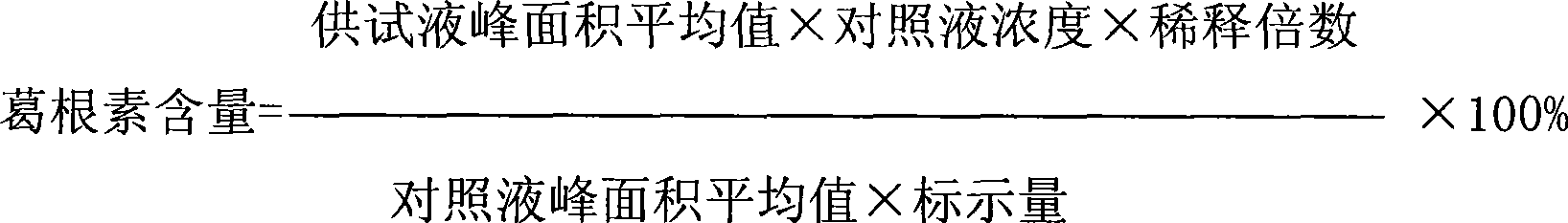Puerarin liquid formulation and preparation method thereof
A liquid preparation, puerarin technology, applied in blood diseases, pharmaceutical formulations, extracellular fluid diseases, etc., can solve problems such as allergies, pyrogens, tumor-like reactions, adverse reactions of propylene glycol, pollution particles and impurities, etc., to overcome adverse reactions Many factors, easy production operation and control, low content effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, the preparation of puerarin injection
[0033] One, the preparation of puerarin injection
[0034] In the aseptic operation room, dissolve 1 g of puerarin in water for injection at 80° C., then add 4 g of sodium chloride and stir until fully dissolved to obtain solution I; adjust the pH value of solution I to 5.0 with 10% hydrochloric acid solution;
[0035] In the aseptic operation room, the temperature of the above-mentioned solution I was lowered to 40°C, and then the solution I was filtered with a 0.22 μm filter to obtain the filtrate a; the filtrate a was ultrafiltered with an ultrafiltration membrane with a molecular weight cut-off of 10,000 Daltons, Obtain filtrate b; make up the filtrate b to 1000ml with water for injection, and then filter with a 0.22 μm filter to obtain filtrate c; then filter the filtrate c with a 0.22 μm filter to obtain filtrate d.
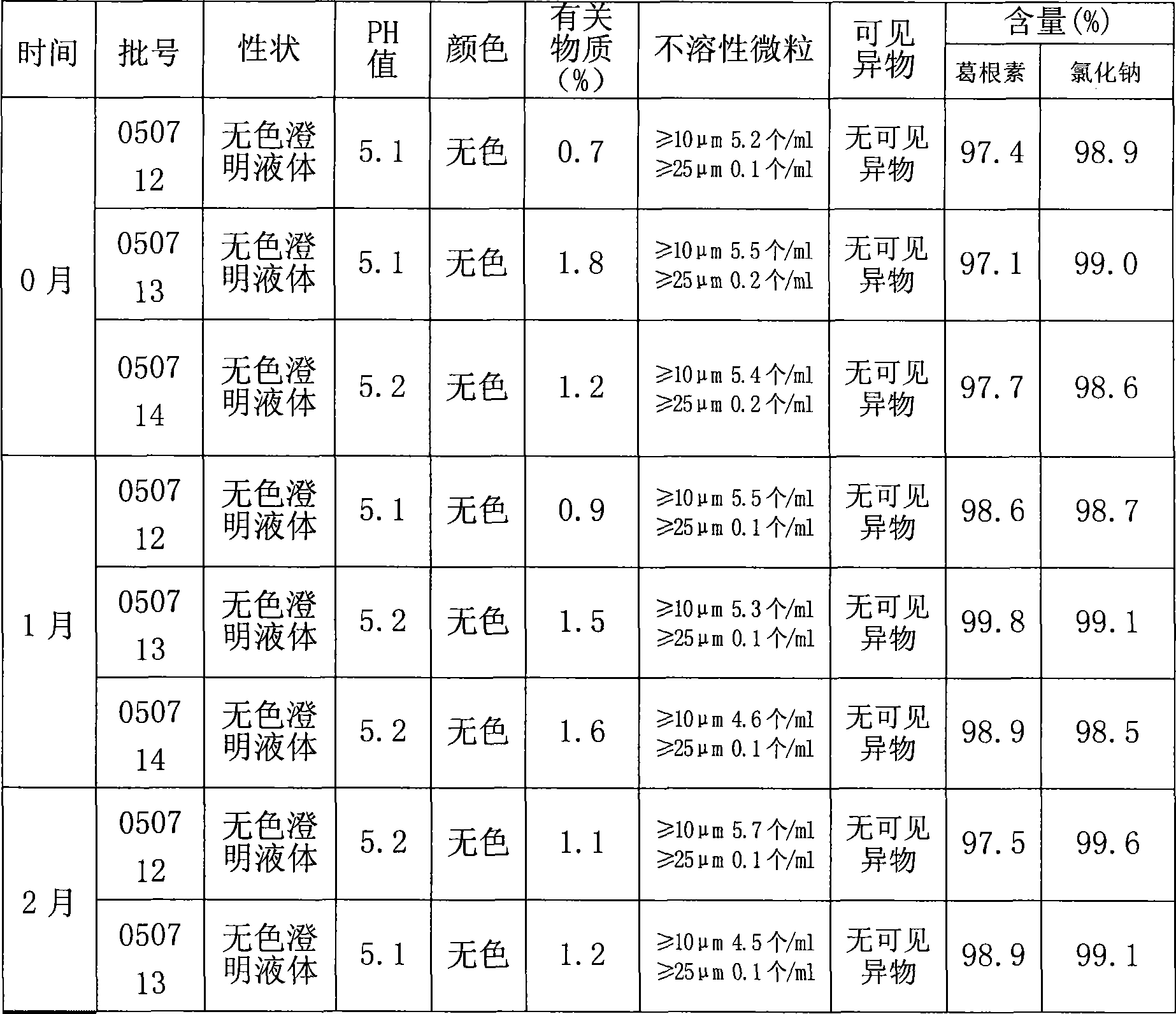
[0036] The filtrate d is inspected for the following indicators: puerarin content, content of...
Embodiment 2
[0072] Embodiment 2, the preparation of puerarin infusion
[0073] The preparation process is carried out in the 10,000-class clean area, and the filling process is carried out under the local 100-class clean area of the 10,000-class clean area.
