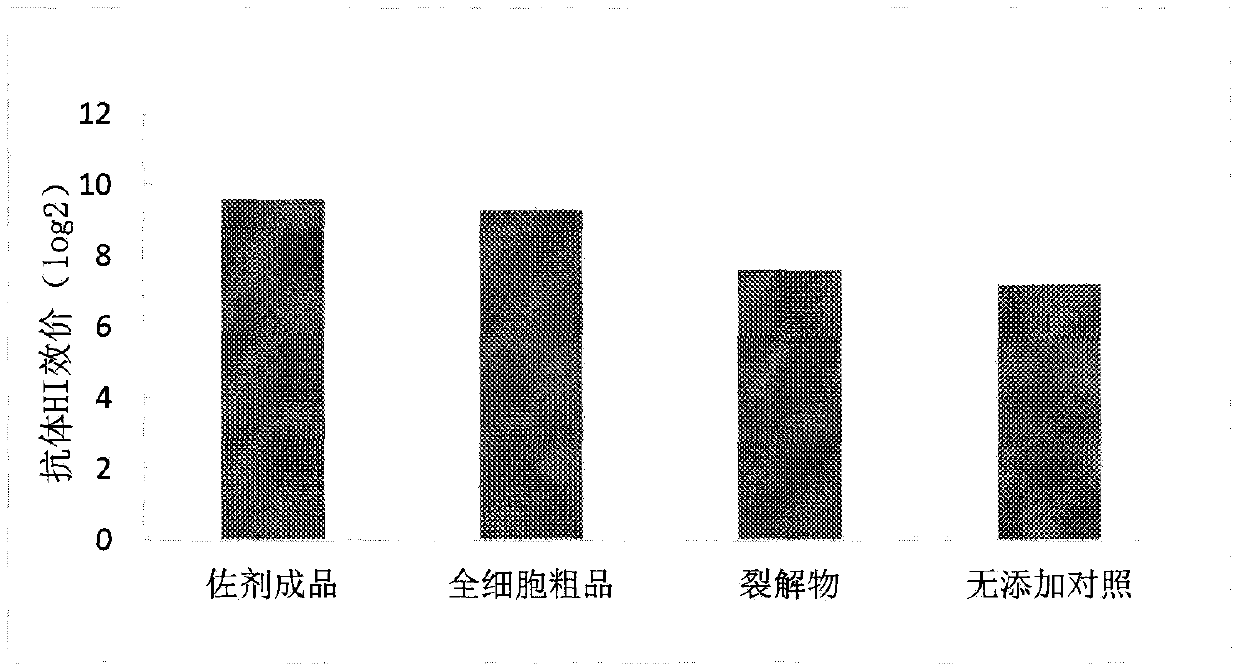
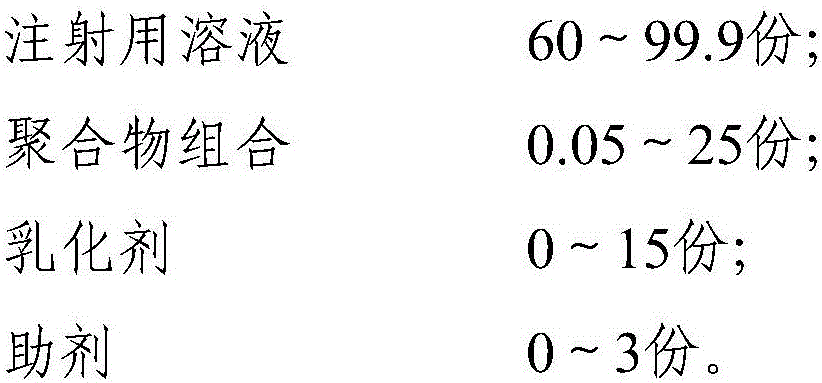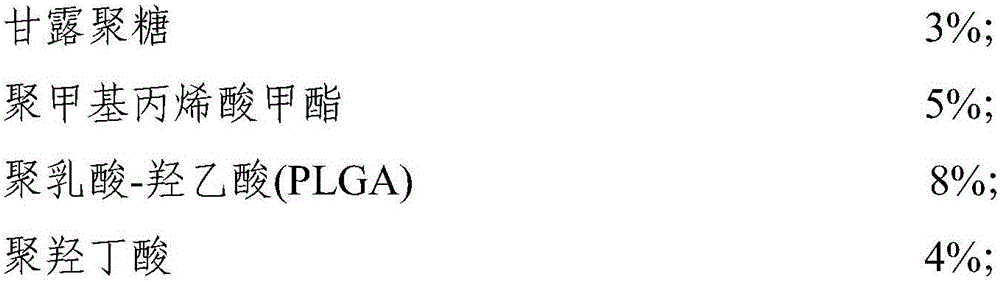Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
209 results about "Animals vaccines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccinating animals reduces animal suffering, reduces the transmission of microorganisms in the animal population, and is often more affordable than paying for the treatment of sick animals. Pets receive vaccines for infectious diseases such as rabies, parvovirus, distemper, and hepatitis.
Live bacterial vaccines for viral infection prophylaxis or treatment
ActiveUS20120142080A1Enhance immune responseAntibacterial agentsSsRNA viruses negative-senseBacteroidesDNA construct
A live bacterium, having a DNA construct stabilized against transduction of other bacteria, having a promoter sequence and encoding a fusion peptide, comprising a bacterial secretion peptide portion and a non-bacterial immunogenic polypeptide portion, having a nucleotide sequence coding for the non-bacterial immunogenic polypeptide portion which has at least one codon optimized for bacterial expression. The bacterium has a secretion mechanism which interacts with at least the bacterial secretion peptide portion to cause a secretion of the fusion peptide from the bacterium, and a genetic virulence attenuating mutation. The bacterium is adapted to act as an animal vaccine, to transiently infect a tissue of the animal, and cause an immunity response to the non-bacterial immunogenic polypeptide portion in the animal to a non-bacterial organism associated with the non-bacterial immunogenic polypeptide portion.
Owner:AVIEX TECH
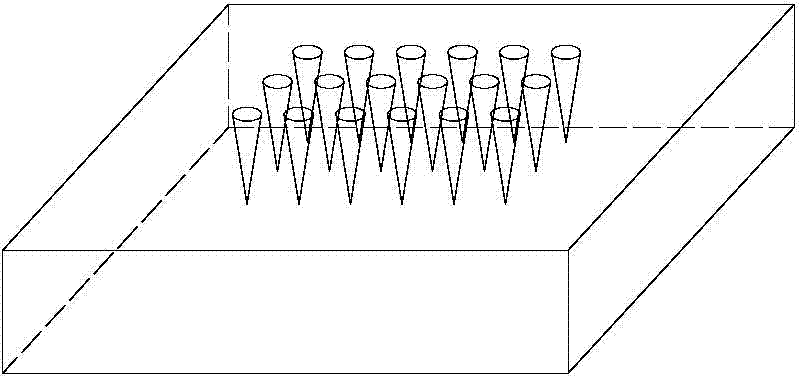
Microneedle patch convenient for administrating animal vaccine and preparation method of microneedle patch
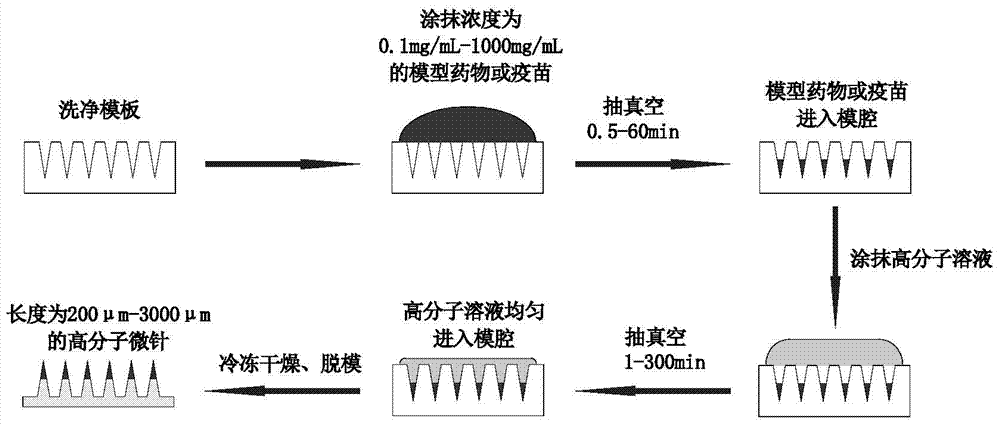
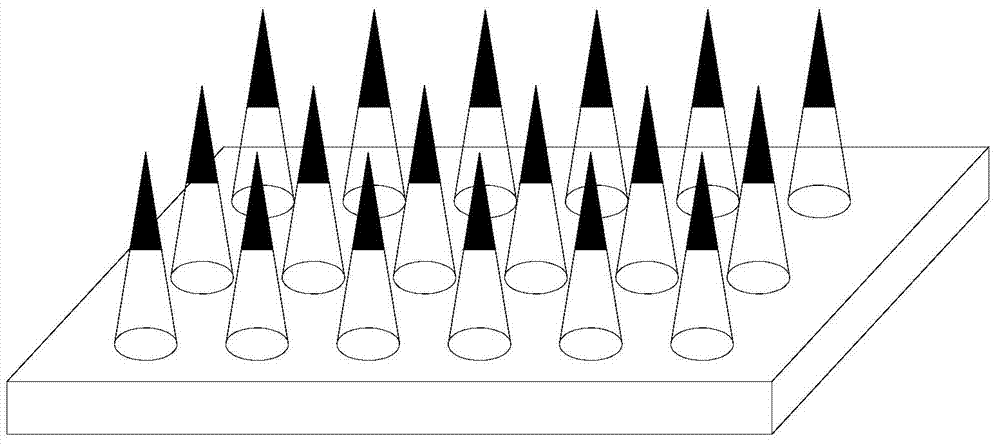
ActiveCN104706626ANothing producedEasy to administerViral antigen ingredientsMicroneedlesAnimals vaccinesMedical waste
The invention belongs to the technical field of administration equipment and particularly relates to a microneedle patch convenient for administrating animal vaccine and a preparation method of the microneedle patch. The microneedle patch comprises a soluble patch substrate and soluble microneedle bodies in array distribution on the patch substrate, wherein the microneedle bodies carry a targeted medicine at the top ends. The preparation material of the microneedle bodies comprises a water soluble degradable macromolecular material. Compared with the prior art, the microneedle patch convenient for administrating animal vaccine provided by the invention does not cause fear responses of animals when a vaccine is inoculated, is easy to administrate and convenient to operate, and inoculating personnel needs not to be trained; the vaccine inoculating work can be completed by a single person, so that the efficiency is high; the animal is not marked in an extra manner when the vaccine is inoculated; the microneedle patch is used at one time, so that the probability of cross infection is avoided; the material used is biodegradable and free of medical wastes; the microneedle patch is simple and economic in preparation method, free of cold preservation or vacuum storage and the like and convenient to transport.
