Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
218 results about "Ambroxol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ambroxol is a drug that breaks up phlegm, used in the treatment of respiratory diseases associated with viscid or excessive mucus. Recently, a hypothesis suggested that it may have a potential role in treatment of Paget's disease of bone, Parkinsonism, and other common diseases of aging-associated diseases involving dysfunction of autophagy. Ambroxol is often administered as an active ingredient in cough syrup.
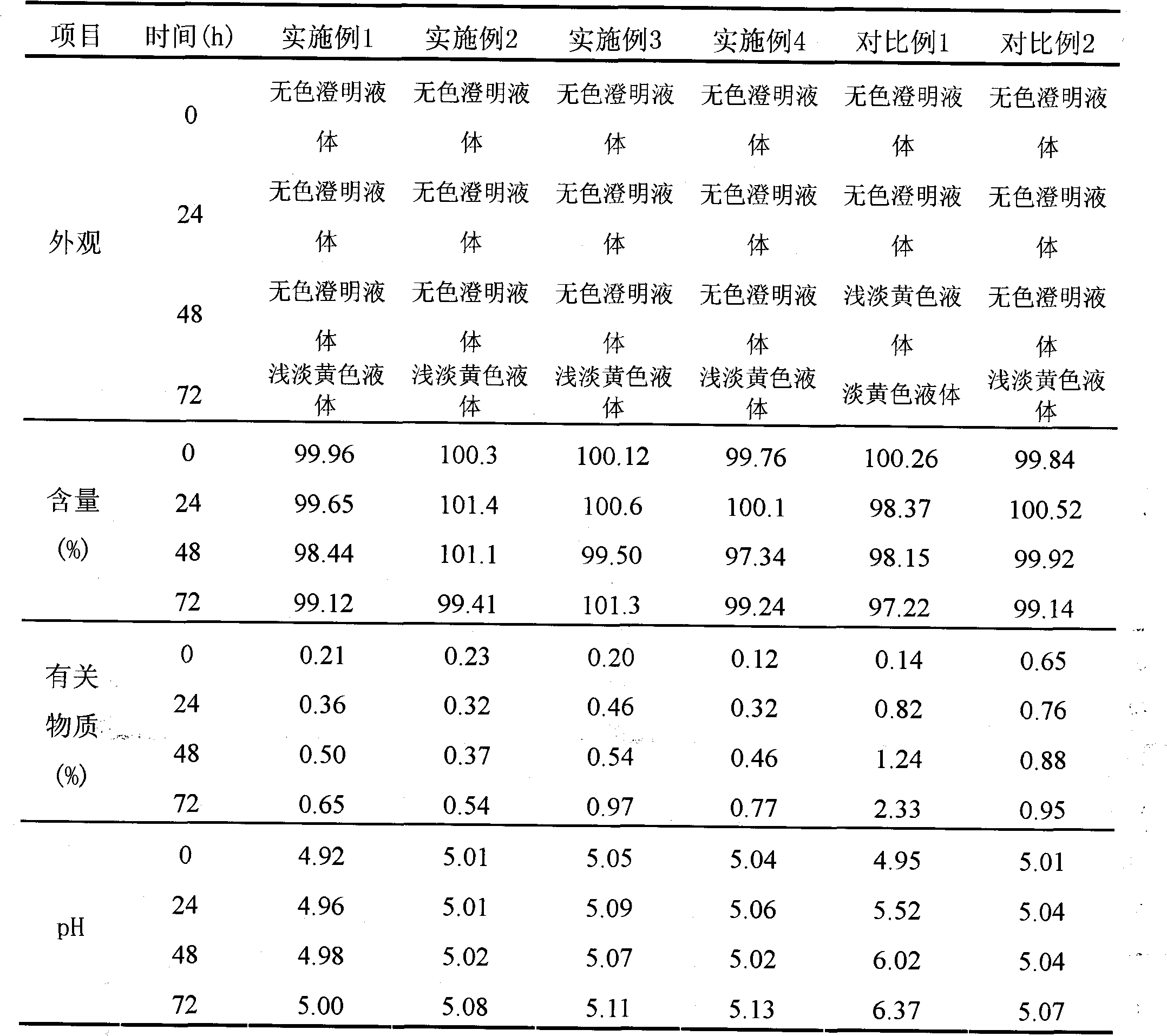
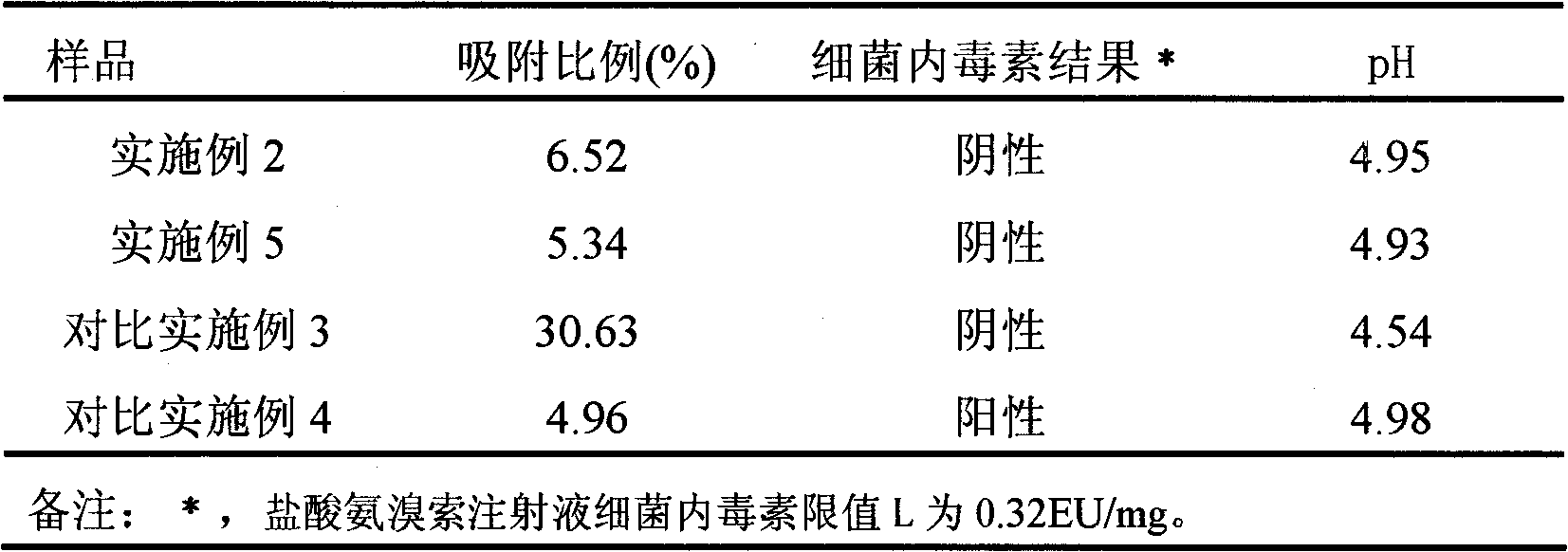
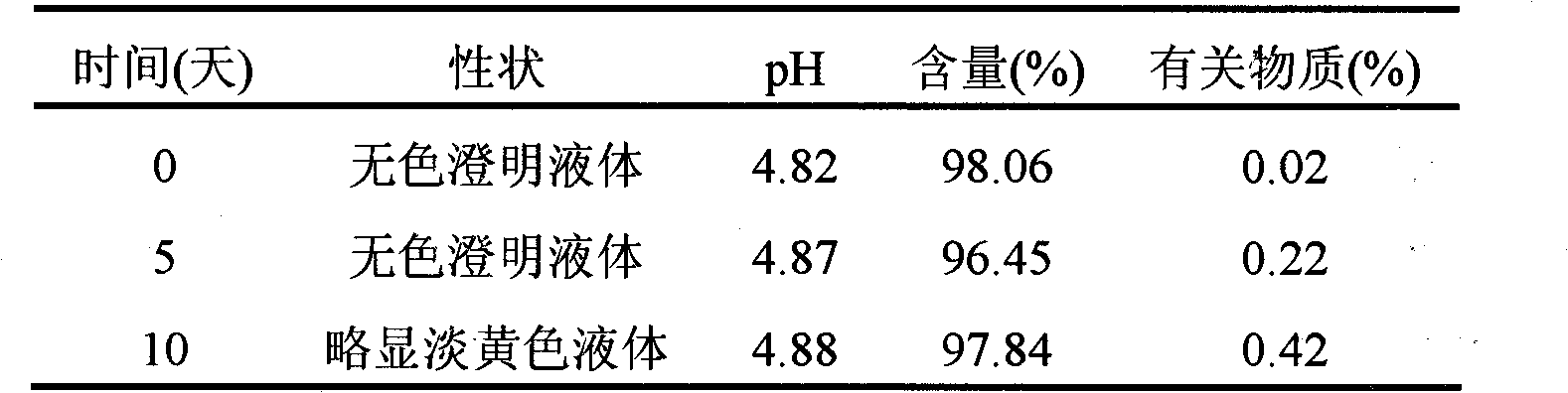
Ambroxol hydrochloride injection
The invention relates to a medicinal preparation, in particular to an ambroxol hydrochloride injection which is a stable medial composition taking ambroxol hydrochloride as an active ingredient to be combined with carriers acceptable in pharmacy. The carriers comprise a water-soluble filling agent, a pH regulating agent, a stabilizing agent, water used