Pepsinogen II recombinant protein and its monoclonal antibody, preparation method and application thereof
A pepsinogen and monoclonal antibody technology, applied in the biological field, can solve the problems of high cost, labor and labor, etc., and achieve the effects of sufficient supply, cost reduction, and simple quantification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
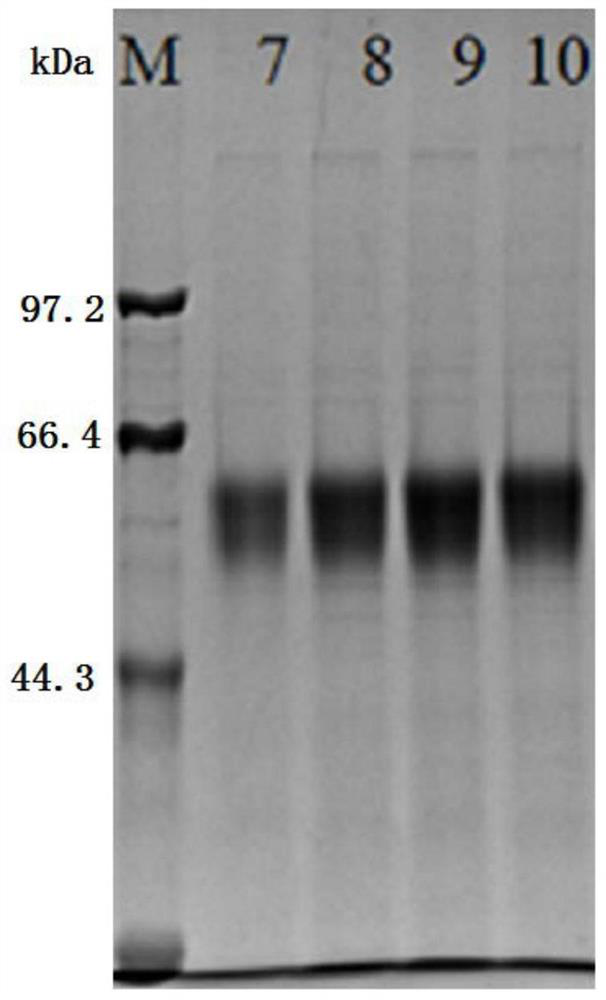
[0019] The preparation of embodiment 1 pepsinogen II
[0020] The codon-optimized nucleotide sequence of pepsinogen II (the specific sequence is shown in SEQ ID NO.1) is cloned into a eukaryotic expression vector (such as ). Using the codon-optimized nucleotide sequence of pepsinogen II (the specific sequence is shown in SEQID NO.1) as a template, EcoRI and Hind III double restriction sites were used to connect to construct pEE12.4-PGII-6His expression plasmid. The recombinant plasmids identified as positive were sent to Huada Biological Company for sequence determination, and software was used to analyze and compare the determined nucleotide sequence and encoded amino acid sequence to check the correctness of the reading frame.
[0021] The correctly identified pEE12.4-PGⅡ-6His expression plasmid was transfected into CHO-K1 cells. Pressurized screening started 24 hours after transfection: Take out the six-well plate cells from the 37°C incubator, discard the supernatant med...
Embodiment 2
[0024] Example 2 Preparation of anti-pepsinogen II monoclonal antibody
[0025] The pepsinogen II recombinant protein prepared in Example 1 was used to immunize 8-week-old BALB / c mice. For the first immunization, the pepsinogen II recombinant protein was emulsified with an equal volume of Freund's complete adjuvant, and the mice were inoculated intraperitoneally, with 100 μg of protein 7 days later, pepsinogen II recombinant protein was emulsified with equal volume of Freund's incomplete adjuvant, and the mice were immunized by intraperitoneal inoculation for the second time, 100 μg protein / mouse; 7 days later, the mice were directly immunized with pepsin for the third time by intraperitoneal route Original Ⅱ recombinant protein, 100 μg / mouse; on the 3rd day after immunization, take mouse splenocytes to fuse with mouse myeloma cell SP2 / 0, and culture in HAT selective medium; 10 days later, use pepsinogen Ⅱ recombinant protein as a package The cell supernatant was detected by a...
Embodiment 3
[0028] Example 3 Application of pepsinogen Ⅱ and anti-pepsinogen Ⅱ monoclonal antibody
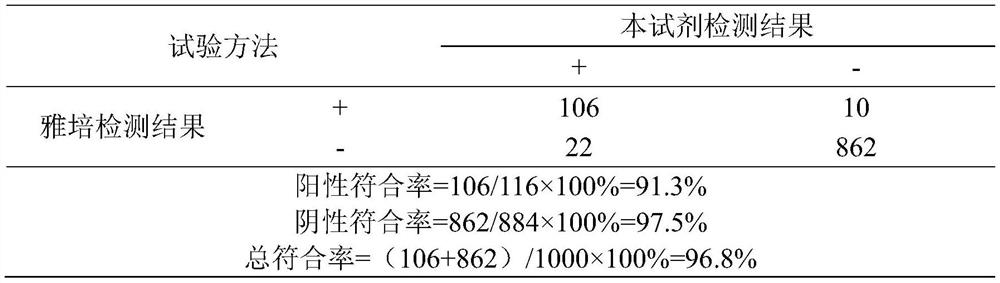
[0029] Using the PGII protein prepared by the invention as a calibrator and two monoclonal antibodies prepared by the invention as detection antibodies, a set of diagnostic reagents for the PGII protein is established. For the specific reagent preparation method, see CN 109307765 A. Use reagent of the present invention and Abbott's test kit to carry out performance comparison, the result is as shown in the table below: the diagnostic reagent prepared with PGⅡ protein of the present invention and monoclonal antibody can be used in the detection of PGⅡ protein, and detection result is very good (specificity good, high sensitivity), and the coincidence rate with imported reagents is as high as 96.8%.
[0030]
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com