Owner:BEIJING UNIV OF CHEM TECH
Bursopoietin extracting method and its use in disease treating and immune
InactiveCN1528783AImprove immunityIncrease body fluidsAnimal feeding stuffTripeptide ingredientsAdjuvantAntimicrobial drug
The invention relates to a bursin extracting method and its application to curing disease and immunity, having important value in application in the aspects of heightening organismal immunity and acting as immunoenhancer, heightening effect of vaccine, etc., and able to heighten body fluid and cell immune functions of mammal at the same time. It can be used to prevent and cure infectious diseases and young animal diseases singly or together with other drugs such as antivirus and antibacterial drugs or immunomodulators, also be applied to animal vaccine as adjuvant or immunoenhancer to strengthen the disease-resistant ability and immunoresponse ability to peculiar antigens, thus heightening the immune effect.
Owner:王爱华 +1
Nanometer adjuvant and preparation method for same
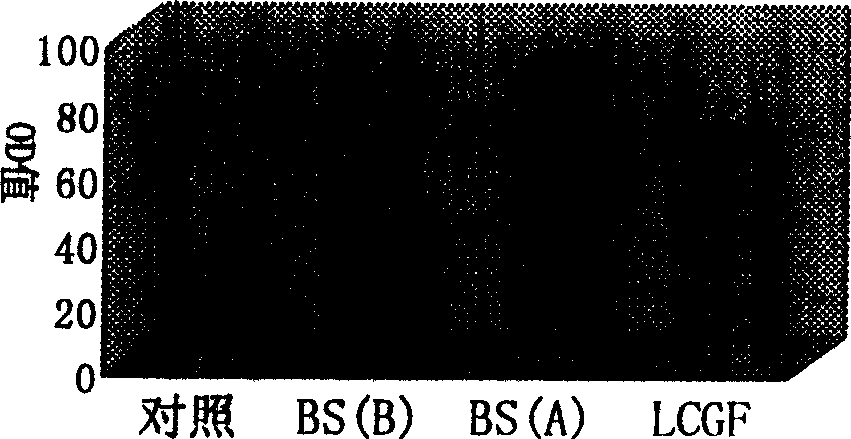
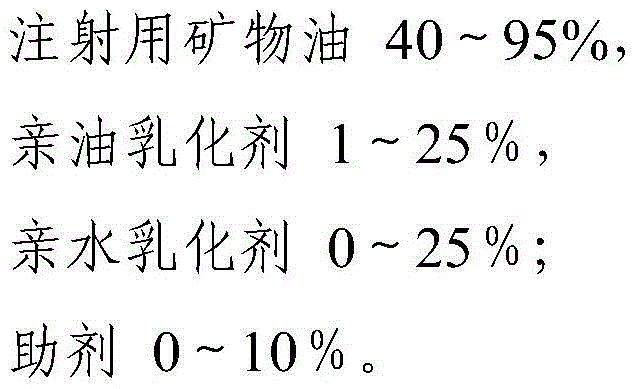
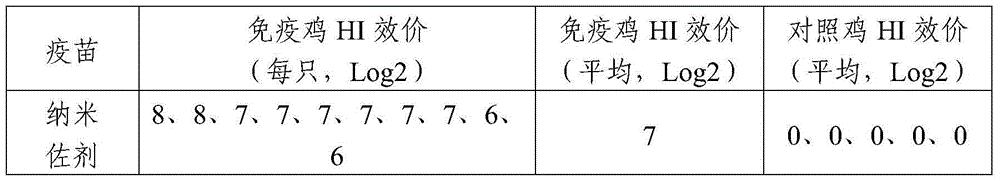
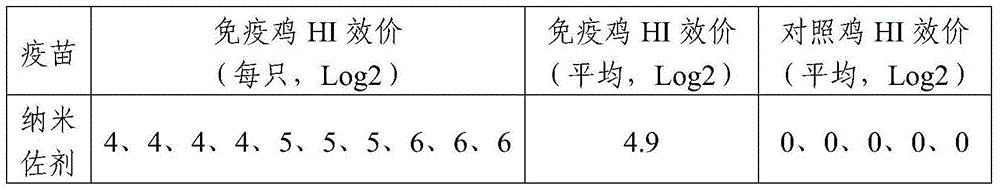
The invention relates to a nanometer adjuvant and a preparation method for the same. The nanometer adjuvant comprises the following components in percentage by weight: 40 to 95 percent of injection mineral oil, 1 to 25 percent of oleophilic emulsifiers, 0 to 25 percent of hydrophilic emulsifiers and 0 to 10 percent of auxiliaries. A nanometer adjuvant animal vaccine prepared from the adjuvant is a novel stable, safe and efficient clinical animal vaccine.
Owner:乾元浩生物股份有限公司
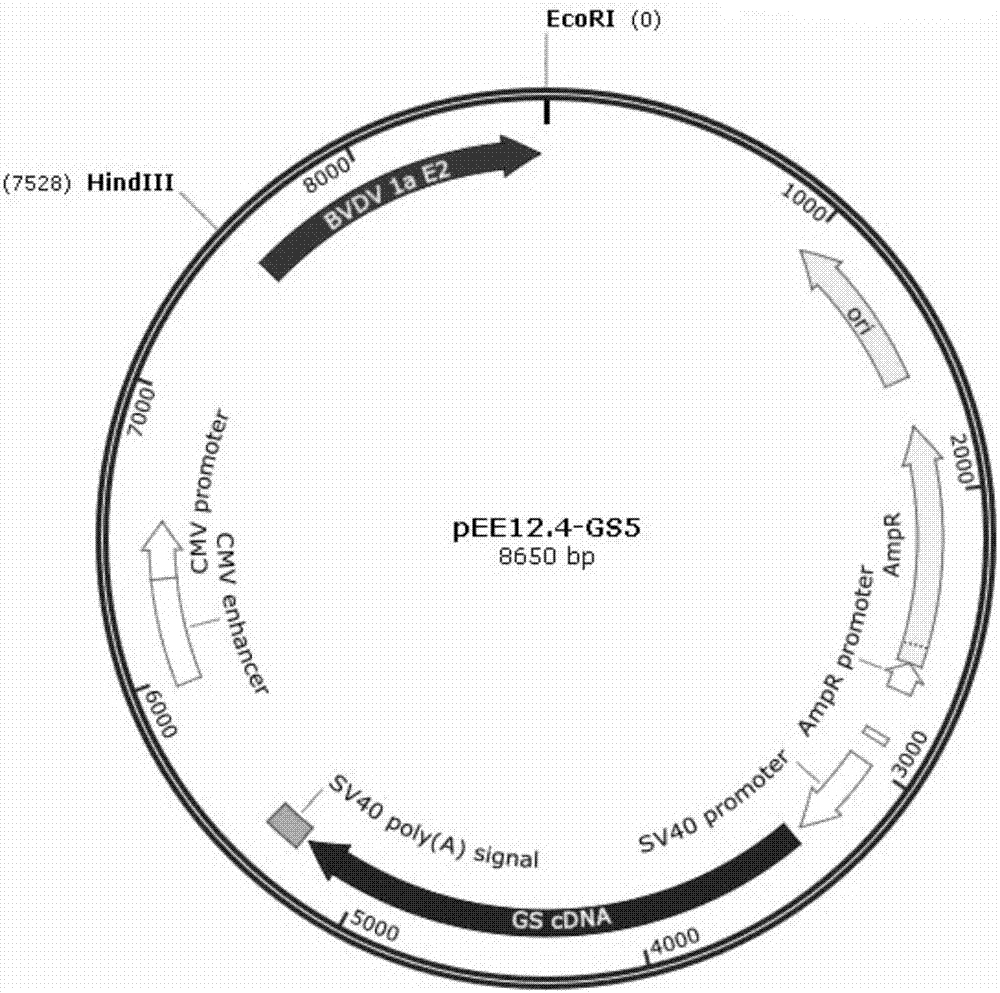
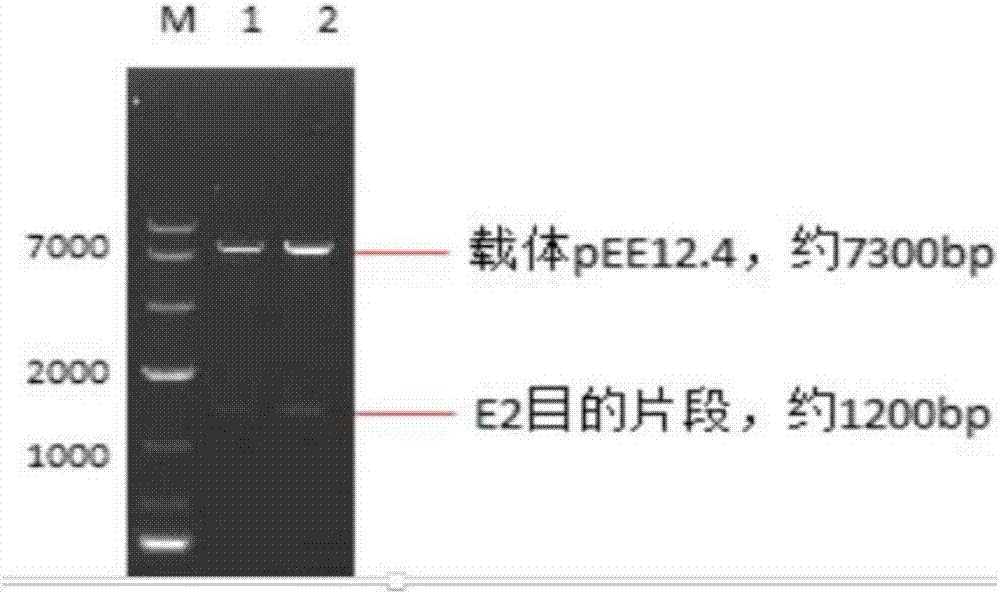
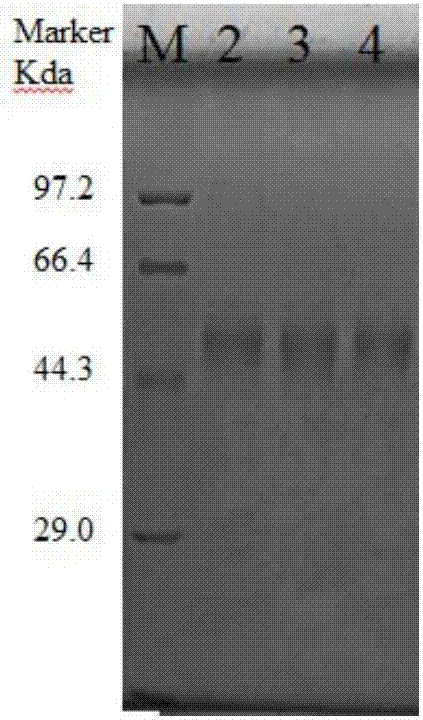
Preparation methods for CHO cell expressed recombinant bovine viral diarrhea virus protein E2 and subunit vaccine and application
ActiveCN107973841AIncrease productionImprove securitySsRNA viruses positive-senseViral antigen ingredientsProtein targetBovine Viral Diarrhea Viruses
The invention discloses preparation methods for CHO cell expressed recombinant bovine viral diarrhea virus protein E2 and a subunit vaccine and applications and belongs to the technical fields of animal vaccines and veterinary biologicals. The object of the invention is to provide a preparation method capable of industrially producing the bovine viral diarrhea virus recombinant subunit vaccine ona large scale. The preparation method for the recombinant subunit vaccine, provided by the invention, comprises the following steps: 1) cloning an eukaryotic expression vector containing a protein E2coding gene; 2) transfecting CHO cells, and performing selection, screening and acclimatizing to obtain suspending CHO cell strains, which stably and efficiently express the protein E2; 3) subjectingthe cell strains obtained in the step 2) to fermentation culture, and carrying out purification, so as to obtain recombinant protein E2; and 4) uniformly mixing the recombinant protein E2 and ISA 201VG thoroughly, thereby obtaining the recombinant subunit vaccine. According to the method provided by the invention, target protein can be obtained from cell culture supernatant, the yield reaches upto 500mg / L, the protein purification time is shortened, the vaccine production steps are simplified, and the vaccine production cost is greatly reduced.
Owner:NOVO BIOTECH CORP
Novel vaccine adjuvant and application
ActiveCN102370977AAdjustable affinityAdjustable specificityGenetic material ingredientsEmulsion deliveryPolyoxyethylene castor oilVaccination
The invention discloses a novel vaccine adjuvant (also called as SPO1 vaccine adjuvant) and a preparation method thereof. The vaccine adjuvant disclosed by the invention mainly comprises squalane, polyoxyethylene castor oil and polyether, and can realize immunization through injection or nasal spray, transdermal non-injection and other approaches; and the vaccination through non-injection has the characteristics of low cost, convenience for administration, avoidance of cross infection and high safety. The SPO1 vaccine adjuvant disclosed by the invention can be used for preparing vaccines along with inactivated whole pathogens, extracted components of cracked pathogen, bacterial vesicles and capsular polysaccharide or polysaccharide-binding protein, recombinant protein, recombinant VLP (Virus-like Particle), DNA (Deoxyribonucleic Acid) or RNA (Ribonucleic Acid) and other different types of antigens, can be suitable for people in different age groups and preparation of different animal vaccines and immunization, has the advantages of no complex structure, simple process, convenience for preparation and sterilization, low cost and suitability for mass production and industrialization.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI +1
Live genetically engineered protozoan vaccine
The invention provides methods for inducing an immune response in a vertebrate host against a protozoan parasite, comprising administering to the host a live protozoan parasite that is genetically engineered to disrupt a stage-specific gene function that is required by the protozoan parasite to establish a secondary infection in the vertebrate host. Representative protozoan parasites belong to the phyla Apicomplexa and Kinetoplastida. The vertebrate host may be a mammal or a bird.
Owner:SEATTLE BIOMEDICAL RES INST
Recombinant plant rhabdovirus vector and construction method thereof
ActiveCN104962580AEasy to operateGood repeatabilityFermentationVector-based foreign material introductionHeterologousPlant rhabdovirus