for injection or an osmotic pressure regulating agent and the like. As the carriers are adopted to carry out scientific preparation, the drug quality and medication safety of freeze-dried powder injections, small-needle injections and small infusion solutions are ensured, and the stability of the preparations during storage is improved.
Owner:天津康哲维盛医药科技发展有限公司
Ambroxol hydrochloride compound and medicine composition thereof
The invention discloses an ambroxol hydrochloride crystal and a medicine composition prepared by the ambroxol hydrochloride crystal. The ambroxol hydrochloride crystal shows specific diffractive peaks in parts of 6.9 degrees, 7.2 degrees, 12.8 degrees, 15.6 degrees, 17.5 degrees, 20 degrees, 21 degrees, 22 degrees and 24 degrees in an X-ray powder diffraction pattern which is shown in form of 2 Theta + / -0.2 degrees. The prepared ambroxol hydrochloride crystal is high in yield and liquidity; and the amount of lubricant in original prescription is halved, so that the weight and uniformity of tablets can meet the specification.
Owner:SHENYANG XINMA PHARMA +2
Tablets containing ambroxol
A tablet comprising a core portion comprising 150 mg to 1200 mg of ambroxol and a film coating surrounding the core portion.
Owner:BOEHRINGER INGELHEIM INT GMBH
Preparation method of ambroxol hydrochloride
InactiveCN103012167AAvoid processing powerAvoid the corresponding processOrganic compound preparationAmino-hyroxy compound preparationBiotechnologyBiochemical engineering
The invention discloses a preparation method of ambroxol hydrochloride. According to the preparation method, a 'one-pot reaction' is adopted in the reaction, so that the separation of an intermediate is avoided, the operation is simple, the production period is short, the production cost is reduced, and the method is suitable for industrial production and application; the operation in refining the ambroxol hydrochloride is easy to carry out; and the ambroxol hydrochloride with purity more than 99.9% can be simply obtained by one-time recrystallization, so that the demand on preparation production can be met.
