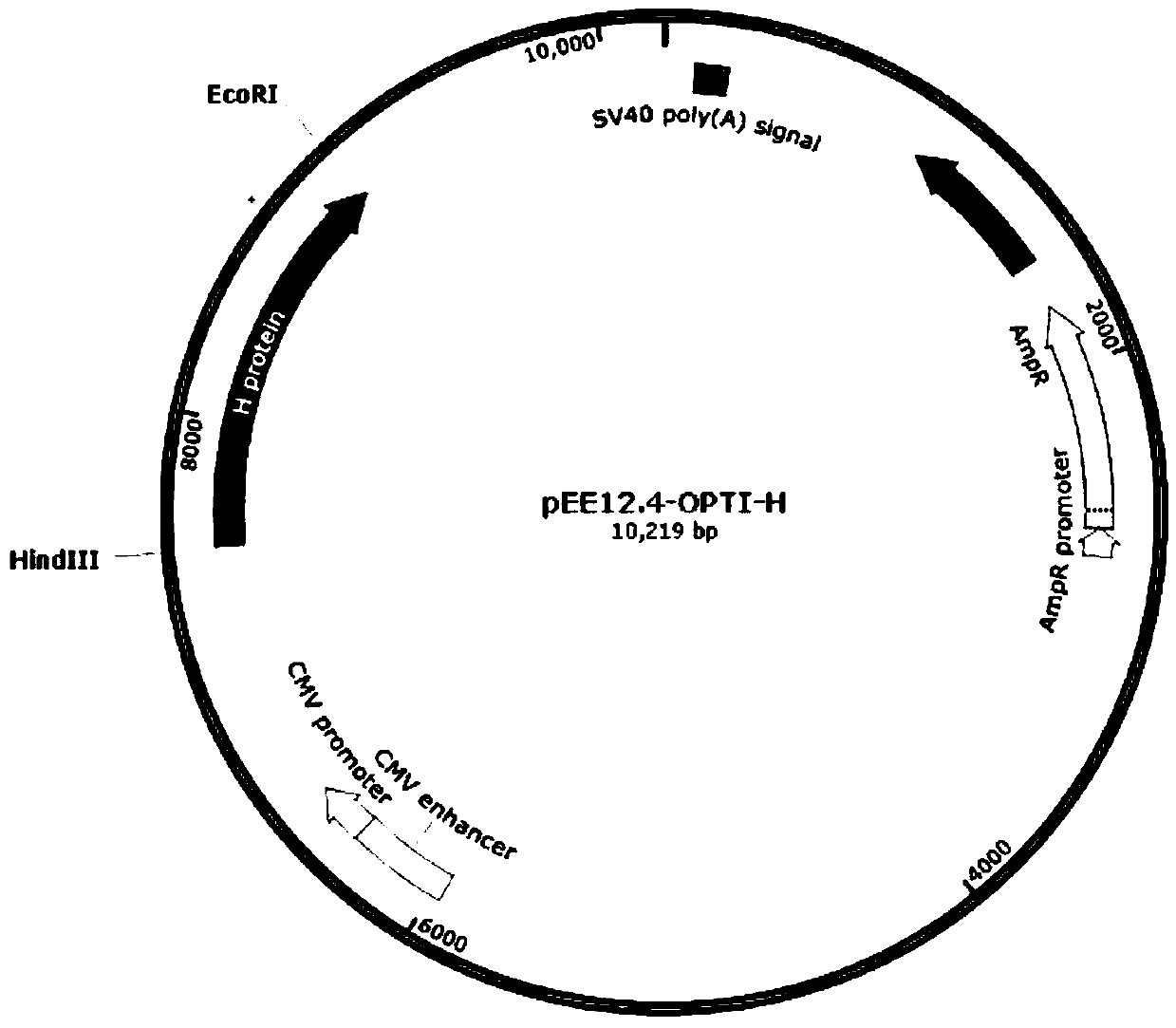
Preparation method and application of classical swine fever virus recombinant subunit vaccine
InactiveCN104826100ANo risk of contaminationImprove securityAntiviralsAntibody medical ingredientsProtein targetVaccine Production
The invention discloses a preparation method and application of a classical swine fever virus recombinant subunit vaccine with the amino acid sequence shown as SEQ ID No.1. The preparation method of the classical swine fever virus recombinant subunit vaccine typically includes the following steps: classical swine fever E2 truncated protein (TE2) coding gene is cloned into baculovirus vector pFastBacTM1, and is then transfected into Sf9 insect cells to obtain recombinant baculovirus capable of expressing protein TE2. The high five insect cells in logarithmic growth phase are infected by the recombinant baculovirus, so that a large amount of the protein TE2 can be expressed in a cell culture supernatant. Finally, the cell culture supernatant is recovered and purified to obtain a large amount of the recombinant protein TE2 with the purity more than 90%. According to the method, the target protein can be harvested from the cell culture supernatant, the time of protein purification is reduced, consumption of a large amount of time can be avoided, and the vaccine production process can be simplified. Under the premise of simplification of the vaccine production process, the recombinant protein TE2 has the advantages of strong immunogenicity and high safety, and the animal experiments prove that the recombinant protein can effectively stimulate the body to produce a highly effective humoral immune response.
Owner:NOVO BIOTECH CORP
Preparation methods and application of recombinant swine fever E2 protein and subunit vaccine of recombinant swine fever E2 protein
PendingCN107674883AIncrease productionImprove securitySsRNA viruses positive-senseViral antigen ingredientsProtein targetVaccine Production
The invention discloses preparation methods and application of recombinant swine fever E2 protein and a subunit vaccine of the recombinant swine fever E2 protein. The preparation method of the recombinant swine fever E2 protein comprises the following steps that (1) a swine fever E2 protein coding gene is cloned into an eukaryotic expression vector to obtain recombinant plasmid containing the swine fever E2 protein coding gene; (2) then, the recombinant plasmid containing the swine fever E2 protein coding gene is transfected into a CHO cell strain; (3) the CHO cell strain obtained in the step(2) is cultured, screened and domesticated; and (4) the cell strain in the step (3) is fermented and cultured; and the recombinant swine fever E2 protein is obtained after purification. The methods provided by the invention have the advantages that the target protein can be obtained from cell culture supernatant; the yield reaches up to 1g / L; the protein purification time is shortened; the vaccineproduction steps are simplified; and the vaccine production cost is also greatly reduced.
Owner:NOVO BIOTECH CORP
Ambroxol hydrochloride liquid preparation and preparation method thereof
ActiveCN101627967AImprove solubilityOvercome the defect of being slightly soluble in waterOrganic active ingredientsPharmaceutical delivery mechanismWater useBULK ACTIVE INGREDIENT
The invention discloses an ambroxol hydrochloride liquid preparation and a preparation method thereof. The method comprises the steps: dissolving ambroxol hydrochloride, stabilizing agent and osmotic pressure regulator into water used for injection, and evenly mixing together to obtain solution I; then, filtering the solution I, and obtaining the ambroxol hydrochloride liquid preparation. The preparation method does not introduce active carbon, so as to avoid the danger of hurting human body since active carbon particle is introduced into the preparation; meanwhile, the active ingredients in the preparation is ensured to be stable, and the safety (namely, the chemical stability of the ambroxol hydrochloride can be effectively improved, the particle content in the preparation is reduced, and the purity of the preparation is improved) of the finished product can be guaranteed.
Owner:上海华源药业(宁夏)沙赛制药有限公司
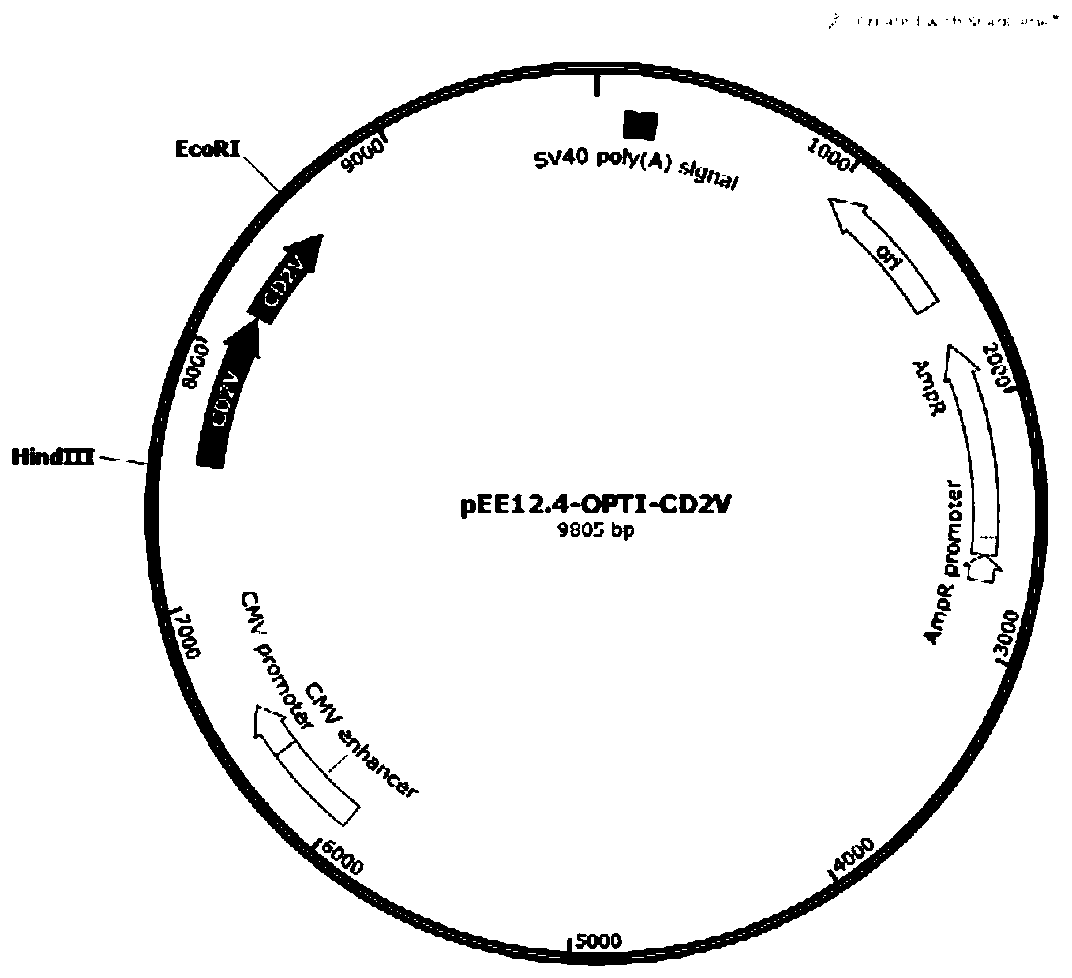
Recombinant African swine fever virus CD2V subunit protein as well as preparation method and application thereof
PendingCN111471089ABatch-to-batch stabilityImprove controllabilityViral antigen ingredientsVirus peptidesAdjuvantAfrican swine fever
The invention discloses a recombinant African swine fever virus CD2V subunit protein as well as a preparation method and application thereof. The protein comprises an extracellular region and an intracellular region of African swine fever virus surface envelope protein, and the amino acid sequence of the protein is shown as SEQ ID NO.3. The preparation method comprises the following steps: 1) cloning a codon-optimized gene sequence shown as SEQ ID NO.1 into an eukaryotic expression vector; 2) transfecting a recombinant expression vector containing the African swine fever virus subunit proteincoding gene into CHO cells; 3) culturing, screening and domesticating a CHO cell strain in the step 2) to obtain a highly-expressed cell strain; 4) fermenting and culturing the cell strain in the step3), and performing purifying to obtain the African swine fever virus CD2V subunit protein; and 5) mixing the CD2V protein with a pharmaceutically acceptable adjuvant to obtain a subunit vaccine. Theinvention can provide the African swine fever surface CD2V subunit protein which can be industrially produced on a large scale, the preparation method is simple and low in cost, and the prepared vaccine can reach the existing national standard.