The invention discloses a recombinant plant rhabdovirus vector and a construction method thereof. The recombinant plant rhabdovirus vector comprises a modified plant rhabdovirus genome, a transcription unit which is induced newly and a heteroduplex nucleotide sequence, wherein the transcription unit is inserted into the plant rhabdovirus genome to express the heteroduplex nucleotide sequence, the heteroduplex nucleotide sequence is replaced into a glycoprotein transcription unit of a recombinant plant rhabdovirus; the recombinant plant rhabdovirus has copying and infecting capacity, and one antigenic polypeptide or other protein is coded by the heteroduplex nucleotide sequence. The virus expression vector is the first one which can express a negative-sense RNA virus vector of foreign protein on plants, and the recombinant plant rhabdovirus vector has the advantages that the expression quantity is high, the expression stability is good, a relative long foreign gene segment can be inserted, and inserted foreign gene segment is not prone to loosing; the recombinant plant rhabdovirus vector can be used for expressing of multiple kinds of foreign protein and can also be used for preparing animal vaccines, and wide application values and application prospects are achieved.
Owner:ZHEJIANG UNIV
Non-antibiotics resistant shuttle plasmid expression carrier and its constructing method and uses
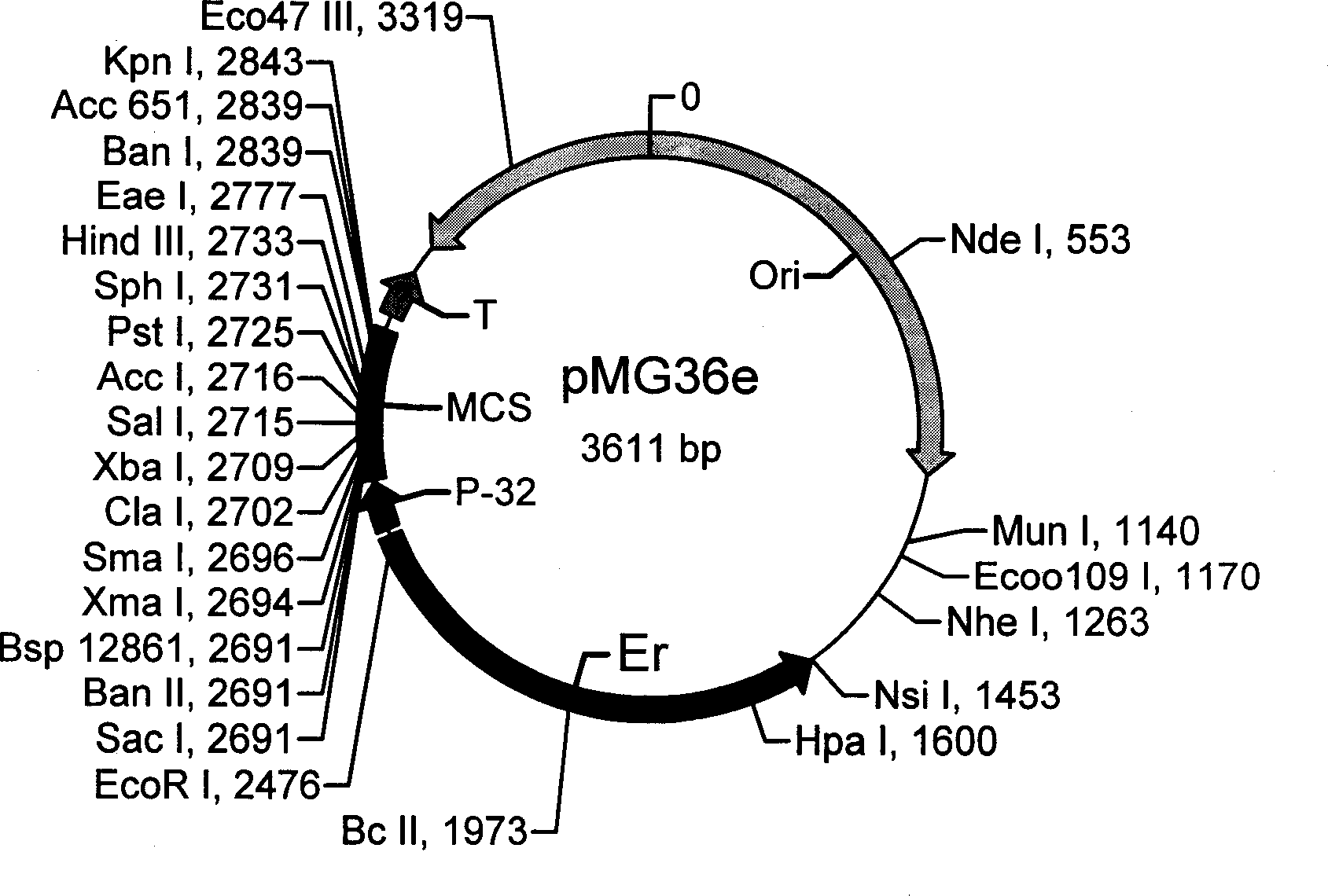
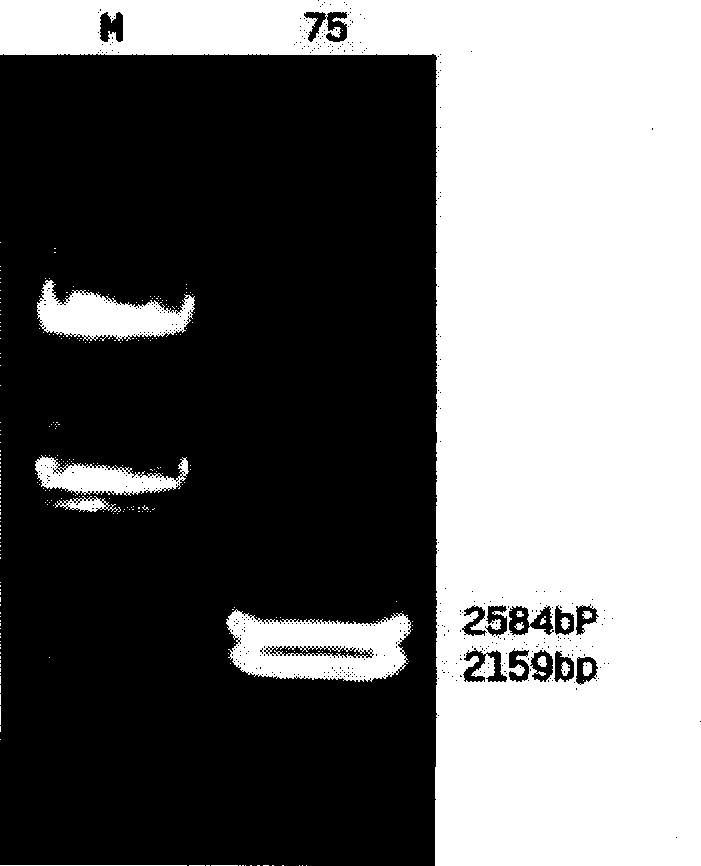
InactiveCN1482247AHighlight environmental protectionProminence effectMilk preparationBacteriaAnimals vaccinesEndonuclease
The present invention belongs to the field of bioengineering technology and is non-antibiotic resistant shuttle plasmid expression vector and its constitution method and application. Basic plasmid carrier pMG36e is enzyme incised in its incising site with endoenzyme EcoRI to become linear. Non-antibiotic resistant gene is connected to the near erythromycin Er end before cyclization into cycle; and the erythromycin gene Er is then incised with endoenzyme on the cycle to form non-antibiotic resistant shuttle plasmid expression vector. The protective antigen gene on human or animal disease inconnected to the non-antibiotic resistant shuttle plasmid expression vector, so as to produce functional food, bacterial suspension with specific disease preventing and treating effect, oral tonic, feed product and animal vaccine. The gene of the present invention may be used in replacing erythromycin gene without problem of antibiotic resistant gene diffusion.
Owner:王春凤 +1
Raccoon Poxvirus Expressing Genes of Feline Antigens
InactiveUS20080299149A1Avoiding adjuvant-related sarcoma side effectBroad protectionSsRNA viruses positive-senseViral antigen ingredientsHemagglutininAnimals vaccines
The present invention relates to new recombinant raccoon poxvirus vectors comprising two or more exogenous nucleic acid molecules, each encoding at least one feline protein, wherein at least two of the nucleic acid molecules are inserted into the hemagglutinin (ha) locus or the thymidine kinase (tk) locus, or at least one of the nucleic acid molecules is inserted into each of the hemagglutinin and thymidine kinase loci. Described herein are monovalent and polyvalent recombinant feline vaccines that encompass an immunologically effective amount of the recombinant raccoon poxvirus vectors and, optionally, a suitable carrier or diluent. The vaccine of this invention optionally includes additional feline antigens to provide broad spectrum protection to cats against a variety of feline pathogens. The invention further concerns the method for inducing a protective immune response to the feline pathogens in a cat by administering the recombinant vaccines.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Compound adjuvant for animal vaccine and application of compound adjuvant
ActiveCN105688207AImprove immune efficiencyGood immune protectionAntibody medical ingredientsSide effectVegetable oil
The invention relates to a vaccine adjuvant and particularly discloses a compound adjuvant for an animal vaccine. The compound adjuvant comprises raw materials in parts by weight as follows: 10-70 parts of vegetable oil for injection, 0.1-20 parts of an emulsifier, 1-10 parts of an additive, 0.01-10 parts of polymers and 0.01-5 parts of an immunopotentiator, wherein the polymers are selected from natural polymers and / or synthetic polymers. According to the animal vaccine prepared from the compound adjuvant, a synergistic effect is realized through compounding of the vegetable oil for injection, the polymers and the immunopotentiator, the immune efficacy of the animal vaccine is improved effectively, and immune protection of the animal vaccine is facilitated. Side effects of the vaccine adjuvant are reduced obviously in the aspect of animal safety compared with a mineral oil adjuvant.
Owner:CHINA ANIMAL HUSBANDRY IND
Hepatitis E virus-like particle vaccine preparation method and application
ActiveCN104857510AEasy to prepareEasy to zoom inDigestive systemImmunoglobulins against virusesEscherichia coliDisease