Owner:石药集团中诺药业(石家庄)有限公司
Ambroxol for the treatment of inflammation in the pharynx
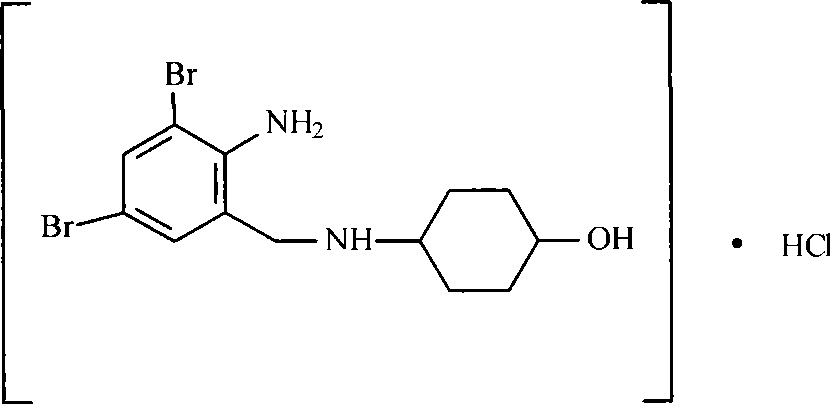
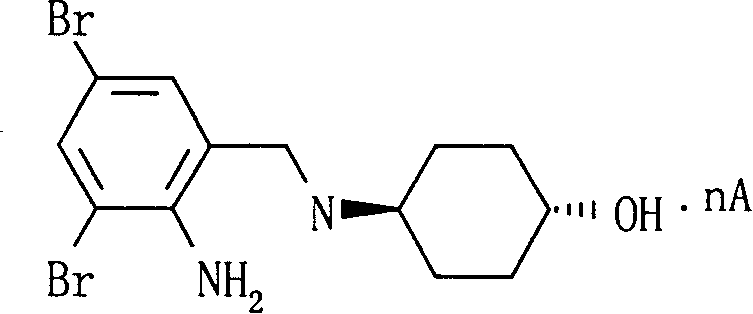
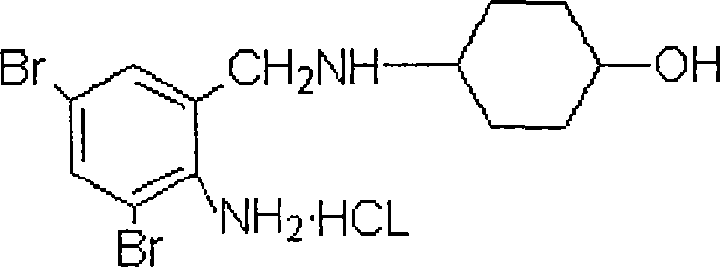
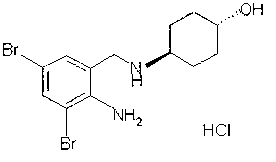
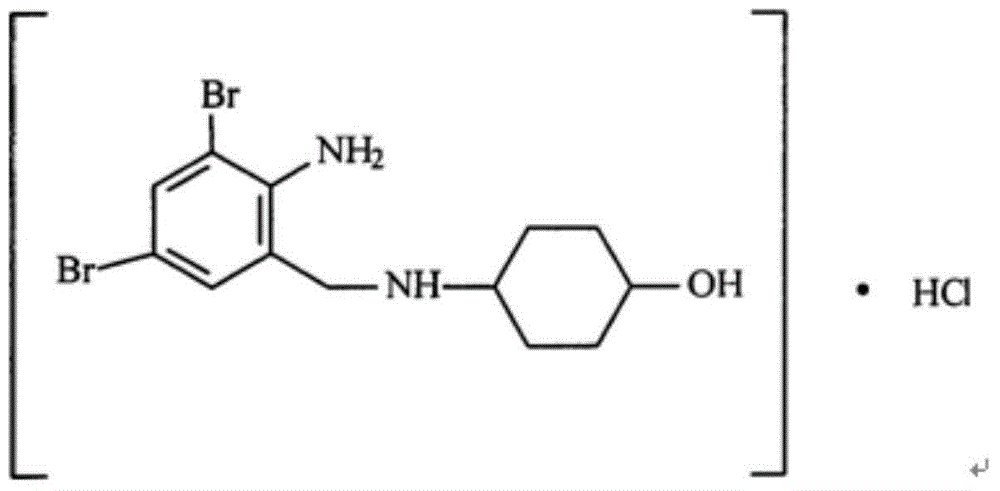
The invention relates to the use of ambroxol (trans-4-(2-amino-3,5-dibromobenzylamino)-cyclohexanole) and the pharmacologically acceptable salts thereof for preparing a pharmaceutical composition for the treatment of inflammation in the pharynx.