Owner:NOVO BIOTECH CORP
Aptamer modified triazine covalent organic framework composite material and preparation method and application thereof
ActiveCN109174009AGood choiceMild preparation conditionsOther chemical processesMaterial analysis by electric/magnetic meansNanoparticleTriazene
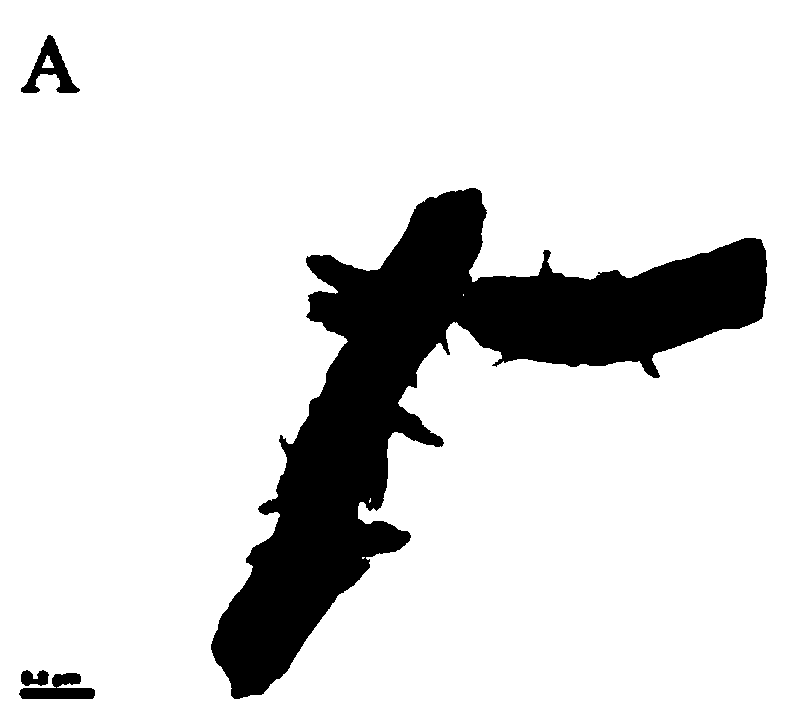
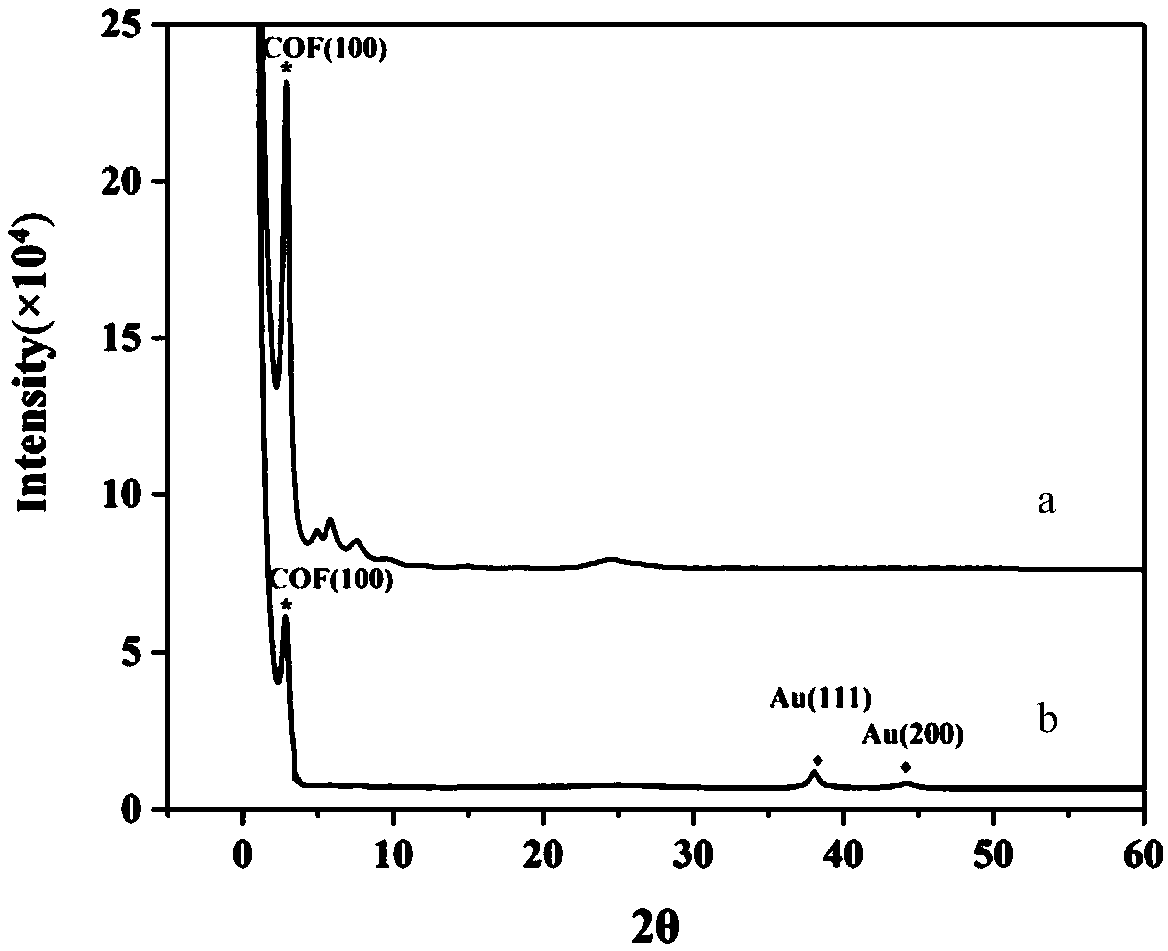
The invention relates to an aptamer modified triazine covalent organic framework composite material and a preparation method and application thereof. The aptamer modified triazine covalent organic framework composite material disclosed by the invention comprises a triazine covalent organic framework, gold nanoparticles and an aptamer, wherein the gold nanoparticles are supported on the triazine covalent organic framework; and the aptamer is bonded onto the surface of the gold nanoparticles. When the sequence of the aptamer is 5'-SH-(CH2)6-(ACAG4TGTG4)2-3', the composite material disclosed by the invention can be applied to enrichment of insulin in biological samples. The aptamer modified triazine covalent organic framework composite material disclosed by the invention combines the advantages of the covalent organic framework such as large specific surface area and multiple adsorbable sites and the advantage of the aptamer such as specific recognition, has excellent selectivity, and canprovide an economic, high-efficiency and high-sensitivity biological analysis method.
Owner:SUN YAT SEN UNIV
Method for continuously preparing large-aperture nitrocellulose film
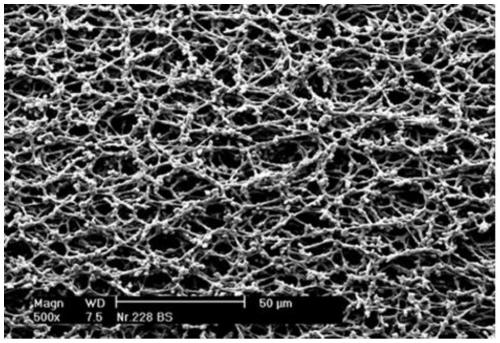
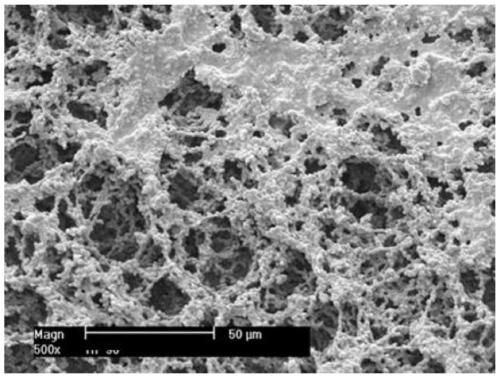
The invention discloses a method for continuously preparing a large-aperture nitrocellulose film, wherein the method comprises the steps: preparing a film solution: adding a cosolvent, a surfactant, water and a high polymer material nitrocellulose into a reaction kettle, mixing, after mixing, adding a solvent, and dissolving and stirring at normal temperature; defoaming and filtering to obtain a uniform film casting solution for later use; carrying out tape casting on the film casting solution through a tape casting machine; carrying out evaporation molding on the tape-casting wet film throughan evaporation section; and drying: drying in a drying section after film formation, and rolling after drying to obtain the large-aperture nitrocellulose film. The large-aperture nitrocellulose filmcan be continuously cast through continuous operation of the tape casting machine. According to the invention, the raw material ratio and the film forming process are improved, and the stable, uniformand firm nitrocellulose film can be produced. A stable and uniform film is formed by blending a film casting solution and controlling film forming conditions through the precise tape casting machine,cleaning and dust removal are not needed, and the film has natural permanent hydrophilicity and can be used for an in-vitro diagnosis test strip with high requirements.