The invention discloses a hepatitis E virus-like particle vaccine preparation method, and belongs to the biological medicine technical field. According to the method, hepatitis E virus truncated capsid protein gene codon optimization is performed, prokaryotic expression system escherichia coli strain B834-pRARE2 is used, by fusion of purification tag MBP expression, different cracking agent screening and combination, bacterial membrane extraction, maltose amylose affinity chromatography, molecular sieve chromatography for removal of MBP and non-virus structural protein, and promotion of virus-like particle assembly, the hepatitis E virus-like particle purity is more than 99.0%, and is free of protein label and bioactive (in the form of non-inclusion body, and soluble). The hepatitis E virus-like particle vaccine produces complete protection to pig, dog and chicken HEV infection, cross protective antigens are between different genotype HEV virus, and the hepatitis E virus-like particle vaccine can be used as an animal vaccine to prevent the diseases and infection caused by pig, dog and poultry HEV.
Owner:张澍 +1
Swine fever-porcine circovirus combined subunit vaccine, as well as preparation method and application thereof
ActiveCN107233566AImproving immunogenicityImprove securitySsRNA viruses positive-senseViral antigen ingredientsAdjuvantAnimals vaccines
The invention discloses a swine fever-porcine circovirus combined subunit vaccine, as well as a preparation method and an application thereof, and belongs to the technical fields of animal vaccines and veterinary biological products. The vaccine is prepared from swine fever virus E2 protein, porcine circovirus type 2 cap protein and a pharmaceutically acceptable adjuvant. The preparation method comprises the following steps: (1) preparing swine fever virus E2 protein and porcine circovirus type 2 cap protein; (2) preparing an antigen solution from the swine fever virus E2 protein and porcine circovirus type 2 cap protein prepared in the step (1); and (3) mixing and stirring the antigen solution and Montanide GEL 01PR adjuvant in a mass ratio of 10:1. The vaccine has the advantages of strong immunogenicity, good safety, no immune interference and the like, can be used for preventing potential biological safety hazard for virus variation and fundamentally purifying swine fever virus and porcine circovirus type 2, and can achieve the effect of dual prevention with one injection by immunizing the vaccine to achieve the aims of saving time, labor and cost.
Owner:NOVO BIOTECH CORP
Prevention, treatment and detection of pig progressive atrophic rhinitis
Disclosed is the animal vaccine for the prevention, treatment and detection of pig progressive atrophic rhinitis, which comprises three polypeptides generated by antibody against Pasteurella multocida related to progressive atrophic rhinitis, these polypeptides all have a amino acid sequence, which mainly corresponds to the 2-486, 486-986 or 986-1281 amino acid residue of the PMT protein. The invention also discloses the multivalent vaccine for the prevention and treatment of at least PAR for animals.
Owner:简茂盛
Compound oil adjuvant as well as preparation method and application thereof
ActiveCN102813922ASolve the difficult problem of preparationEasy injectionViral antigen ingredientsAntiviralsAnimals vaccinesEngineering
The invention relates to the technical field of vaccine adjuvant, in particular relates to a compound oil adjuvant. The compound oil adjuvant comprises three ingredients, namely white oil, oleyl alcohol polyoxyethylene ether and polyoxyethylene ether stearate. The white oil accounts for 80-95%, the oleyl alcohol polyoxyethylene ether accounts for 1-10%, and the polyoxyethylene ether stearate accounts for 1-10%. Molecular weight of the oleyl alcohol polyoxyethylene ether is 400-600, and the molecular weight the polyoxyethylene ether stearate is 400-600. The preparation method of the compound oil adjuvant disclosed by the invention comprises the steps of respectively weighing the white oil, the oleyl alcohol polyoxyethylene ether and the polyoxyethylene ether stearate in proportion of a prescription and mixing to be uniform. The invention also discloses an application of the compound oil adjuvant in preparation of an animal vaccine. The invention successfully solves the problem that a W / O / W type emulsion vaccine is difficult to prepare, the prepared emulsion vaccine is convenient for injection and also has good immunogenicity, and stability of the prepared emulsion vaccine is better than that of the W / O / W type emulsion vaccine prepared by ISA206.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Rhodococcus ruber fermentation method and application thereof as adjuvant in animal vaccines
ActiveCN109666609AUnusual humoral immunomodulatory effectsImprove securityBacteriaMicroorganism based processesAdjuvantAnimals vaccines
The invention relates to a special rhodococcus ruber (CGMCC NO. 17012) fermentation method, a preparation process of adjuvants with different dosage forms and an application as an adjuvant in animalvaccines. Rhodococcus ruber strains are separated from a farm and obtained through single colony cloning purification and identification, the invention provides a special fermentation process where the strain inactivation product is used as an animal vaccine adjuvant, and provides a preparation process of different dosage forms of adjuvants containing the strain and the product. Animal experimentsprove that when the adjuvant product is used for univalent or multivalent animal vaccines, particularly Newcastle disease inactivated vaccine, avian influenza inactivated vaccine and swine fever livevaccine, definite non-specific immune enhancement effect can be provided, specifically, the antibody peak value level induced by the animal vaccine is obviously improved, the time for producing the protective antibody level is advanced, the antibody maintenance period is prolonged, and the immune effect of the animal vaccines is enhanced.