Owner:BOEHRINGER INGELHEIM PHARM KG
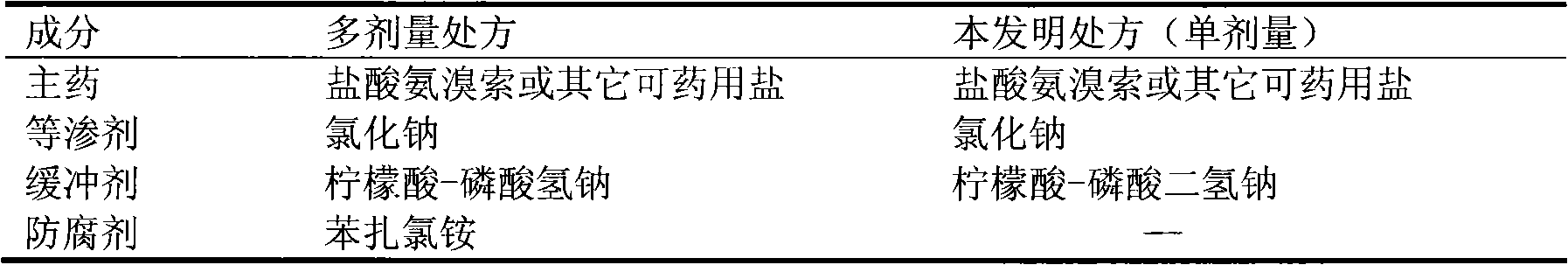
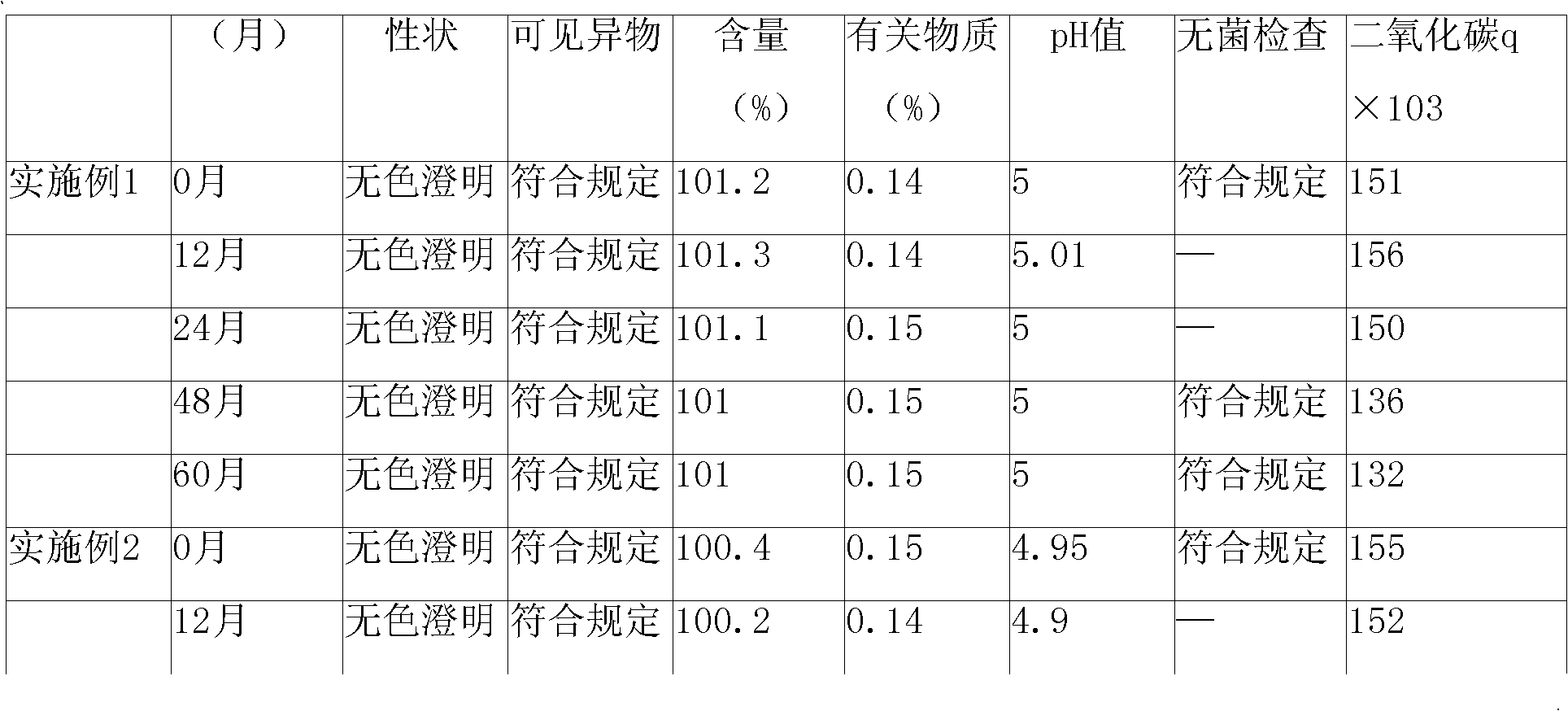
Ambroxol injection and preparation method thereof
ActiveCN101836952AImprove buffering effectLess irritatingPowder deliveryOrganic active ingredientsCITRATE ESTERPharmacology
The invention provides an ambroxol injection and a preparation method thereof; the injection contains ambroxol and the pharmaceutical acceptable salt thereof, disodium hydrogen phosphate and citrate, wherein the weight ratio of the ambroxol and the pharmaceutical acceptable salt thereof, the disodium hydrogen phosphate to the citrate is 1:0.18 to 0.22:0.12 to 0.16; and preferably, the weight ratio