Owner:上海市新亚净化器件厂
Puerarin liquid formulation and preparation method thereof
ActiveCN101416939AImprove solubilityOvercome the defect of being slightly soluble in waterOrganic active ingredientsSugar derivativesPuerarinMicroparticle
The invention discloses a puerarin liquid preparation and a preparation method thereof. The method comprises the following steps: puerarin is dissolved in water and then added with an iso-osmotic regulator, thus obtaining a solution I by mixing evenly; the solution I is filtered with the aperture of 0.22 Mum, thus obtaining a filtering solution a; the filtering solution a is treated with ultrafiltration by an ultrafiltration membrane with the MWCO of 10000 Dalton, thus obtaining a filtering solution b; the filtering solution b is filtered by the aperture of 0.22 Mum, thus obtaining a filtering solution c; and the filtering solution c is filtered by the aperture of 0.22 Mum, thus obtaining the puerarin liquid preparation. The preparation method of the puerarin liquid preparation overcomes the defect that the existing preparation has a plurality of untoward reaction factors. Experiments prove that the preparation prepared by the method does not contain a solubilizing agent, the content of other particles and bacteria is low, the puerarin has high purity and good stability; in addition, products of each batch produced by the method of the invention meet quality standards, have batch to batch stability and reliable quality; the method of the invention has simple process and is convenient for production operation and control and suitable for mass production.
Owner:上海华源药业(宁夏)沙赛制药有限公司
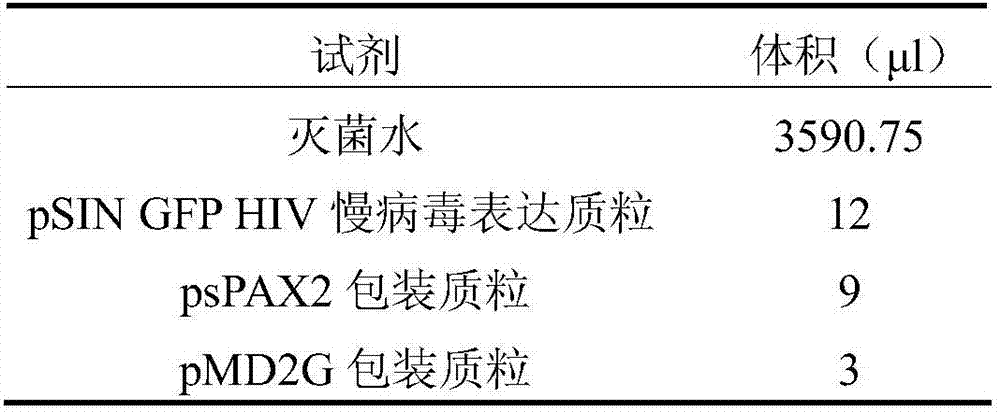
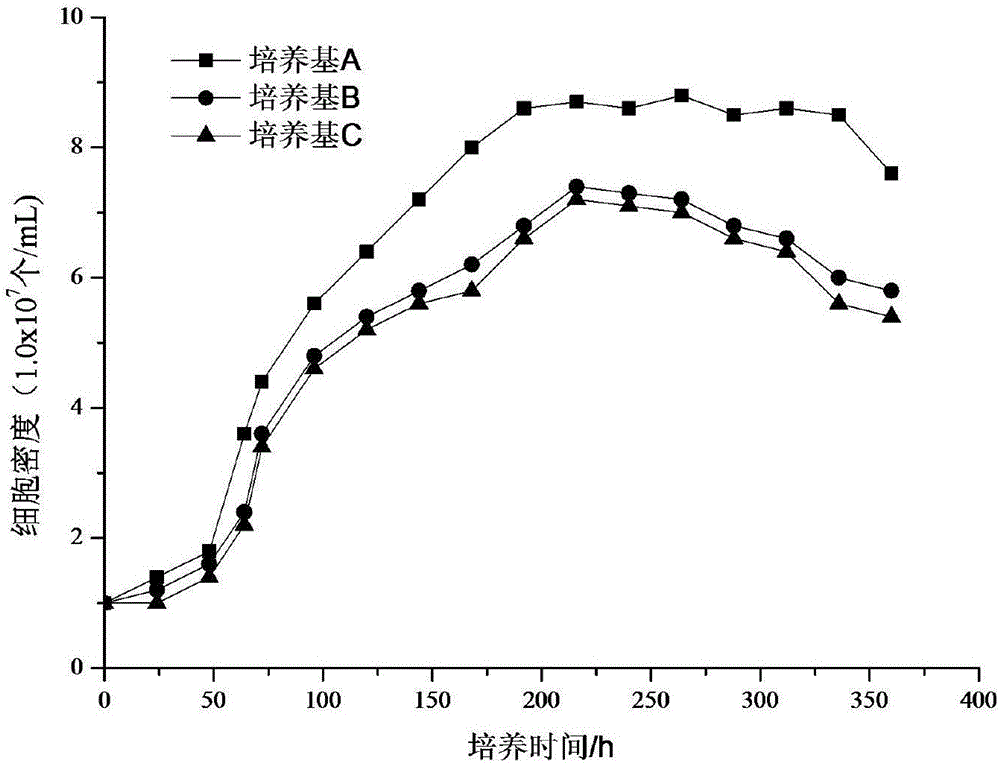
Culture solution for culturing 293T cells in serum-free suspension mode
ActiveCN108004202ABatch-to-batch stabilityQuality is easy to controlCulture processEpidermal cells/skin cellsInsulin-like growth factorVitamin C
The invention discloses a culture solution for culturing 293T cells in a serum-free suspension mode. According to the culture solution for culturing the 293T cells in the serum-free suspension mode, ahigh-glucose DEMEM-F12 culture substrate without L-glutamine serves as a base solution, and L-alanyl-L-glutamine, vitamin C, HEPES, an insulin-like growth factor, an epidermal growth factor, lipid concentrate, human serum albumin, transferrin, trehalose, heparin sodium and Pluronic F-68 are added. The culture solution for culturing the 293T cells in the serum-free suspension mode contains clear components without any serum components, is explicit in component, stable in batch, controllable in quality, low in cost and suitable for not only routine culture in laboratories but also large-scale production culture, and has broad application prospects.
Owner:济南赛尔生物科技股份有限公司
Glycyrrhiza polysaccharide-containing and animal origin-free low protein culture medium
InactiveCN106754693AReasonable collocationLow protein concentrationBlood/immune system cellsAnimal proteinsChloride sodium
The invention belongs to the technical field of cell culture in vitro and particularly relates to a glycyrrhiza polysaccharide-containing and animal origin-free low protein culture medium. The culture medium is prepared from the following components: glycyrrhiza polysaccharide, glucose, soybean hydrolysate, putrescine, spermine, ornithine, sodium pyruvate, amino acid, biotin, vitamin D, folic acid, ferrous sulfate, ferric nitrate, EDTA (Ethylene Diamine Tetraacetic Acid).2Na, cholamine, glycerinum, oleum morrhuae, cholesterol, linoleic acid, thymidine, hypoxanthine, hydrocortisone, sodium selenite, tocopherol, sodium bicarbonate, calcium chloride, magnesium chloride, magnesium sulfate, potassium chloride, sodium dihydrogen phosphate, sodium chloride, disodium hydrogen phosphate, zinc sulfate and copper sulfate. The culture medium disclosed by the invention comprises added glycyrrhiza polysaccharide, and does not contain any added supplementary animal origin protein and / or recombinant animal protein; rapid proliferation of cells can be realized, and the expression ability of the cells is effectively improved.
Owner:严志海
GB subunit recombinant protein of porcine pseudorabies virus, and preparation method and application of gB subunit recombinant protein
ActiveCN112142827ABatch-to-batch stabilityImprove controllabilityVirus peptidesDsDNA virusesBaculovirus expressionCell strain
The invention provides a gB subunit recombinant protein of a porcine pseudorabies virus, and a preparation method and application of the gB subunit recombinant protein. The preparation method comprises the following steps of 1) cloning an optimized gB gene sequence into an eukaryotic expression vector to obtain a recombinant plasmid containing a gB subunit protein coding gene of the pseudorabies virus; 2) transfecting the recombinant plasmid containing the gB subunit protein coding gene of the pseudorabies virus into an expression cell; 3) culturing, screening and domesticating the expressioncell in the step 2) to obtain a highly expressed cell strain; and 4) fermenting and culturing the cell strain in the step 3), and performing purification to obtain the gB subunit protein of the pseudorabies virus. The invention provides the gB subunit protein of the porcine pseudorabies virus, and the gB subunit protein can be industrially produced in a large scale; the preparation method is simple; the cost is low; and the yield is much higher than that of an existing baculovirus expression system.
Owner:NOVO BIOTECH CORP
Mammalian cell culture technology for enhancing monoclonal antibody ADCC activity
InactiveCN105713946AIncrease the degree of basementImprove ADCC activityMicroorganism based processesFermentationMonoclonal antibodyMammalian cell
The invention discloses a process approach suitable for mammalian cell culture and rapidly and effectively improving the ADCC activity of monoclonal antibodies. The process is characterized in that the glycosylation of monoclonal antibodies is controlled by adding nucleosides and metal ions during the culture process level, and increase the ADCC activity of the monoclonal antibody. The process has the characteristics of good stability, strong operability, easy scale-up, and small difference in results between batches. It can be applied to large-scale mammalian cell culture to express monoclonal antibodies, especially for industrial production of therapeutic monoclonal antibodies.