Owner:刘春郁
Swine Streptococcosis trivalent inactivated vaccine and preparation method thereof
ActiveCN102949714AImprove survival rateReduce morbidityAntibacterial agentsBacterial antigen ingredientsStreptococcus suis serotypeAnimals vaccines
The invention belongs to the technical field of preparation of animal vaccines and particularly relates to a trivalent inactivated vaccine for Streptococcus equi subsp. zooepidemicus, Streptococcus suis serotype 2 and Streptococcus suis serotype 7, as well as a preparation method and application thereof. The inactivated vaccine disclosed by the invention is mainly applicable to prevention and treatment of swine Streptococcosis for sows, boars and piglets. The invention discloses separation, identification and application of three strains special for preparing the swine Streptococcosis trivalent inactivated vaccine, wherein the three strains for the vaccine are respectively a Streptococcus suis serotype 2-LT strain (with the collection number being CCTCC NO:M2011282), a Streptococcus suis serotype 7-YZ strain (with the collection number being CCTCC NO:M2011160) and a Streptococcus equi subsp. Zooepidemicus XS strain (with the collection number being CCTCC NO:M2011405). The inactivated vaccine disclosed by the invention can achieve multiple prevention effects with one injection, so that the stresses of swine are reduced, and the inactivated vaccine is safe and reliable and is free from the potential hazards of toxin spreading.
Owner:HUAZHONG AGRI UNIV +1
Animal vaccine immunopotentiator and production method thereof
InactiveCN101255189AOvert stimulating activitySignificant immune enhancing activityBacteriaMicroorganism based processesSmall peptideAnimals vaccines
The invention provides a bursatin of immunopotentiator kind which is a small peptide which is a monomer with amino acid sequence COOH-Gly-His-Lys-NH2 or a multimer with end-to-end of a plurality of the sequences. The immunopotentiator of the invention has sufficient stimulating activity to various of animal lymphocyte in vitro, and has sufficient immuno-enhancing activity to newcastle disease (ND) vaccine, H5 and H9 Avian Influenza vaccine.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA
Adjuvant composition comprising (poly-gamma-glutamate)-chitosan nanoparticles
The present invention relates to an adjuvant composition comprising (poly-gamma-glutamate)-chitosan nanoparticles and a vaccine composition comprising the adjuvant composition, and more specifically to an adjuvant composition comprising nanoparticles by an ionic bond between (poly-gamma-glutamate) of proven safety and chitosan and a vaccine composition comprising the (poly-gamma-glutamate)-chitosan nanoparticles and an antigen. The adjuvant composition comprising (poly-gamma-glutamate)-chitosan nanoparticles according to the present invention has little or no toxicity and side effects and is advantageous in increasing the rate of antibody production when added to the vaccine for virus and bacterial infections and to the vaccine for prevention and treatment of cancer in humans or animals.
Owner:BIOLEADERS CORP +4
Construction and application of H9N2-subtype avian influenza virus cell high-yield vaccine strain
ActiveCN105671002AHigh titerImprove adaptabilityAntiviralsViruses/bacteriophagesAnimals vaccinesEmbryo
The invention belongs to the technical field of animal vaccine preparation and particularly relates to construction and application of an H9N2-subtype avian influenza virus cell high-yield vaccine strain. The cell vaccine strain is prepared through artificially induced mutation screening of an avian influenza isolate and is named H9N2-subtype avian influenza virus W1-HA358 strain, of which an original isolate is A / duck / Hubei / W1 / 2004 (H9N2) strain. The 3rd, 5th and 8th loca of a 3'-terminal of an HA gene non-coding region of the isolate are subjected to artificial site-specific mutagenesis, and a mutation strain is saved through reverse genetic operation, wherein the mutation strain is subjected to passage on an MDCK cell until a stable generation to obtain a high-proliferation-titer virus strain, thereby producing the H9N2-subtype avian influenza virus cell vaccine. The invention solves the problem of instable proliferation of the H9N2-subtype avian influenza on the MDCK cell, and overcomes a defect of shortage of large quantity of chick embryo during outbreak of the avian influenza, thereby increasing preparation efficiency of the vaccine.
Owner:HUAZHONG AGRI UNIV
Virus-like particle of senecavirus A and preparation method and application thereof
ActiveCN108642021AHigh expressionImprove assembly efficiencySsRNA viruses positive-senseVirus peptidesSolubilityProtein target
The invention discloses a virus-like particle of senecavirus A and a preparation method and application thereof. The virus-like particle of the senecavirus A is assembled by structural protein VP0, structural protein VP1 and structural protein VP3 of the senecavirus A, wherein the gene sequence of the encoding structural protein VP0 is shown in SEQ ID NO.1, the gene sequence of the encoding structural protein VP1 is shown in SEQ ID NO.2, and the gene sequence of the encoding structural protein VP3 is shown in SEQ ID NO.3. The method tries to combine different fusion tags to use so as to improve expression quantity of target proteins and assembling efficiency of the virus-like particle, and the result shows that after the N end of an SUMO VP1 gene combines with GST again, the solubility ofproteins expressed by expression bacterium which is transfected jointly by the combination of the obtained recombinant vector pGST / VP1, pSMK / VP0 and pSMC / VP3 is best, and the assembling efficiency ofVLPS is highest. The method provides technical support for further research and application of virus-like particle vaccines and accelerated transformation of animal vaccines from traditional inactivated vaccines to genetic engineering vaccines.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
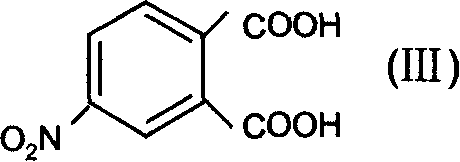
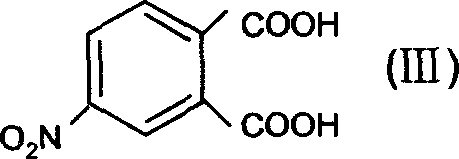
Dipropyl phthalic ester hapten derivant and its preparation method
InactiveCN1762983AThe synthesis method is simpleHigh purityOrganic compound preparationAmino-carboxyl compound preparationAnimals vaccinesEsterification reaction
The dipropyl phthalate half-antigen derivative has the structure as shown and the preparation process disclosed. The preparation process includes the following steps: A. esterification reaction of 4-nitro phthalic acid and n-propanol under acid condition to obtain one kind of dipropyl phthalate half-antigen derivative; and B. reaction of the dipropyl phthalate half-antigen derivative and reductant under acid condition to obtain one other kind of dipropyl phthalate half-antigen derivative. The preparation process is simple, and the product has high purity and may be used in preparing antigen for animal vaccine.