of the ambroxol and the pharmaceutical acceptable salt thereof, the disodium hydrogen phosphate to the citrate is 1:0.20:0.14. The pharmaceutical acceptable salt of the ambroxol is preferred to be hydrochloride. The used amount of active carbon adopted in the preparation is 0.2 to 0.3 percent.
Owner:云南龙海天然植物药业有限公司
High dose Ambroxol hydrochloride freeze-dried preparation and preparation method
InactiveCN1954808AGood solubilization effectInhibition of precipitation and crystallizationOrganic active ingredientsPowder deliveryFreeze-dryingHigh doses
A freeze-dried powder injection of high-dosage (500mg) ambroxol hydrochloride and its preparing process are disclosed.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Ambroxol for the treatment of acute pain
A method of treating acute pain in a patient in need thereof, the method comprising orally administering to the patient ambroxol or a pharmacologically acceptable salt thereof.
Owner:BOEHRINGER INGELHEIM INT GMBH
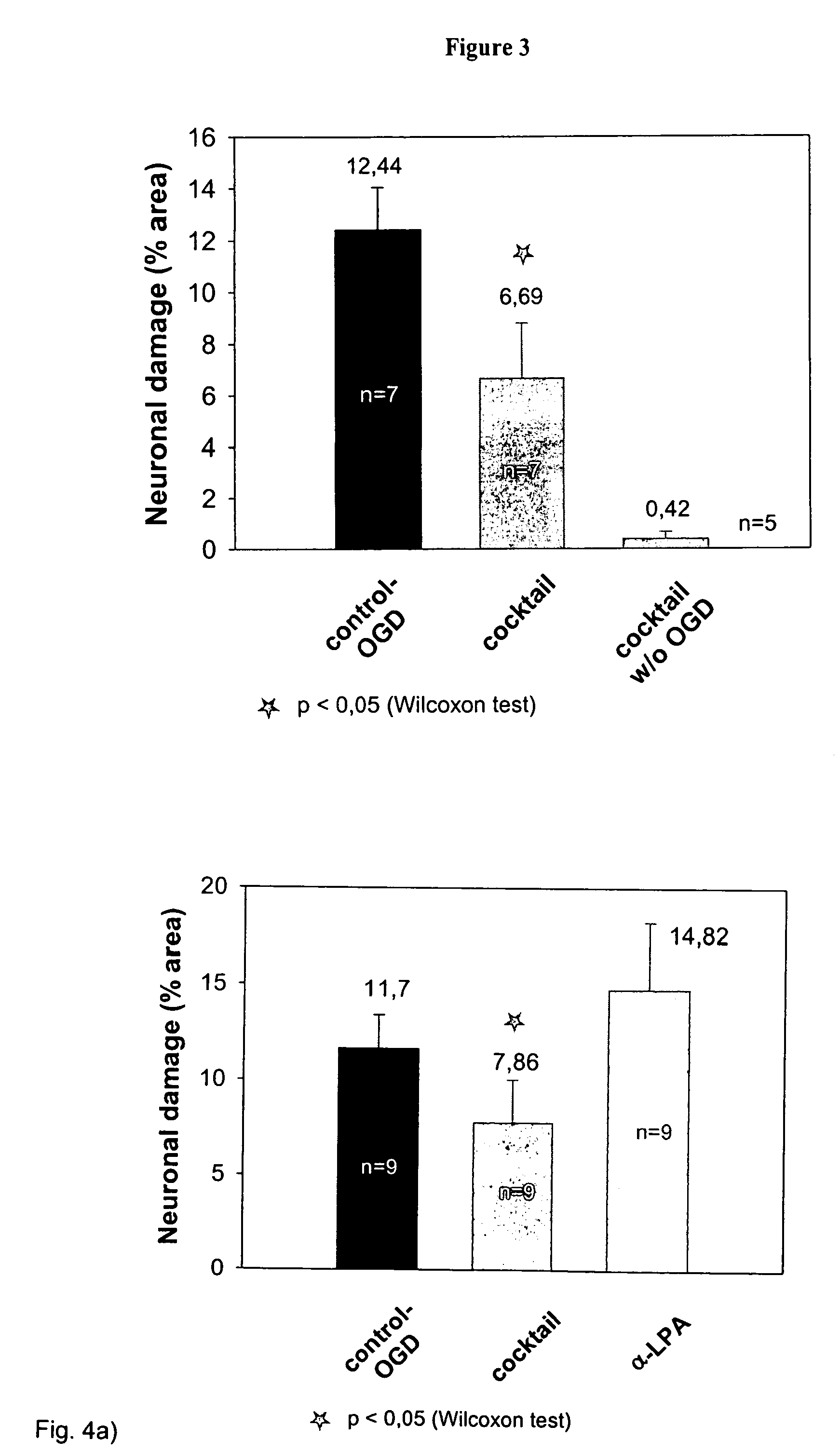
Drug preparation comprising α-lipoic acid, ambroxol and/or inhibitors of the angiotensin-converting enzyme (ACE) and its use for the treatment of neurodegenerative diseases
InactiveUS7858655B2Reducing neuronal damaging processBiocideNervous disorderAngiotensin-converting enzymeDrugs preparations