Owner:HAISCO PHARMA GRP INC
Novel gene engineering subunit vaccine for mycoplasma gallisepticum
ActiveCN109999191ANot pathogenicReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsNucleotideVaccine Production
The invention provides an immunological composition and a subunit vaccine. The immunological composition comprises a protein which is selected from one or an arbitrary combination of two or more of mycoplasma gallisepticum-associated proteins encoded with nucleic acid molecules of SEQ ID NO: 1 or 3 or 5 or 7 or 9 or nucleic acid molecules which are 95% or above identical to the nucleotide sequenceof SEQ ID NO: 1 or 3 or 5 or 7 or 9. The vaccine adopts eukaryotic expression, the antigenicity and immunogenicity of the product are similar to those of a natural protein, the expression level is high, the immunogenicity is strong, the protective effect is good, and the vaccine has no pathogenicity to chickens; besides, large-scale serum-free suspension culture preparation of the vaccine can berealized through a bioreactor, and meanwhile the vaccine production cost is greatly reduced.
Owner:苏州沃美生物有限公司
Soybean peptide effervescent tablets and preparation method thereof
PendingCN110604248AGreat tasteEasy to shapeFood ingredient as mouthfeel improving agentProtein food ingredientsEffervescent tabletFruit juice
The invention relates to the field of food, and particularly provides soybean peptide effervescent tablets and a preparation method thereof. The soybean peptide effervescent tablets provided by the invention are prepared from acid particles, alkali particles and mixed auxiliary materials in parts by weight, wherein the alkali particles are prepared from, in parts by weight, 600-700 parts of soybean peptide powder, 250-330 parts of lactose, 45-55 parts of a sweetening agent, 450-550 parts of sodium carbonate and 50-100 parts of potassium chloride; the acid particles are prepared from, in partsby weight, 250-300 parts of maltodextrin, 1600-1750 parts of citric acid and 100-250 parts of glucose; and the mixed auxiliary materials are prepared from, in parts by weight, 150-250 parts of fruit juice powder, 35-50 parts of essence and 15-65 parts of a bean product defoaming agent. The soybean peptide effervescent tablets are better in tablet shape, accessible to more people and short in disintegration time. Most importantly, according to the formula, the proportion of effective nutrient substance soybean peptides in the soybean peptide effervescent tablets is greatly increased, and the overall efficacy of a product is improved.
Owner:博凯药业有限公司
Novel genetic engineering subunit vaccine for avian Newcastle disease viruses
ActiveCN111154778AGood immune effectProcess safetySsRNA viruses negative-senseViral antigen ingredientsAdjuvantF protein
Owner:苏州沃美生物有限公司
Preparation of CHO cell expressed infectious bovine rhinotracheitis virus protein gD and subunit vaccine thereof and application
PendingCN107973840AIncrease productionImprove securityViral antigen ingredientsVirus peptidesProtein targetVaccine Production
The invention discloses preparation of CHO cell expressed recombinant infectious bovine rhinotracheitis virus protein gD and a subunit vaccine thereof and an application and belongs to the technical fields of animal vaccines and veterinary biologicals. The condition that the vaccine can generate relatively high humoral immunity in bovine bodies is proven. The object of the invention is to providea preparation method capable of industrially producing the infectious bovine rhinotracheitis virus recombinant subunit vaccine on a large scale. The reparation method for the recombinant subunit vaccine comprises the following steps: 1) cloning an eukaryotic expression vector containing a protein gD coding gene; 2) transfecting CHO cells, and obtaining suspending CHO cell strains, which stably andefficiently express the protein gD, in a selecting, screening and acclimatizing manner; 3) subjecting the cell strains obtained in the step 2) to fermented culture, and carrying out purification, soas to obtain recombinant protein gD; and 4) uniformly mixing the recombinant protein gD and ISA 201 VG thoroughly, thereby obtaining the recombinant subunit vaccine. According to the method provided by the invention, target protein can be obtained from cell culture supernatant, the yield reaches up to 2g / L to 3g / L, the protein purification time is shortened, the vaccine production steps are simplified, and the vaccine production cost is greatly reduced.
Owner:NOVO BIOTECH CORP
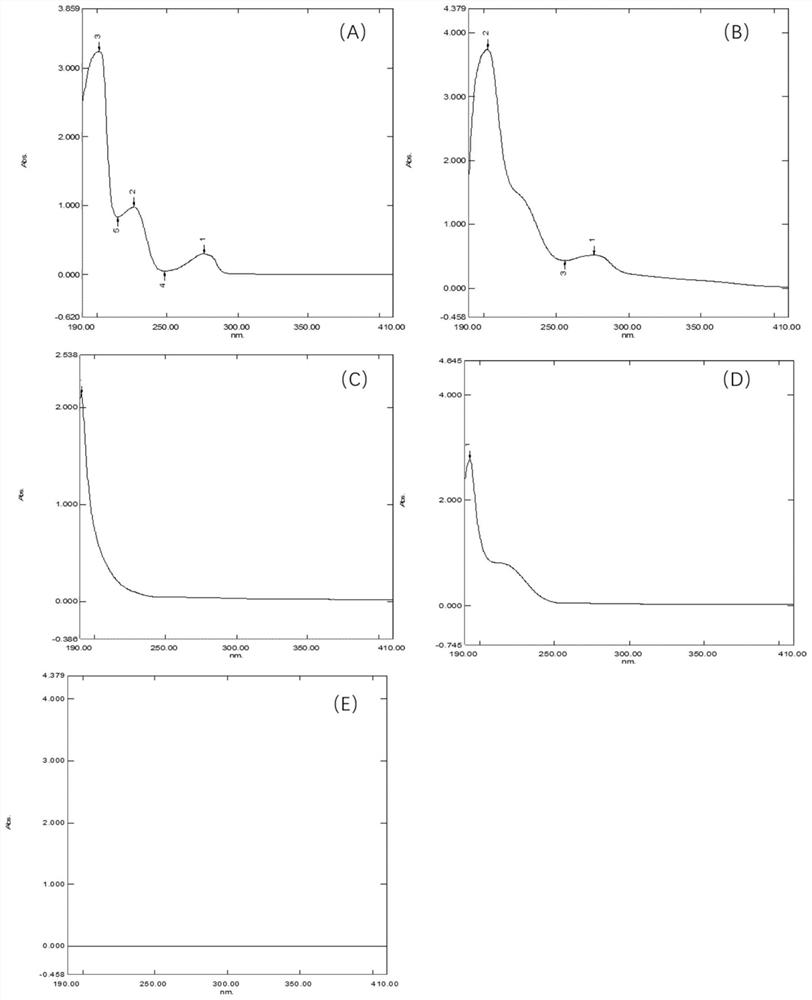
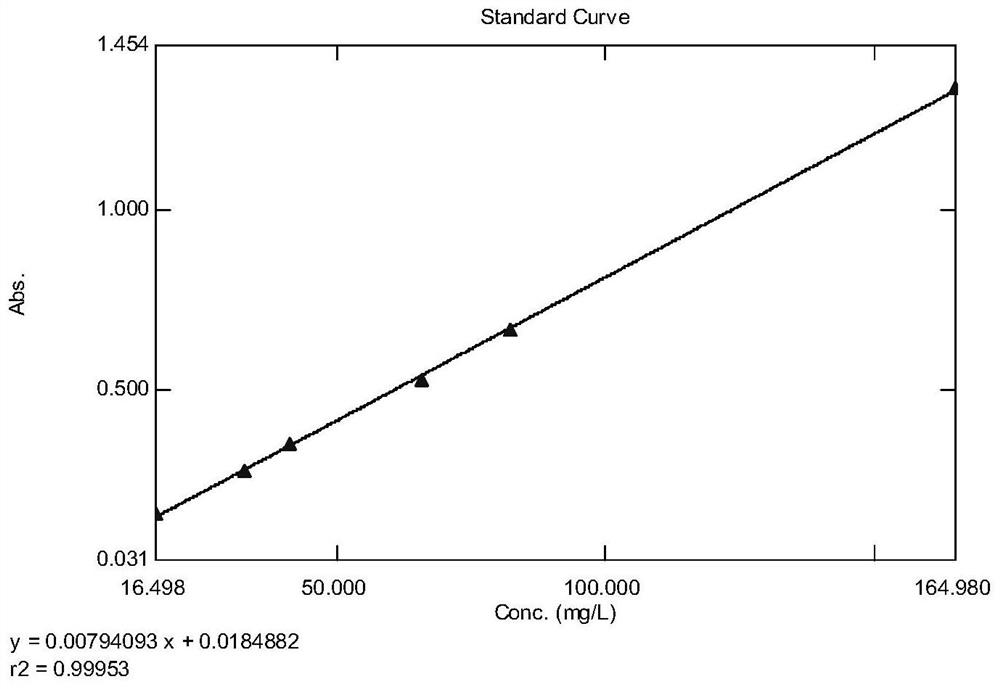
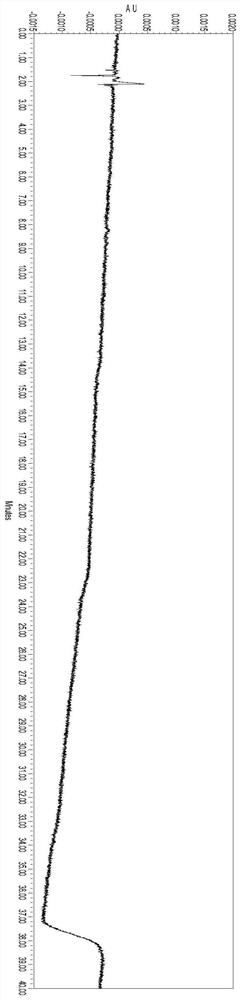
Method for determining total lignans in Shuxuening injection
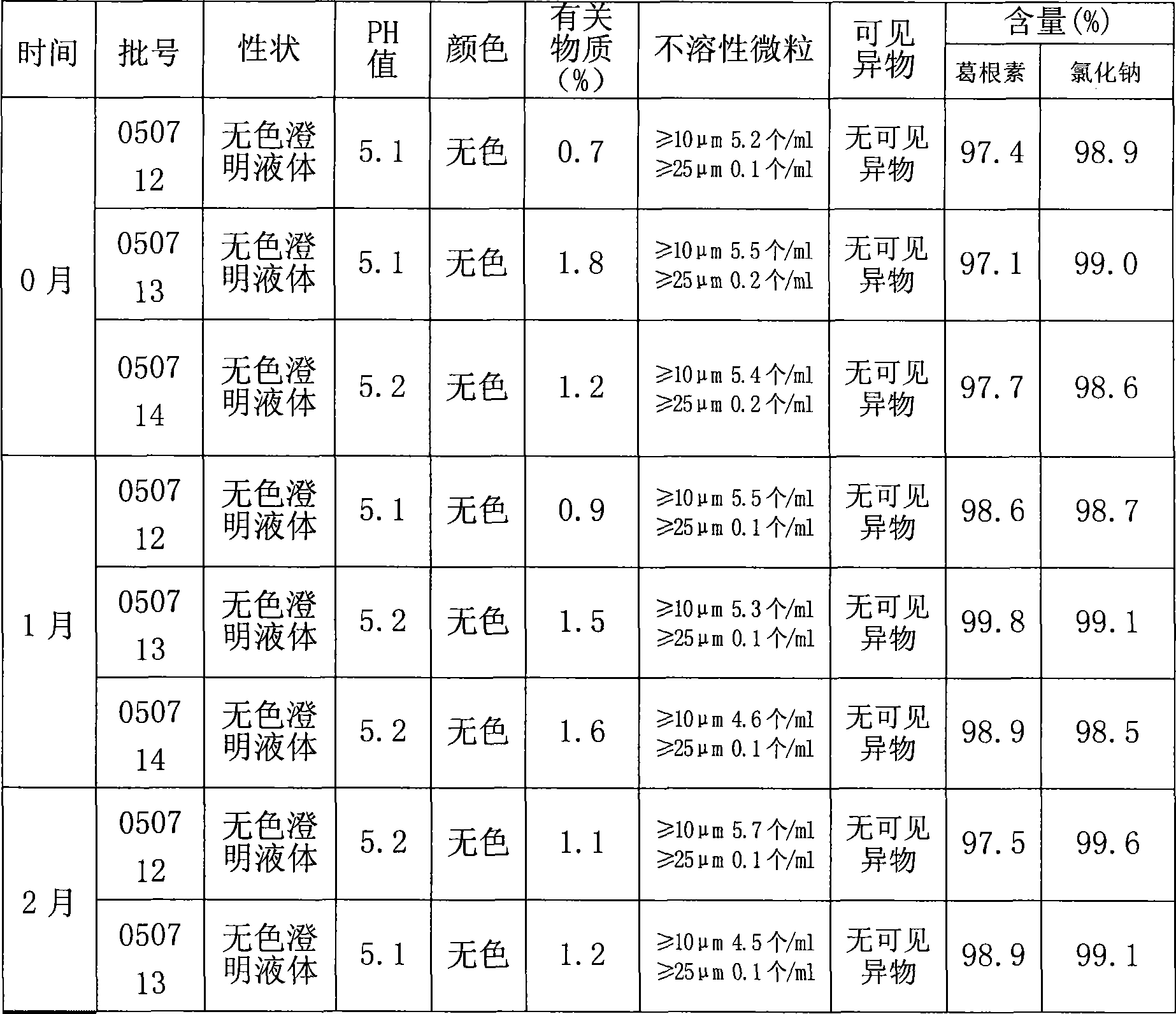
PendingCN112067726ATotal lignan content controlUniformity between batchesComponent separationGinkgo bilobaLignan