Owner:DONGHUA UNIV
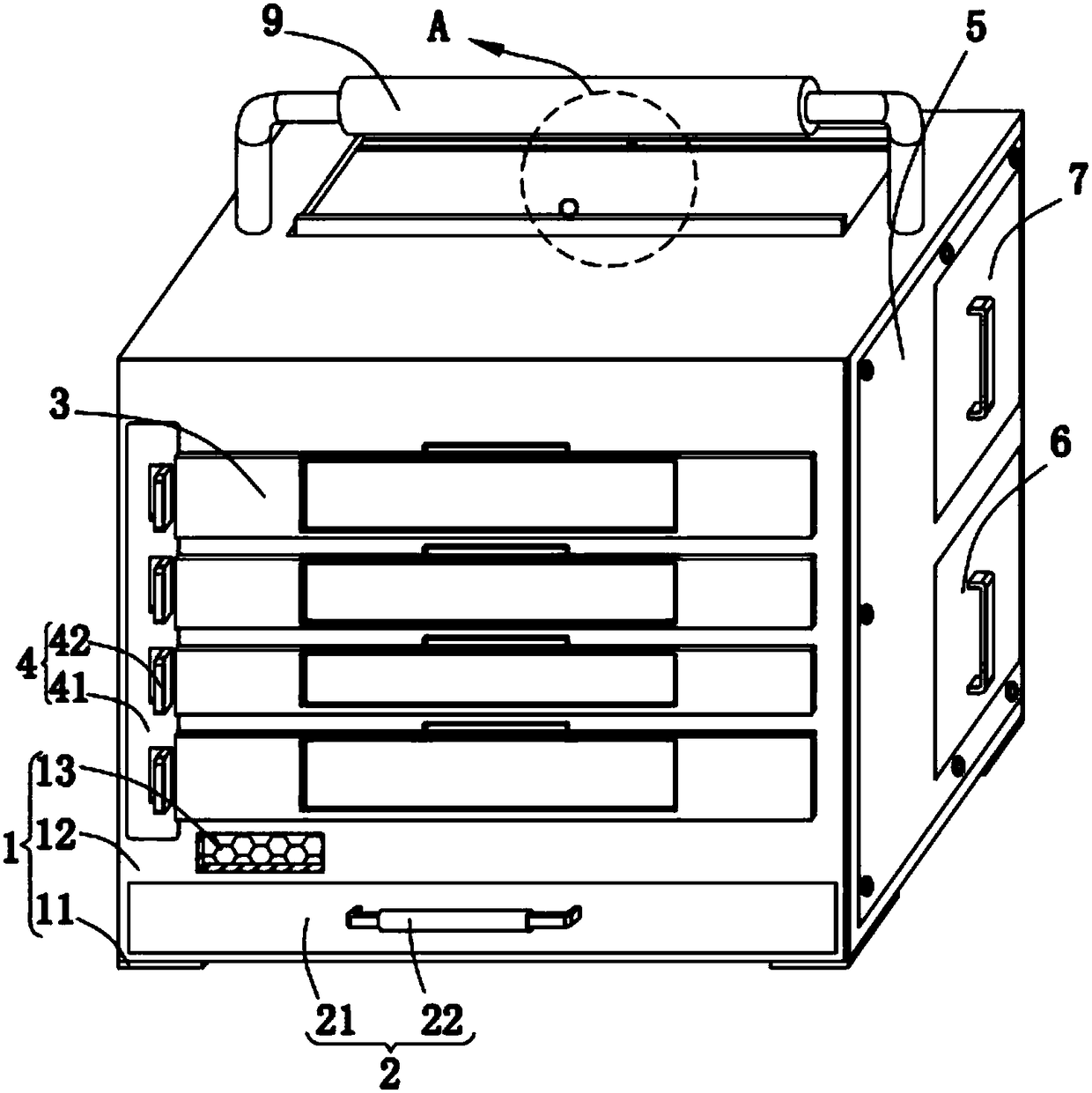
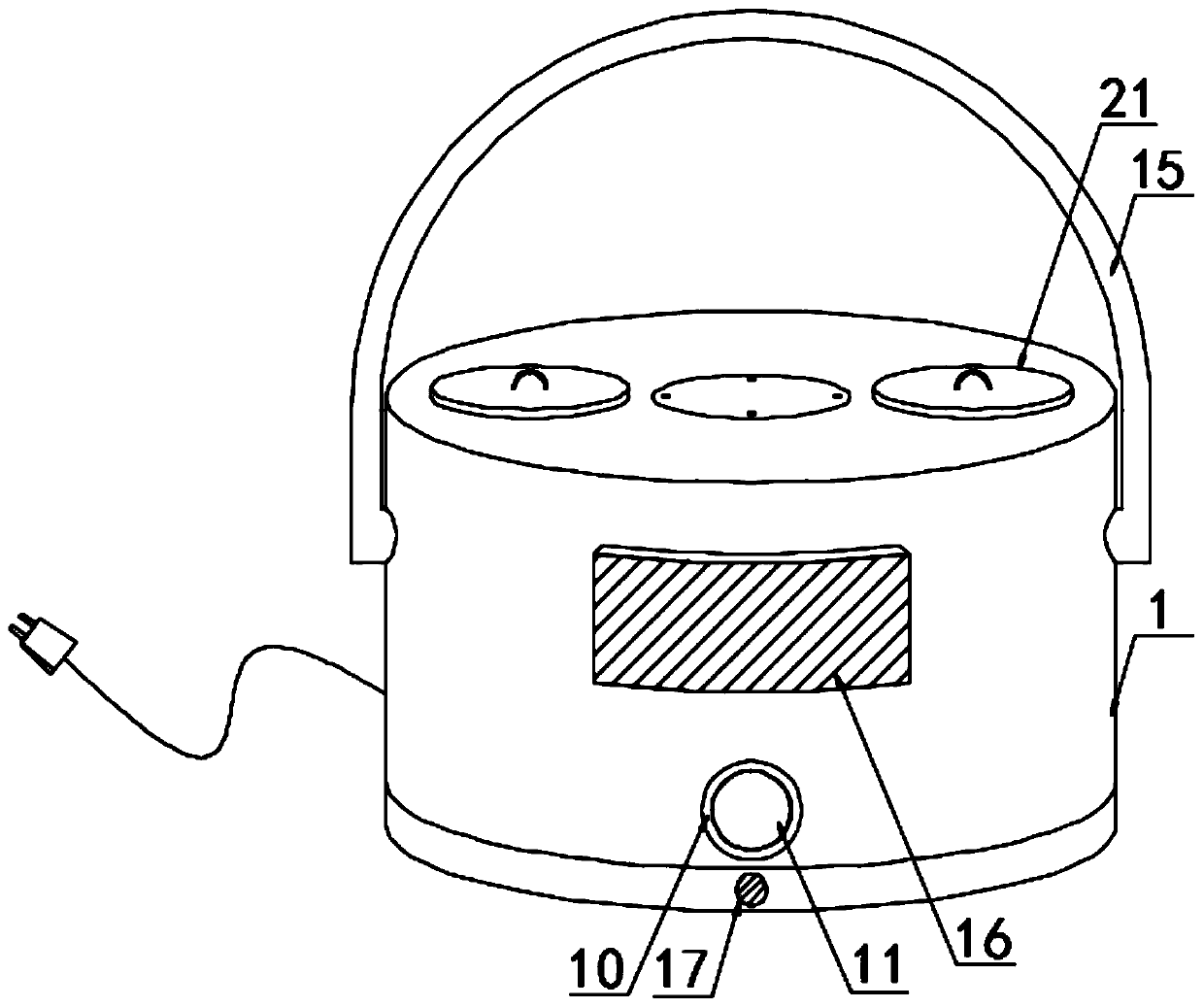
Animal vaccine inoculation box
ActiveCN108482866AEasy to placeEasy to useDomestic cooling apparatusLighting and heating apparatusAnimals vaccinesLitter
The invention relates to the field of biomedicine, in particular to an animal vaccine inoculation box. The animal vaccine inoculation box comprises a heat insulation structure, a refrigeration structure, classification storage structures, first fixing structures, a second maintenance board, a storage box, an edged tool storage structure, a second fixing structure and a handle, wherein the heat insulation structure is used for heat insulation and heat preservation, the refrigeration structure facilitates the placement of dry ice, the classification storage structures facilitates the storage ofvaccine bottles, the first fixing structures are used for limiting the classification storage structures, the edges tool storage structure is used for containing medical waste, the second fixing structure is used for controlling the edged tool storage structure to communicate with the heat insulation structure, and the second maintenance board facilitates maintenance of the animal vaccine inoculation box. According to the animal vaccine inoculation box, the refrigeration structure is in sliding connection with the heat insulation structure, meanwhile, the refrigeration structure is used by matching with the heat insulation structure so that refrigeration and heat insulation can be carried out on vaccine inside the classification storage structures, the classification storage structures facilitate the storage of the vaccine bottles with different sizes, and the edged tool storage structure is used so that vaccine waste can be conveniently classified after inoculation is finished.
Owner:汇智赢华医疗科技研发(北京)有限公司
Preparation method and application of flagellin FliC from salmonella abortus equi
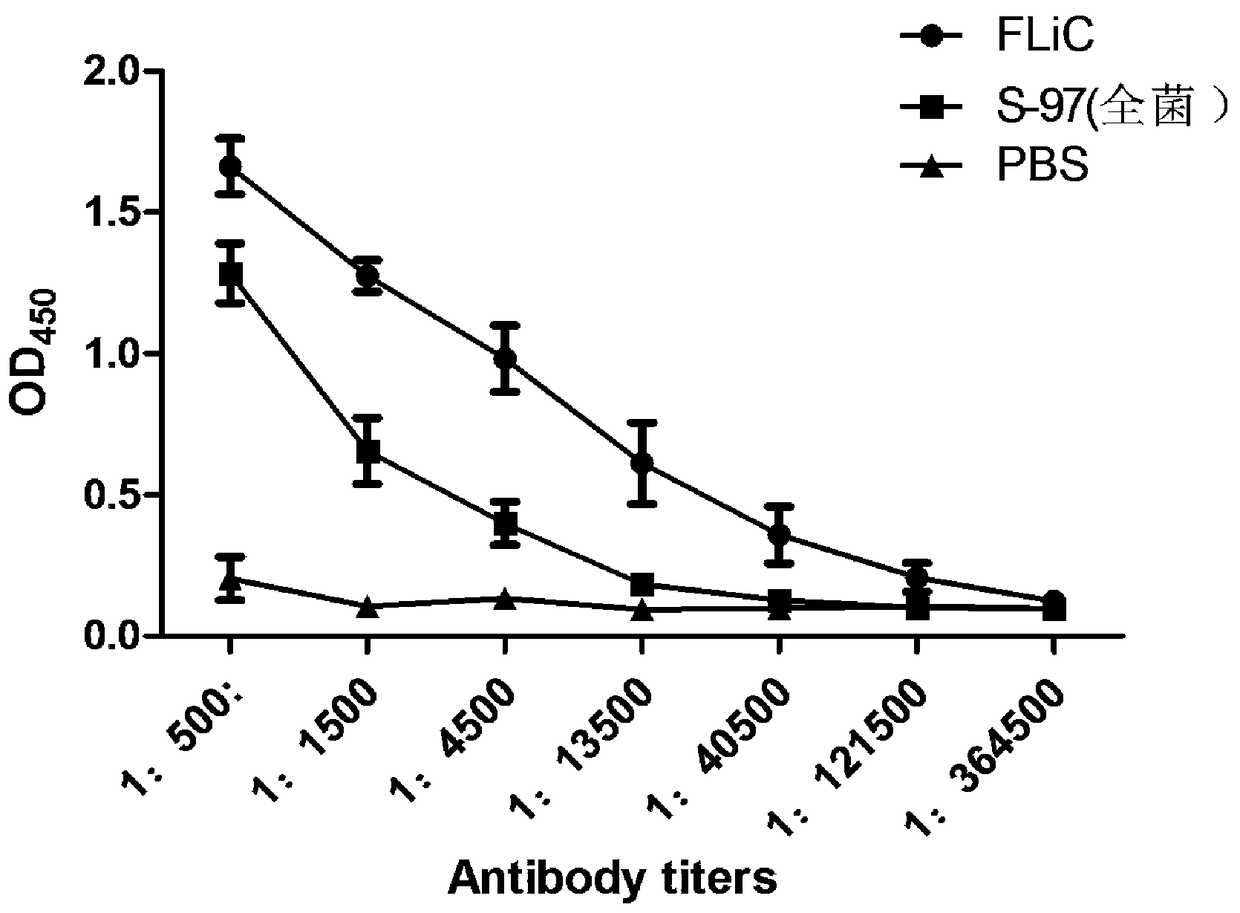
ActiveCN108218965AImproving immunogenicityExcellent levelBacteriaMicroorganism based processesAdjuvantAnimals vaccines
The invention discloses a preparation method and possible application of flagellin FliC from salmonella abortus equi to salmonella abortus equi immunity. Through cloning and connecting a flagellin FliC gene from the salmonella abortus equi to a prokaryotic expression vector pGEX-4T-2, a prokaryotic recombinant of a protein is obtained; the prokaryotic recombinant is transformed into engineering bacteria BL-21, and the protein is subjected to induced expression and purification; the protein has an amino acid sequence in a sequence table 1; the purified FliC recombinant protein has good immunogenicity after being used for the immunization of experimental animals, can overcome the defects of low antibody level and poor safety generated by inactivated vaccines and can be used for preparing subunit vaccines for preventing and treating equine paratyphoid infection and can be used as equine animal vaccine adjuvants, thereby having good development and application prospect.
