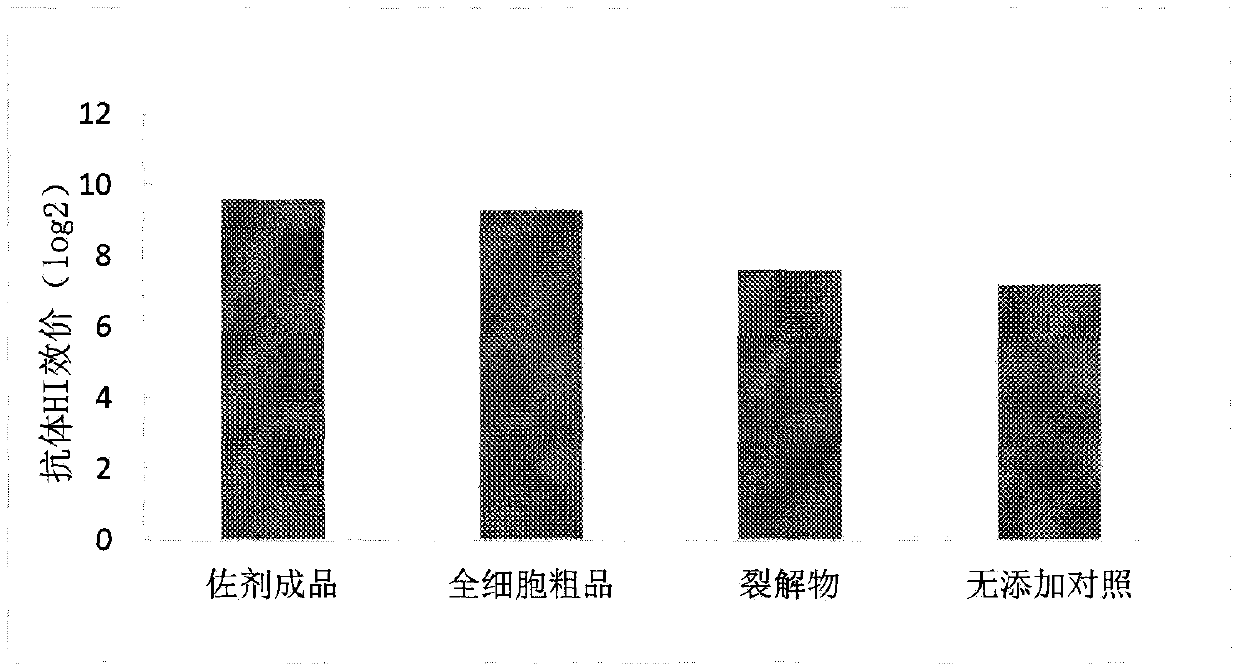
Rhodococcus ruber fermentation method and application thereof as adjuvant in animal vaccines
A technology of Rhodococcus rhodochrous and adjuvant, applied in the field of microbiology and immunology, can solve the problems of low biological safety, edema, and poor targeting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] A method for fermenting Rhodococcus erythrococcus, specifically carried out according to the following steps:
[0061] 1) Preparation of eluted strains: Inoculate the Rhodococcus rubidococcus in a solid agar medium square bottle, and culture at 30°C for 4 days in aerobic or aerobic manner, until the cells in the square bottle appear light yellow or orange yellow or light red or deep red Afterwards, aseptically elute with an appropriate amount of normal saline, harvest the bacterial cells, and use them as eluted strains for later use;
[0062] 2) Preparation of the primary seed liquid: take the eluted strain prepared in step 1), inoculate the shake flask with 2% volume of the liquid culture medium, and cultivate it in a constant temperature shaker with continuous oscillation at a speed of 200 rpm, and cultivate it in aerobic or aerobic conditions at 30°C 48h, as a first-grade seed solution for subsequent use;
[0063] 3) Preparation of the secondary seed liquid: take th...
Embodiment 2
[0066] A method for fermenting Rhodococcus erythrococcus, specifically carried out according to the following steps:
[0067] 1) Preparation of eluted strains: Inoculate the Rhodococcus rubidococcus in a solid agar medium square bottle, and culture at 30°C for 4 days in aerobic or aerobic manner, until the cells in the square bottle appear light yellow or orange yellow or light red or dark red Finally, aseptically elute and harvest the thalline with an appropriate amount of normal saline, and use it as an eluting strain for subsequent use; the percentage by weight of the solid agar medium contained in the solid agar medium in the step 1) is: tryptone 1%, soybean peptone 0.5% , yeast extract powder 0.5%, NaCl 0.5%, sodium glutamate 0.5%, citric acid-citrate 0.25%, glycerin 2%, agar powder 1.5%;
[0068] 2) Preparation of the primary seed liquid: take the eluted strain prepared in step 1), inoculate the shake flask with 2% volume of the liquid culture medium, and cultivate it in...