The invention relates to the technical field of detection of active ingredients of ginkgo leaves, in particular to a method for determining total lignans in a Shuxuening injection. The determination method adopts ultraviolet dual-wavelength spectrophotometry, and comprises the following steps of: providing a Shuxuening injection; removing flavonoid components in the Shuxuening injection; and detecting the Shuxuening injection without the flavonoid components as a test solution. The method provided by the invention can accurately determine the content of the total lignans in the Shuxuening injection, so that the content of the total lignans in the Shuxuening injection is effectively controlled, and the uniformity and stability among batches of products are ensured.
Owner:HEILONGJIANG ZBD PHARMA +1
Free triiodothyronine measurement kit and fabrication method thereof
InactiveCN109239371AHigh affinityStrong specificityChemiluminescene/bioluminescenceBiological testingMicrosphereHook effect
The invention discloses a free triiodothyronine measurement kit and a fabrication method thereof, and belongs to the technical field of vitro inspection. By the free triiodothyronine measurement kit,the technical problems of long reaction time, slow measurement speed, narrow of linear range and hook effect easy to generate of the prior art when a tested substance is high in concentration are solved. The kit comprises the following reagents of a solid-phase reagent 1 and a liquid-phase reagent, wherein the solid-phase reagent R1 is a suspension liquid including a T3 analogue-coated paramagnetism microsphere, the liquid-phase reagent R2 is a suspension liquid including an acridinium ester labeled T3 antibody, the paramagnetism microsphere in the R1 is Fe3O4 with a surface wrapped by a carboxyl active group, and the particle size of the paramagnetism microsphere is 0.1-5 micrometers. With the free triiodothyronine measurement kit and the fabrication method thereof, provided by the invention, free triiodothyronine in blood / plasma is quantitatively detected by a chemiluminescence immunity analysis method, the kit has the advantages of high sensitivity, rapid detection, wide linear range, low cost and the like and is simple and convenient to operate, and automation is facilitated.
Owner:DIRUI MEDICAL TECH CO LTD
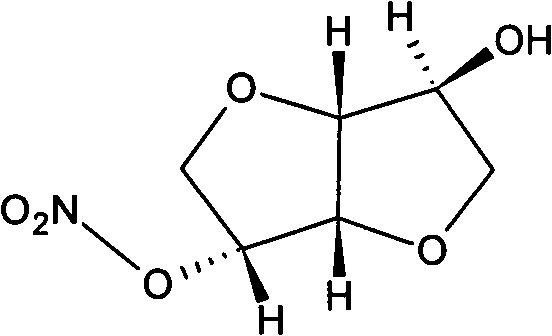
Isosorbide mononitrate liquid preparation and preparing method thereof
ActiveCN101637448AImprove solubilityOvercome the defect of being slightly soluble in waterInorganic non-active ingredientsPharmaceutical delivery mechanismWater useActivated carbon
The invention discloses an isosorbide mononitrate liquid preparation and a preparing method thereof. The method comprises the steps: dissolving isosorbide mononitrate, stabilizing agent and osmotic pressure regulator into water used for injection, and evenly mixing together to obtain solution I; then, filtering the solution I, and obtaining isosorbide mononitrate injection. As active carbon is notintroduced into the preparing method, the active carbon particles are prevented from being introduced into the preparation to be harmful to the human body, and the stability of the active ingredientsin the preparation as well as the safety of the finished product can be ensued (namely, the chemical stability of the isosorbide mononitrate is effectively improved, the particulate content in the preparation is reduced, and the purity of the preparation is increased).
Owner:上海华源药业(宁夏)沙赛制药有限公司
Kit for determining thyrotropin and preparation method of kit
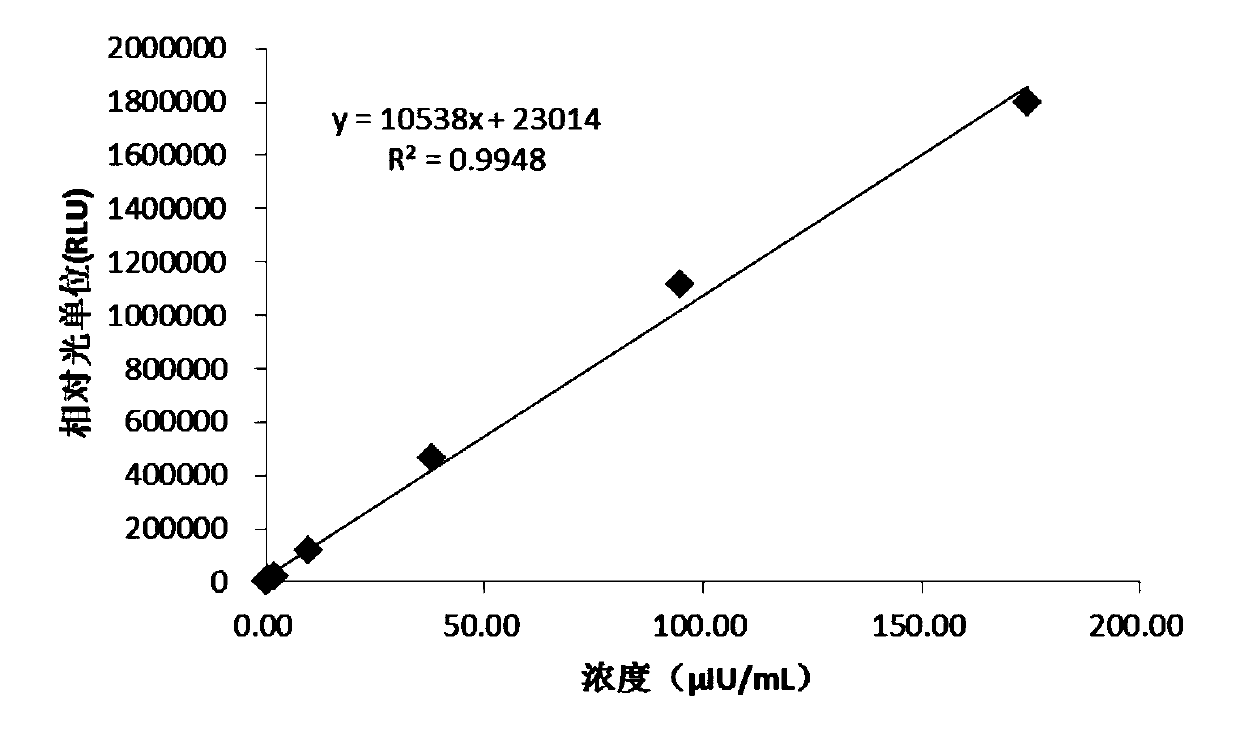
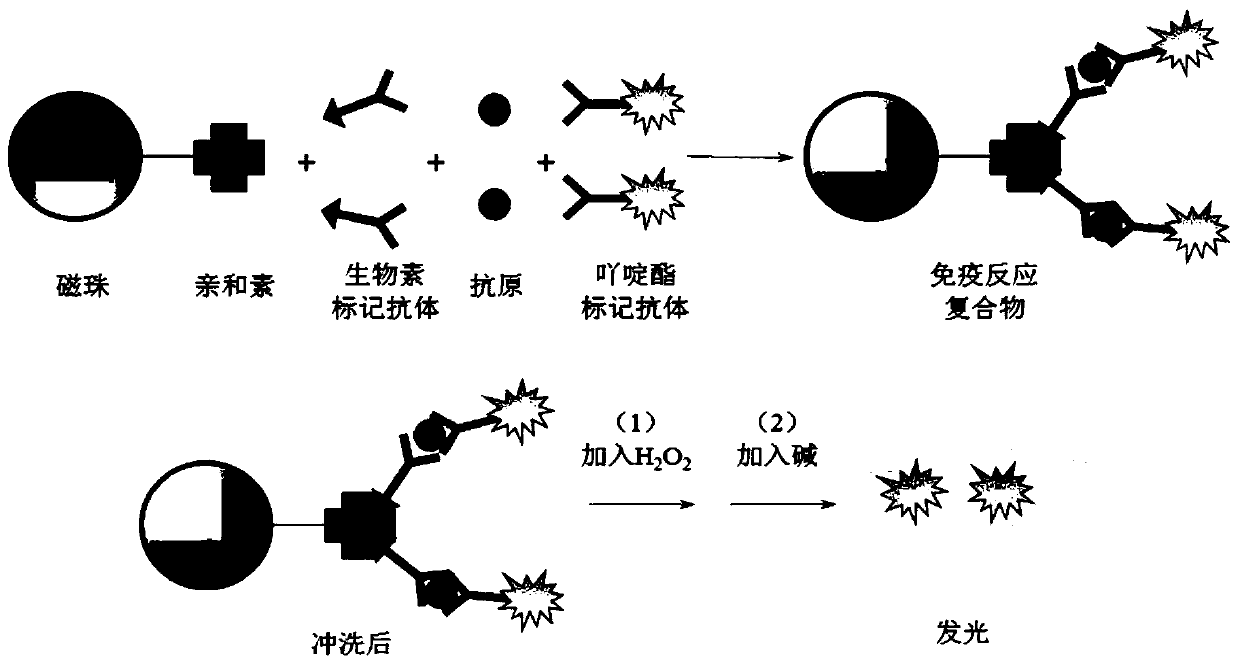
InactiveCN110618280AHigh affinityStrong specificityBiological testingBiotin-streptavidin complexLuminous intensity
The invention discloses a kit for determining thyrotropin and a preparation method of the kit. The kit comprises the following reagents of: a reagent R1, including a streptavidin magnetic particle solution, a reagent R2 including a chemiluminescence marker-labelled thyrotropin antibody, and a reagent R3 including a biotin-labeled thyrotropin antibody, wherein the mass percent of streptavidin magnetic particles in the reagent R1 is 0.07-0.2% and the particle sizes are 1-3 microns; the molar ratio of the thyrotropin antibody to a chemiluminescence marker in the reagent R2 is 1:1.5 to 1:10; and the molar ratio of the thyrotropin antibody to biotin in the reagent R3 is 1:5 to 1:50. The kit has the advantages of being high in sensitivity, high in specificity, free of a catalyst, stable in labelled conjugate, free of radioisotope damage and contamination, short in luminous time, stable in luminous intensity, high in reaction speed and high in specificity, and does not affect binding of a to-be-determined object after being connected with the antibodies.