Owner:XINJIANG AGRI UNIV
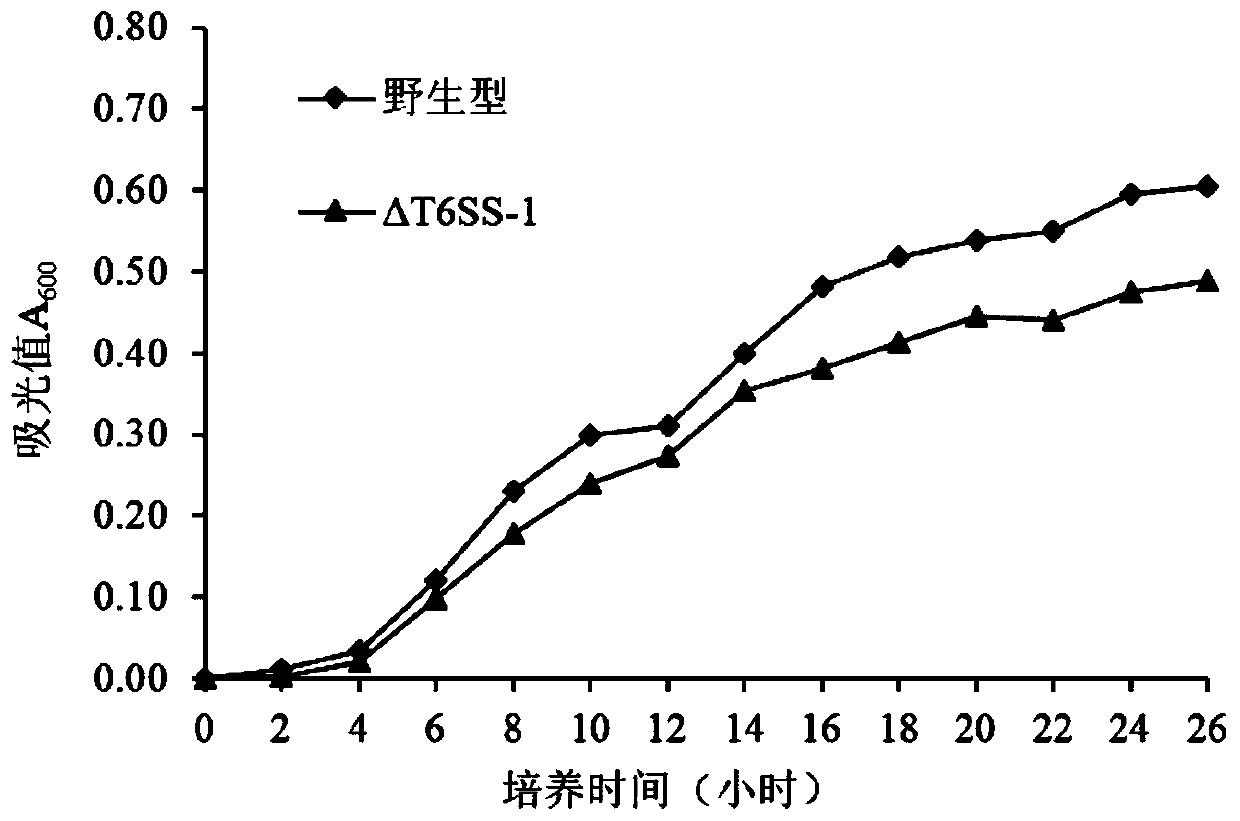
Pseudomonas plecoglossicida attenuated vaccine for fish with T6SS-1 gene cluster knocked out
ActiveCN109825464AAnimal safeImproving immunogenicityAntibacterial agentsBacteriaEscherichia coliAnimals vaccines
The invention provides a pseudomonas plecoglossicida attenuated vaccine for fish with a T6SS-1 gene cluster knocked out, and belongs to the technical field of animal vaccines. The vaccine comprises aknockout strain of one set of VI-secreting system gene clusters of a pseudomonas plecoglossicida genome. A construction method includes adopting an overlapped PCR way to obtain a splicing fragment ofupstream and downstream sequences of the T6SS-1 gene cluster; connecting the splicing fragment to pK18mobSacB to construct a knockout vector and transferring the knockout vector to the competent escherichia coli, transferring the knockout vector to pseudomonas plecoglossicida cells by conjugation and conducting homologous recombination exchange twice to achieve knockout of a target gene. The T6SS-1 gene cluster is knocked out to achieve the pseudomonas plecoglossicida attenuated vaccine with the virulence significantly weakened relative to a wild strain while good immunogenicity is maintained.The vaccine has a good immune protection effect on a host of inoculated fish, and the virulence of the knockout strain does not recover.
Owner:ZHEJIANG OCEAN UNIV
Preparation method of identifiable inactivated vaccine for animals
InactiveCN104069487AAchieve purificationImprove purification effectAntibody medical ingredientsAntigenAnimals vaccines
The invention discloses a preparation method of identifiable inactivated vaccine for animals, and relates to the technical field of animal vaccines, in a preparation process, a gene engineering method, a natural subculture method or a chemical synthesis method is adopted to reduce one or multiple segments of genes from antigenic substance to generate new properties, and the antigenic substance with new properties is prepared into the identifiable inactivated vaccine. The preparation method disclosed by the invention has the beneficial effects that after the vaccine is inoculated to an animal, an antibody can be verified by detecting the properties, by verifying whether the antibody is generated by the non-treated antigen (namely, wild virus) or by treated antigen (namely, vaccine virus), the antibodies generated by the vaccine and the wild virus can be distinguished to diagnose whether the animal is infected with wild virus strain, so as to eliminate and remove animals with virus to eliminate diseases, thereby being beneficial to eliminate diseases in farms.
Owner:SHANGHAI CHUANG HONG BIOTECH
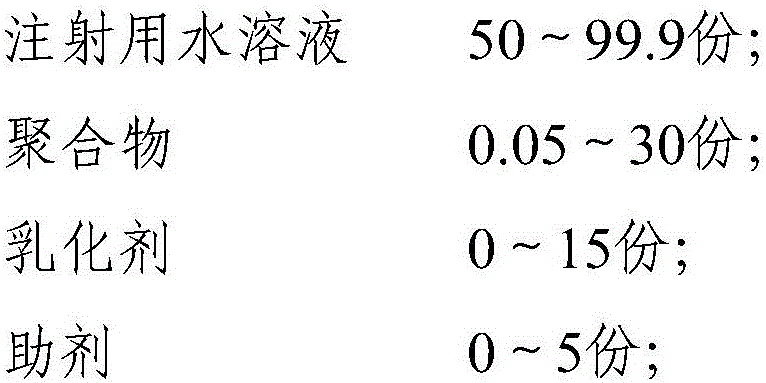
Polymer combined adjuvant of animal vaccine and applications thereof
ActiveCN105816873AFacilitate process quality controlEase of industrial productionImmunological disordersAntibody medical ingredientsMedicineAnimals vaccines
The invention relates to vaccine adjuvant, and specifically discloses a polymer combined adjuvant of animal vaccine and applications thereof. The polymer combined adjuvant comprises an injection water solution, polymer, an emulsifier, and auxiliary agents. The provided polymer combined adjuvant is stable and efficient. Compared with the known vaccine adjuvant, the provided adjuvant has the characteristics of safety and efficiency, the product technology and quality can be controlled easily, and thus the adjuvant has a wide application prospect. The provided polymer combined adjuvant can be applied to industrial production of vaccine easily. The used polymer can be water solution or suspension liquid that can form a particle structure such as capsule or micelle. The antigen for preparing vaccine can be wrapped and stored into cavities formed by polymer or can also be evenly absorbed by polymer so as to be evenly distributed in the polymer.