Embodiment 3
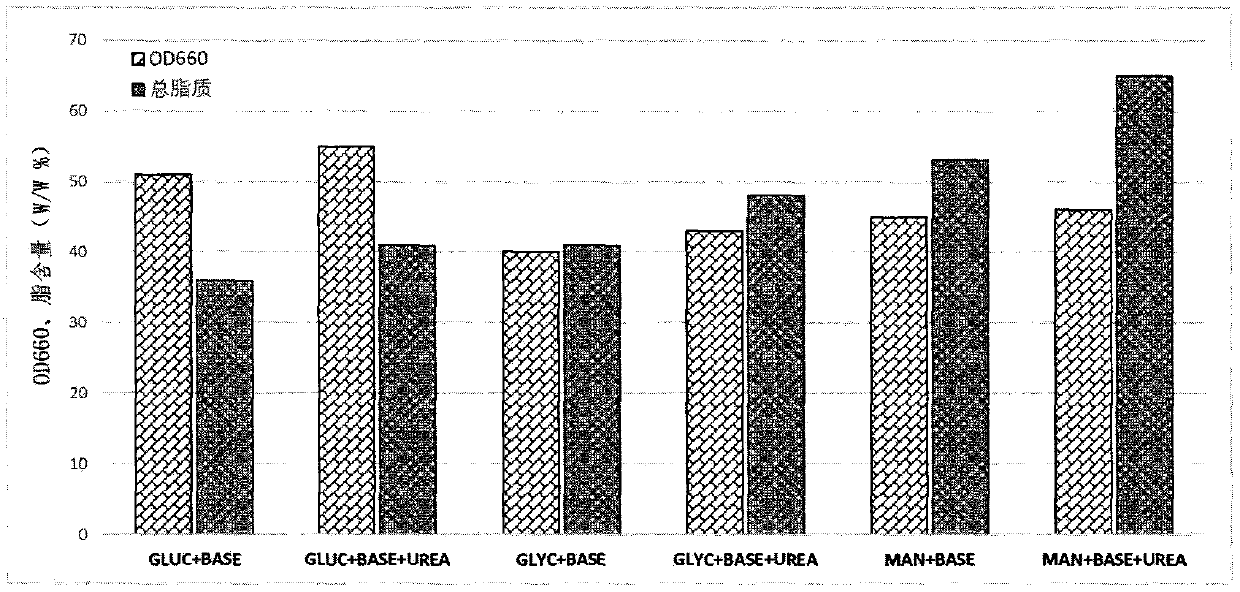
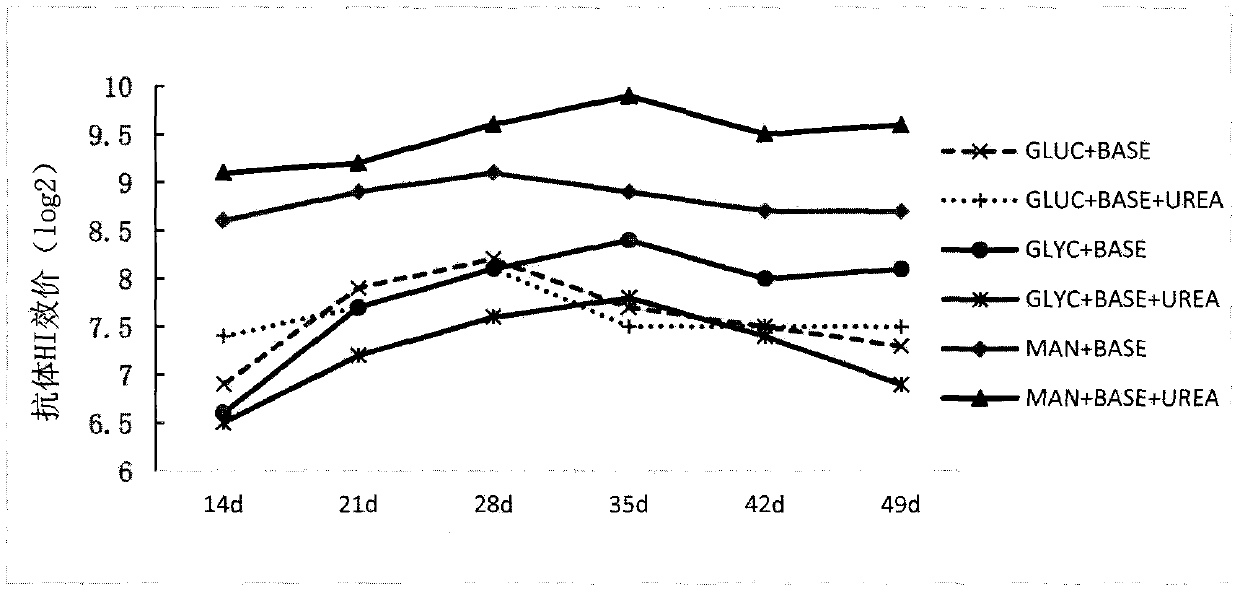
[0072] A liquid culture medium specially used for fermenting and cultivating Rhodococcus Erythrococcus described in this application. The components and weight percentages of the liquid fermentation medium are: 1% tryptone, 1% soybean peptone, 0.1% urea, 1% yeast extract powder, NaCl 1%, sodium glutamate 1%, citric acid-citrate 0.5%, mannitol 5%, and the balance is water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com