Owner:DIRUI MEDICAL TECH CO LTD
Pepsinogen II recombinant protein and its monoclonal antibody, preparation method and application thereof
InactiveCN113046324AIncrease productionImprove controllabilityVector-based foreign material introductionPeptidasesAntigen epitopeAntigen
The invention discloses a CHO-K1 cell strain, which contains a gene capable of efficiently secreting and expressing PG II recombinant protein. The invention also discloses a method for preparing the CHO-K1 cell strain, and the method comprises the following steps: 1) cloning a gene sequence as shown in SEQ ID NO.1 into an eukaryotic expression vector to obtain a recombinant plasmid containing the gene encoding the PG II recombinant protein; (2) transfecting the recombinant plasmid into a CHO-K1 cell, so as to obtain a CHO-K1 cell strain; and 3) culturing, screening and domesticating the CHO-K1 cell strain in the step 2) to obtain the cell strain capable of efficiently secreting and expressing the PG II recombinant protein. The invention also discloses an anti-PG II monoclonal antibody, wherein the antigen epitope combined with a monoclonal antibody 1 is located at aa78-aa90 of the pepsinogen II; and the antigen epitope combined with a monoclonal antibody 2 is located at aa280-aa291 of the pepsinogen II. The protein provided by the invention is high in expression quantity, good in quality and low in cost; the monoclonal antibody can be paired and used for detecting the PG II protein, and is good in specificity and high in sensitivity.
Owner:黎榕萍
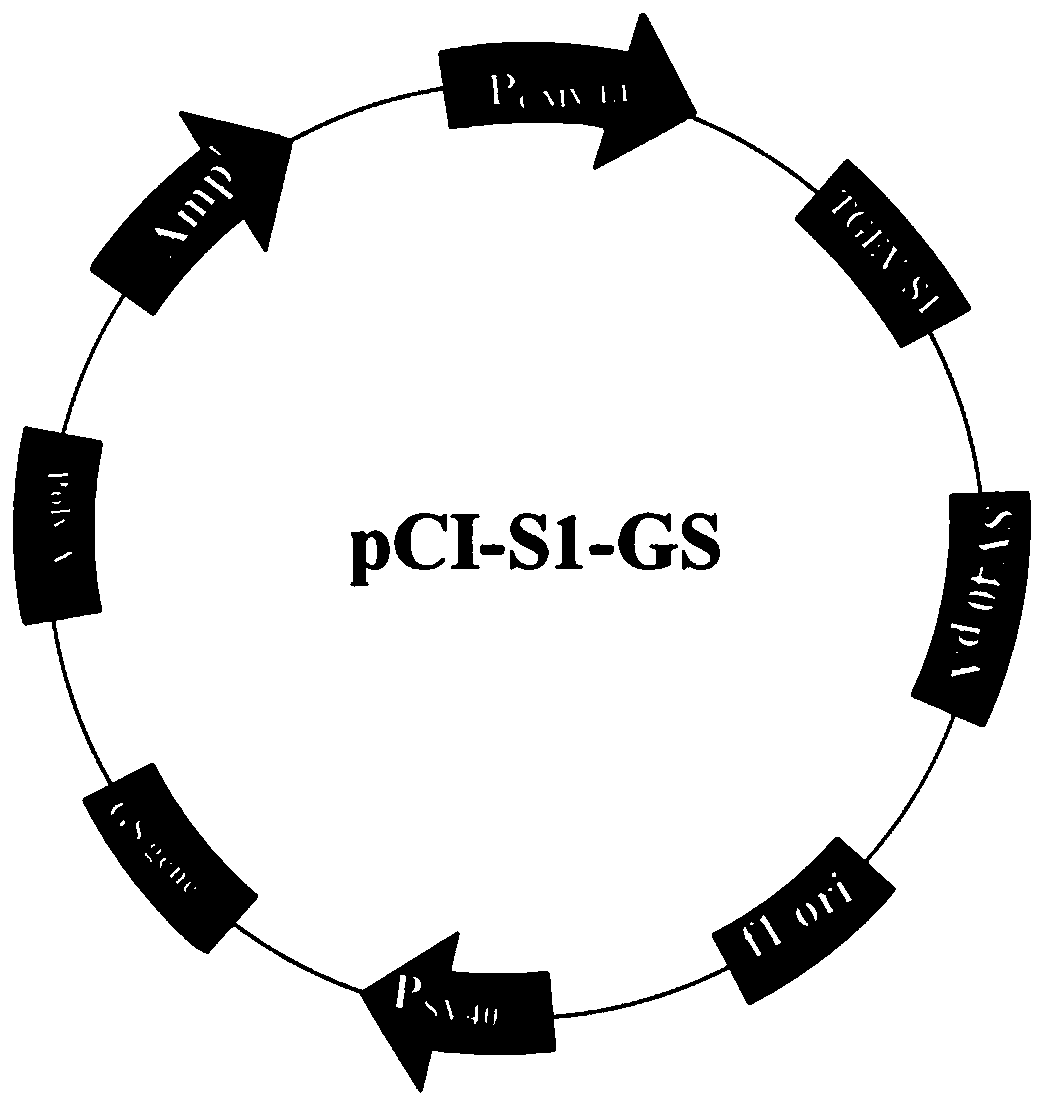
New genetic engineering subunit vaccine for transmissible gastroenteritis virus of swine
InactiveCN110041410AReduce purification timeSimplify production stepsSsRNA viruses positive-senseVirus peptidesNucleotideSwine Transmissible Gastroenteritis
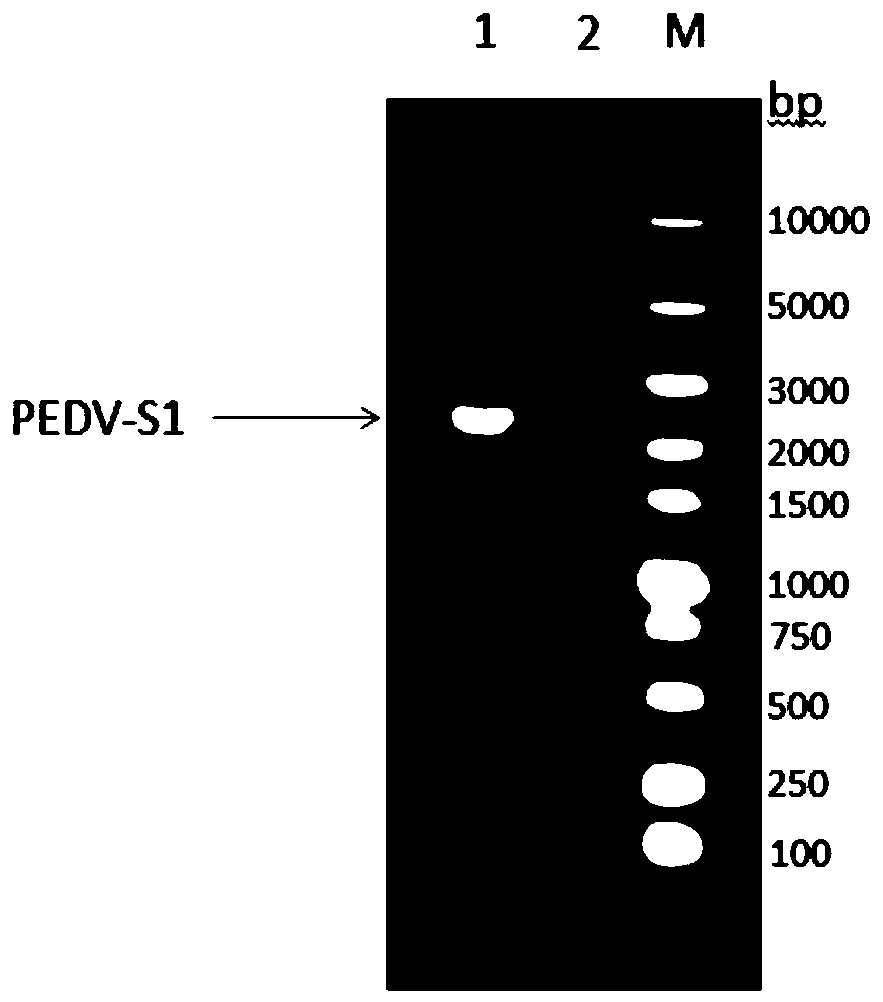
The present application provides an immunological composition and a subunit vaccine. The immunological composition comprises: a swine transmissible gastroenteritis virus S1 protein encoded by a nucleic acid molecule of SEQ ID NO:1 or a nucleic acid molecule of 95% or more identical to the nucleotide sequence of the SEQ ID NO:1. The vaccine uses an eukaryotic expression of CHO cells, is sufficientin protein glycosylation, good in antigen protein immunogenicity, and also very high in expression levels, and reaches 2-3 g / L. The recombinant cells can be subjected to suspension cultivation in a large scale, which greatly reduces complexity of vaccine preparation and saves production costs.