Owner:CHINA ANIMAL HUSBANDRY IND
Recombinant pseudorabies virus for expressing S1 proteins of porcine epidemic diarrhea viruses, construction method and application thereof
ActiveCN107345222ASsRNA viruses positive-senseViral antigen ingredientsEpidemic diarrheaAnimals vaccines
The invention discloses a recombinant pseudorabies virus for expressing S1 proteins of porcine epidemic diarrhea viruses. The virus is inserted into S1 genes of optimized porcine epidemic diarrhea viruses, and the nucleotide sequence of the recombinant pseudorabies virus is shown in SEQ ID NO.1 of a sequence table. The invention further discloses a construction method of the recombinant pseudorabies virus and an application thereof in preparing animal vaccines. The recombinant pseudorabies virus is good in safety, and after immunization, the recombinant pseudorabies virus not only can generate antibodies for the mutant pseudorabies virus, but also can generate S1 protein antibodies for the porcine epidemic diarrhea viruses, so that the market prospect is wide.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Multi-component subunit vaccine for African swine fever, and preparation method and application of multi-component subunit vaccine
PendingCN111658768APrevent and protect against infectionImproving immunogenicityViral antigen ingredientsAntiviralsAdjuvantAfrican swine fever
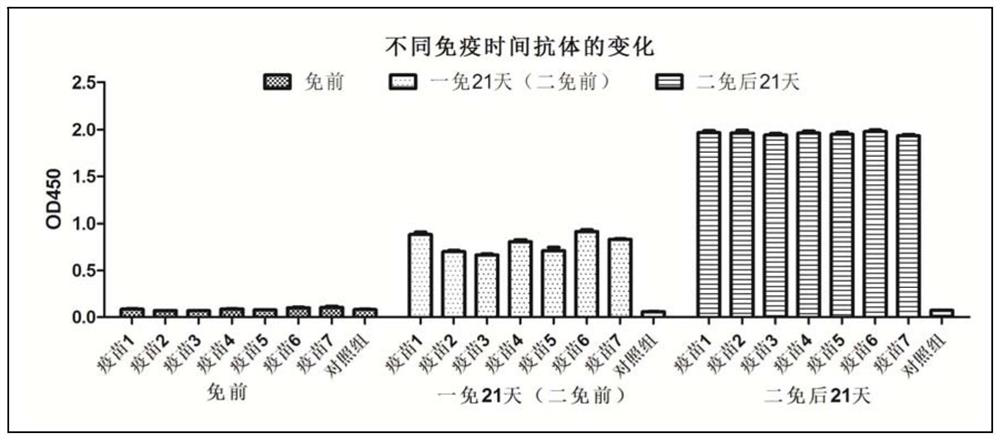
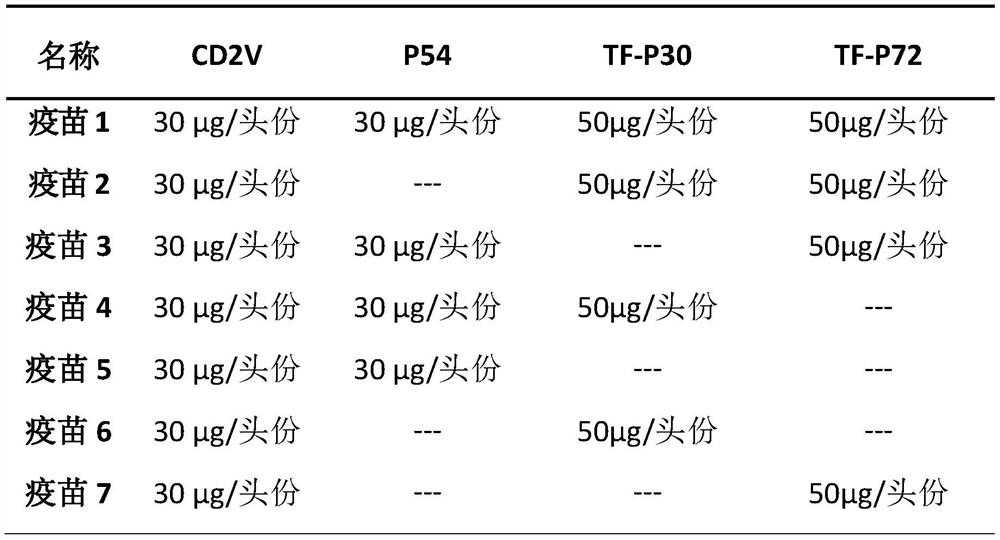
The invention discloses a multi-component subunit vaccine for African swine fever, and a preparation method and application of the multi-component subunit vaccine, and belongs to the technical field of animal vaccines and veterinary biological products. The multi-component subunit vaccine comprises an African swine fever virus surface envelope protein CD2V, at least one protein selected from an African swine fever virus P72 protein, an African swine fever virus P30 protein and an African swine fever virus P54 membrane structure protein, and a pharmaceutically acceptable adjuvant, wherein the African swine fever virus surface envelope protein CD2V is an African swine fever virus CD2V protein without a transmembrane region, or a fusion protein CD2V-Fc of an African swine fever virus CD2V extracellular region protein and a swine antibody Fc. The multi-component subunit vaccine provided by the invention has strong immunogenicity and good safety, and can significantly induce humoral immuneresponse.
Owner:NOVO BIOTECH CORP
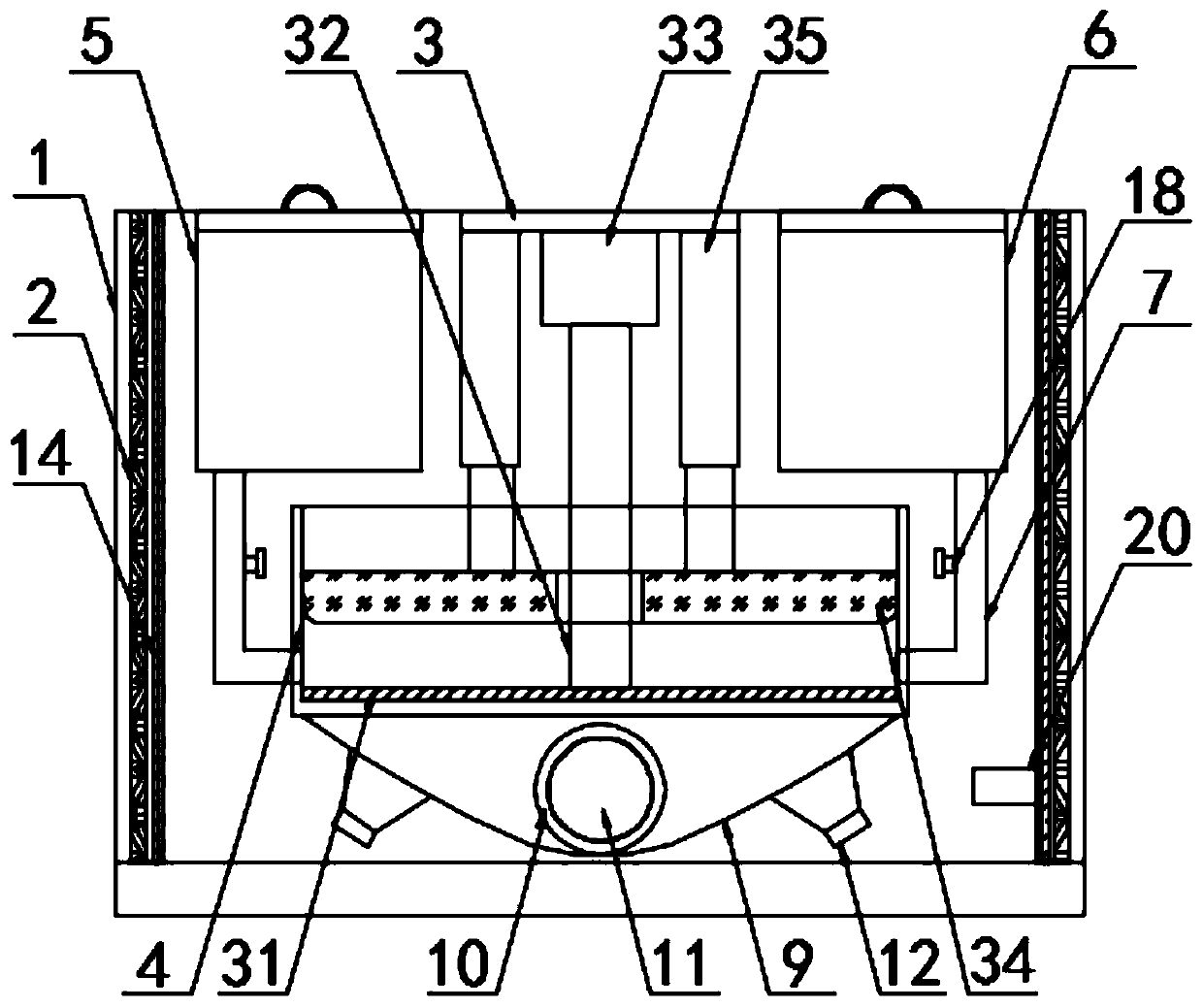
Animal vaccine quick inoculator
InactiveCN109966010AAchieve drainagePrecise control of mixing ratioAmpoule syringesPharmaceutical containersAnimals vaccinesEngineering
The invention discloses an animal vaccine quick inoculator and particularly relates to the field of vaccine inoculation. The animal vaccine quick inoculator comprises a box body; an insulation refrigerating layer is fixed to the inner wall of the box body; a mixing box is arranged in the box body; a mixture feeding mechanism is arrange in the mixing box; a vaccine chamber and a vaccine adjuvant chamber are arranged on the two sides of the top of the mixing box respectively; each of the bottom of the vaccine chamber and the bottom of the vaccine adjuvant chamber is communicated with the mixingbox through a feeding pipe; the bottom surface of the mixing box is provided with a plurality of through holes I; the bottom of the mixing box is provided with a receiving bucket communicated with themixing box through the through holes I. An ultraviolet generator is provided; a vaccine and a vaccine adjuvant are mixed well; the mixture is fed into a receiving bucket; the mixture is filtered witha filter net, and large bubbles are preliminarily eliminated with a small needle; the ultrasonic generator operates to eliminate small bubbles; it is avoided that massive bubbles in the mixed vaccinesolution affect injection; the danger is also avoided that injection of air into vessels causes air embolism.
Owner:CHONGQING UNIV OF ARTS & SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com