[0074] One, the preparation of puerarin infusion
[0075] In the aseptic operation room, dissolve 2g of puerarin in water for injection at 80°C, then add 50g of glucose and stir until fully dissolved; obtain solution I; adjust the pH value of solution I to 5.1 with 10% glacial acetic acid solution;
[0076] In the aseptic operation room, the temperature of the above-mentioned solution I was lowered to 45°C, and then the solution I was filtered with a 0.22 μm filter to obtain the filtrate a; the filtrate a was ultrafiltered with an ultrafiltration membrane with a molecular weight cut-off of 10,000 Daltons, Obtain filtrate b; make up the filtrate b to 1000ml with water for injection, and then filter with a 0.22 μm filter to obtain f...
Embodiment 3
[0104] Embodiment 3, the preparation of puerarin infusion
[0105] The preparation process is carried out in the 10,000-class clean area, and the filling process is carried out under the local 100-class clean area of the 10,000-class clean area.
[0106] One, the preparation of puerarin infusion
[0107] In the aseptic operation room, dissolve 2g of puerarin in water for injection at 90°C, then add 9g of sodium chloride and stir until fully dissolved to obtain solution I; adjust the pH value of solution I to 5.2 with 10% hydrochloric acid solution;
[0108] In the aseptic operating room, the temperature of the above solution I was lowered to 42°C, and then the solution I was filtered with a 0.22 μm filter to obtain the filtrate a; the filtrate a was ultrafiltered with an ultrafiltration membrane with a molecular weight cut-off of 10,000 Daltons, Obtain filtrate b; make up the filtrate b to 1000ml with water for injection, and then filter with a 0.22 μm filter to obtain filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com