Owner:苏州世诺生物技术有限公司
Subunit H protein of peste des petits ruminants virus and preparation method and application of subunit H protein
ActiveCN111378016ASolve technical problems with high production costsLow viral loadSsRNA viruses negative-senseViral antigen ingredientsAnimals vaccinesImmunogenicity
The present invention belongs to the technical field of animal vaccines and veterinary biological products, and particularly relates to a subunit H protein of a peste des petits ruminants virus and apreparation method and application of the subunit H protein. The subunit H protein is a truncated head functional protein of an H protein of the peste des petits ruminants virus, amino acid sequencesof the subunit H protein are an amino acid sequence (1) as shown as SEQ ID NO.1 and a derived amino acid sequence (2) having the immunogenicity and obtained by performing substitution, deletion or addition of multiple amino acids on the sequence SEQ ID NO.1, and the derived amino acid sequence and the sequence SEQ ID NO.1 have the sequence identity of 80-100%. The subunit H protein is prepared mainly by constructing recombinant plasmids, transfecting cell strains with the recombinant plasmids, screening the high-expression cell strains and purifying the subunit H protein of the peste des petits ruminants virus, can be better suitable for subunit vaccines or diagnostic reagents of peste des petits ruminants viruses, and has the characteristics of efficient secretory expression, high proteinpurity, easy purification, low production cost, high safety performance and the like.
Owner:NOVO BIOTECH CORP
Genetic engineering subunit vaccine of avian infectious bronchitis
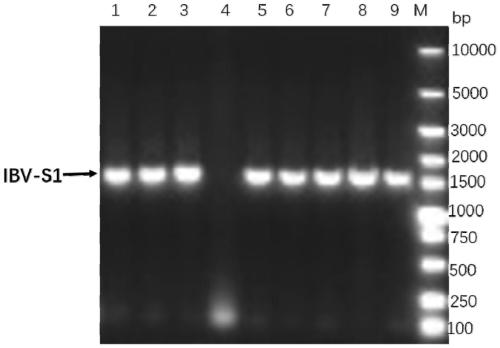
PendingCN109985235AReduce purification timeSimplify production stepsSsRNA viruses positive-senseViral antigen ingredientsNucleotideFhit gene
The invention provides a genetic engineering subunit vaccine of the avian infectious bronchitis. The genetic engineering subunit vaccine of the avian infectious bronchitis comprises avian infectious bronchitis virus S1 protein and S2 protein coded respectively by nucleic acid molecules shown as SEQ ID NO:1 or nucleic acid molecules identical with more than 95% of a nucleic acid sequence of SEQ IDNO:1 and nucleic acid molecules shown as SEQ ID NO:2 or nucleic acid molecules identical with more than 95% of a nucleic acid sequence of SEQ ID NO:2. A heterodimer structure can be formed between theS1 protein and the S2 protein and is similar to a virus surface structure, the S1 protein and the S2 protein are in eukaryotic expression and are sufficient in protein glycosylation, high in antigenprotein immunogenicity and quite high in expression quantity reaching 2-3g / L, recombinant cells are supportive of large-scale suspension culture, vaccine preparation complexity is lowered greatly, andproduction cost is lowered.
Owner:苏州世诺生物技术有限公司
Subunit fusion protein mG on surface of rabies virus as well as preparation method and application of subunit fusion protein mG
PendingCN112430273ABatch-to-batch stabilityIncrease productionSsRNA viruses negative-senseAntibody mimetics/scaffoldsExpression proteinMolecular biology
The invention provides a subunit fusion protein mG on the surface of rabies virus as well as a preparation method and application of the subunit fusion protein mG. The preparation method comprises thesteps: 1) cloning a gene sequence shown as SEQ ID NO.4 into an eukaryotic expression vector to obtain a recombinant plasmid containing a fusion protein MBP-G encoding gene; 2) transfecting the recombinant plasmid containing the fusion protein MBP-G encoding gene into an expression cell; 3) culturing, screening and domesticating the expression cell in the step 2) to obtain highly expressed cell strains; and 4) fermenting and culturing the cell strain in the step 3), and purifying to obtain the subunit fusion protein mG. MG can be expressed in a large amount in a soluble mode, protein is stable, the problems that rabies virus surface G protein cannot be expressed on a large scale and the like in the prior art are solved, and the preparation method is simple and low in cost.
Owner:NOVO BIOTECH CORP
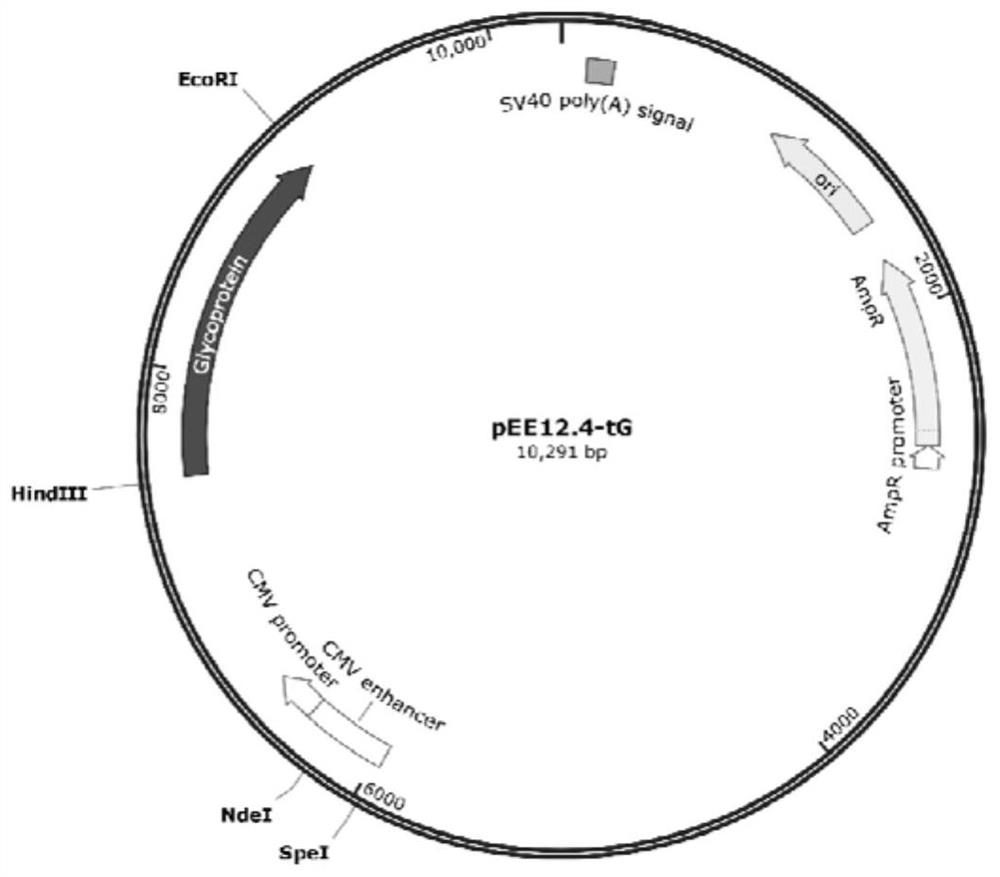
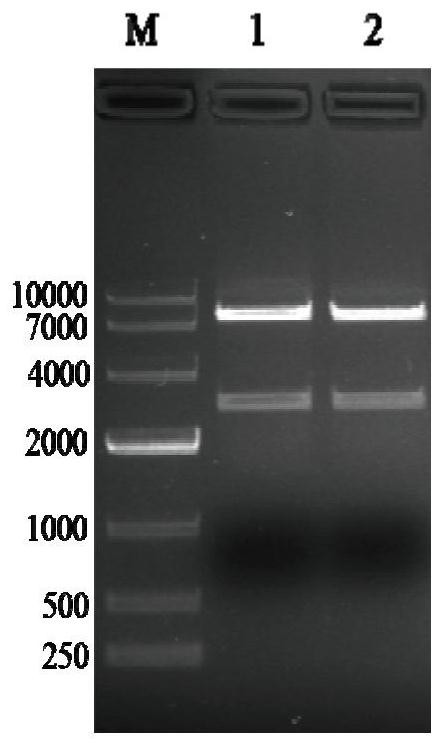
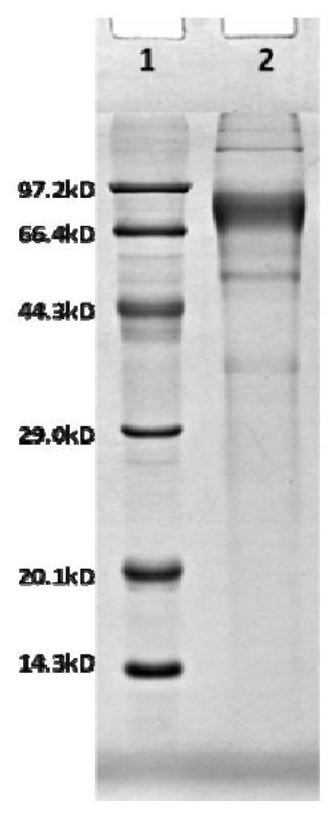
Subunit fusion protein tG on surface of rabies virus, and preparation method and application of subunit fusion protein tG
ActiveCN112142851ABatch-to-batch stabilityIncrease productionSsRNA viruses negative-senseAntibody mimetics/scaffoldsExpression proteinMolecular biology
The invention provides a subunit fusion protein tG on the surface of a rabies virus, and a preparation method and application of the subunit fusion protein tG. The preparation method comprises the following steps of 1) cloning a gene sequence shown as SEQ ID NO.4 into an eukaryotic expression vector to obtain a recombinant plasmid containing a fusion protein tG coding gene; 2) transfecting the recombinant plasmid containing the fusion protein tG coding gene into an expression cell; 3) culturing, screening and domesticating the expression cell in the step 2) to obtain a highly expressed cell strain; and 4) fermenting and culturing the cell strain in the step 3), and after purification, obtaining the subunit fusion protein tG. The tG can be expressed in a large amount in a soluble manner; the protein is stable; various problems that the G protein on the surface of the rabies virus cannot be expressed in a large scale and the like in the prior art are solved; and the preparation method issimple, and the cost is low.
Owner:NOVO BIOTECH CORP
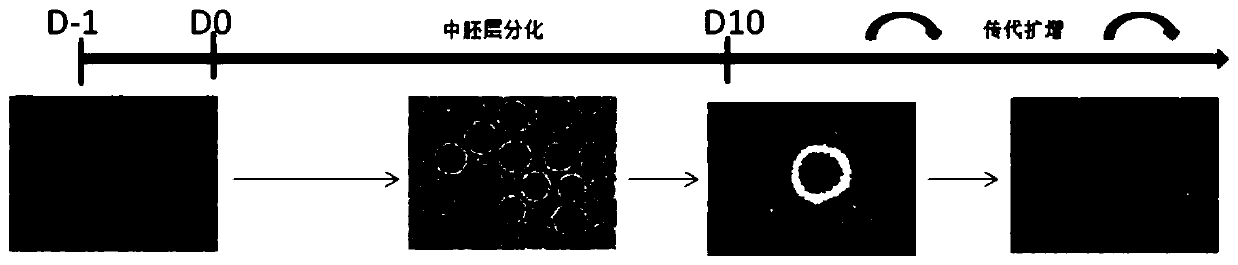
Mesenchymal stem cell, and preparation method and application thereof
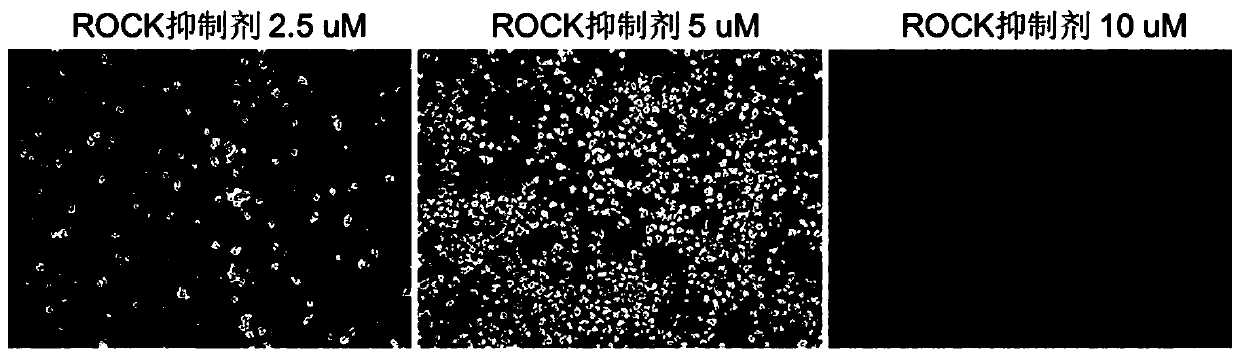
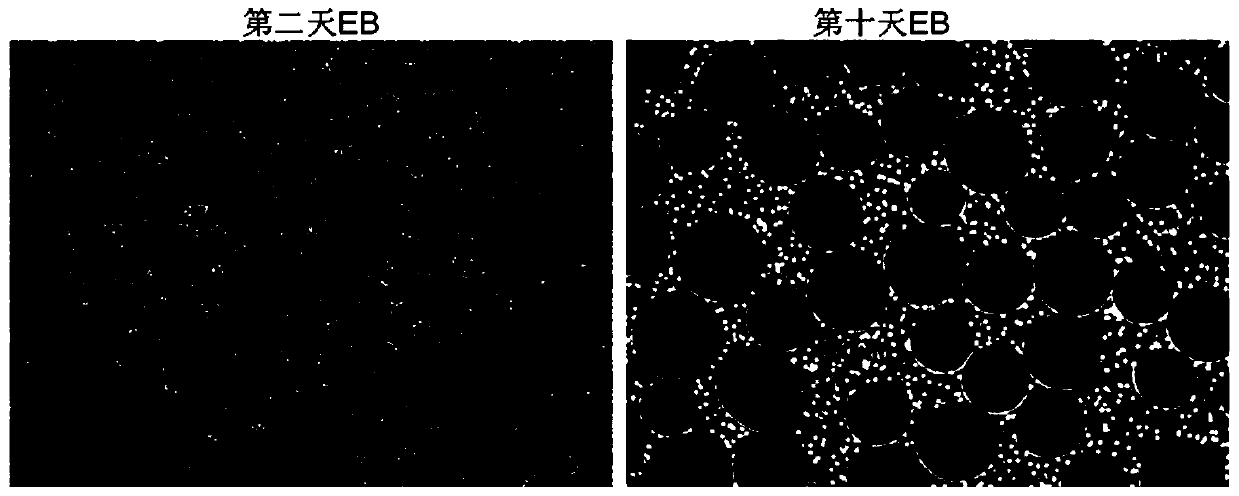
ActiveCN110592007AStable differentiationEfficient differentiationCulture processSkeletal/connective tissue cellsMesodermTrophoblastic cell
The present invention belongs to the field of stem cell biology, relates to lineage-specific differentiation of human multipotential or pluripotent stem cells, and particularly relates to a mesenchymal stem cell, and a preparation method and an application thereof. The method for preparing the mesenchymal stem cell comprises the following steps: S1, human multipotential stem cells form embryoid bodies; S2, the embryoid bodies differentiate into mesoderm cells; and S3, the mesodermal cells differentiate into the mesenchymal stem cells. The preparation method has a clear cell differentiation path, high differentiation efficiency, and stable differentiation effect and does not use serum-containing culture system or trophoblast cells, and the obtained cell population has high purity and largenumber, and thus is suitable for subsequent production and application of clinical-grade cell preparations.
Owner:安徽中盛溯源生物科技有限公司
Method for industrially preparing high-purity ergothioneine
PendingCN113666873AEasy to removeLarge amount of processingOrganic chemistryIon exchangeUltrafiltration
The invention provides a method for industrially preparing high-purity ergothioneine. The method comprises the following steps of: carrying out hot water treatment on mycelium, carrying out solid-liquid separation, and performing collecting to obtain feed liquid containing ergothioneine; carrying out vacuum concentration, filtration, ultrafiltration, decoloration, desorption and concentration on the feed liquid containing ergothioneine; performing chromatographic purification on an ergothioneine desorption concentrated solution, collecting a target peak, performing concentrating until the solution is dry, and dissolving a product in water to obtain an ergothioneine sample solution; desalting and decolorizing the ergothioneine sample solution through ion exchange resin, and performing collecting to obtain a desalted ergothioneine concentrated solution; and carrying out coarse crystallization and recrystallization on the desalted ergothioneine concentrated solution, and performing drying to obtain high-purity ergothioneine crystal powder. According to the method, a large amount of high-purity ergothioneine is prepared from the mycelia of the pleurotus ostreatus CGMCC No.23071, the product quality of the ergothioneine is ensured. The method is particularly suitable for large-scale production of the ergothioneine, so that the requirements of the product in the fields of food, cosmetics, health care, medicine and the like are met.
Owner:天津富麦生物科技发展有限公司
Genetic engineering subunit vaccine for porcine epidemic diarrhea viruses
InactiveCN109908336ANaturally safeIncrease gene copy numberAntiviralsDepsipeptidesNucleotideChinese hamster
The invention provides an immunological composition and a genetic engineering subunit vaccine. The immunological composition comprises porcine epidemic diarrhea virus protein S1 encoded by a nucleic acid molecule of SEQ ID NO: 1 or by a nucleic acid molecule 95% or more identical to the SEQ ID NO: 1 in the nucleotide sequence. The vaccine is subjected to eukaryotic expression by CHO (Chinese hamster ovary) cells, the protein glycosylation is sufficient, the immunogenicity of antigen protein is high, and the expression quantity is very high, reaching 2-3g / L; suspension cultivation of recombinant cells can be conducted on a large scale, the complexity of vaccine preparation is greatly reduced, and the production cost is reduced.
Owner:苏州世诺生物技术有限公司
Swine fever virus recombinant antigen as well as preparation method and application thereof
ActiveCN111718400AImproving immunogenicityReduce allergic reactionsSsRNA viruses positive-senseViral antigen ingredientsClassical swine fever virus CSFVSwine Fever Virus
The invention relates to a swine fever virus recombinant antigen as well as a preparation method and application thereof. The swine fever virus recombinant antigen is E2 protein modified by protein, wherein the protein modification comprises point mutation, and the point mutation is used for mutating 166th amino acid of the E2 protein into asparagine and mutating 229th amino acid into alanine. According to the swine fever virus recombinant antigen, the 229th Asn amino acid of a key glycosylation locus of the E2 protein is mutated into Ala, so that glycosylation can be removed; and furthermore,the 166th Asp amino acid is mutated into Asn, a new glycosylation locus is introduced, so that the immunogenicity of the protein can be increased, and the anaphylactic reaction of the protein can bereduced.
Owner:苏州世诺生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com