The present invention concerns the use of Provided is a drug composition and a method for using the composition for the prevention and the therapy of neurodegenerative diseases. The combination comprises at least two of the following substances: α-lipoic acid; ambroxol and one or several inhibitor(s) of the angiotension-converting enzyme (ACE).
Owner:INSTITUT FUR MEDIZINTECH MAGDEBURG IMTM
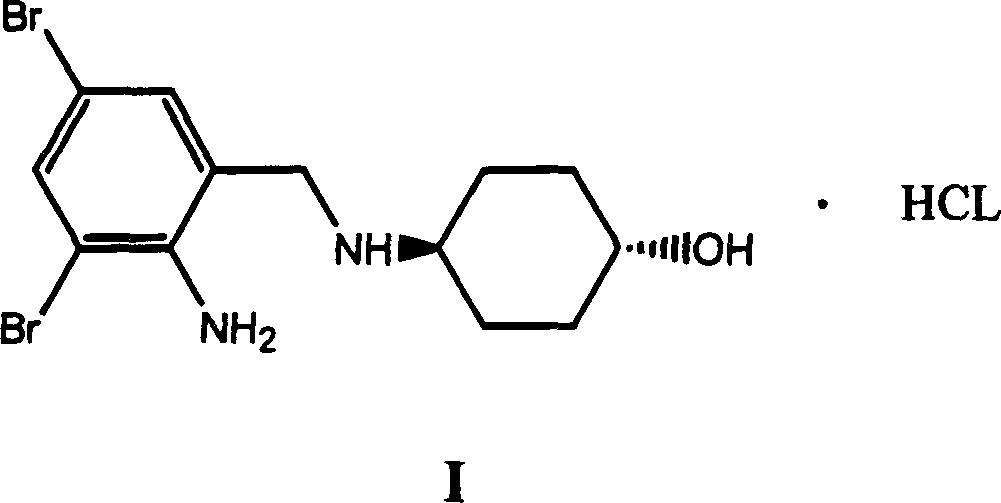
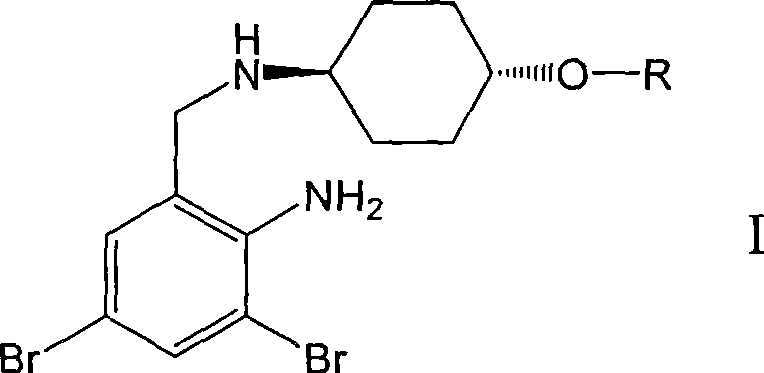
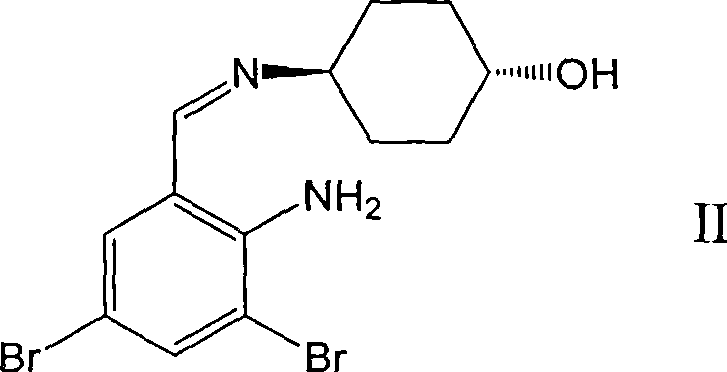
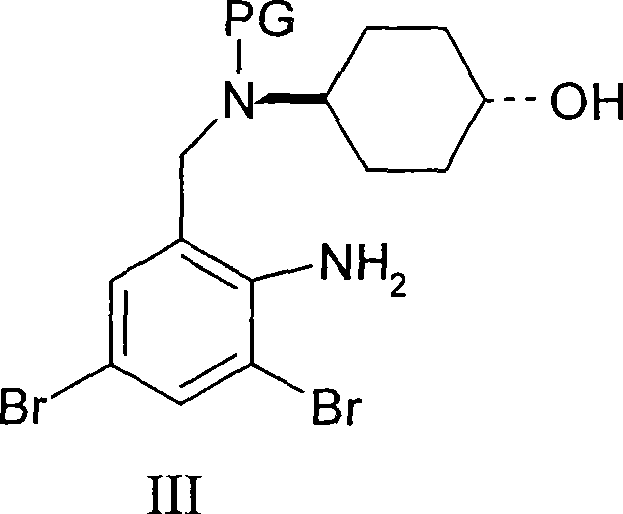
Ambroxol derivative and method for preparing same
InactiveCN101544572AOrganic active ingredientsOrganic compound preparationMedicinal chemistryMedical treatment
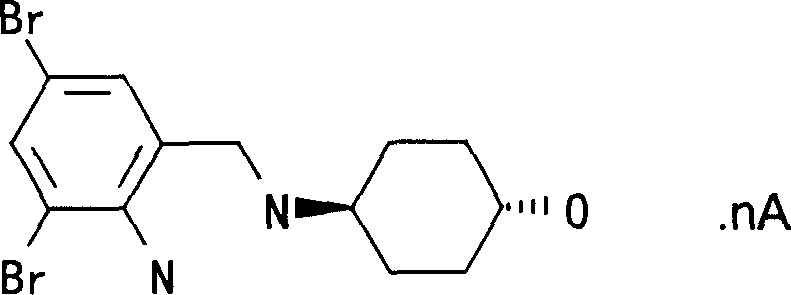
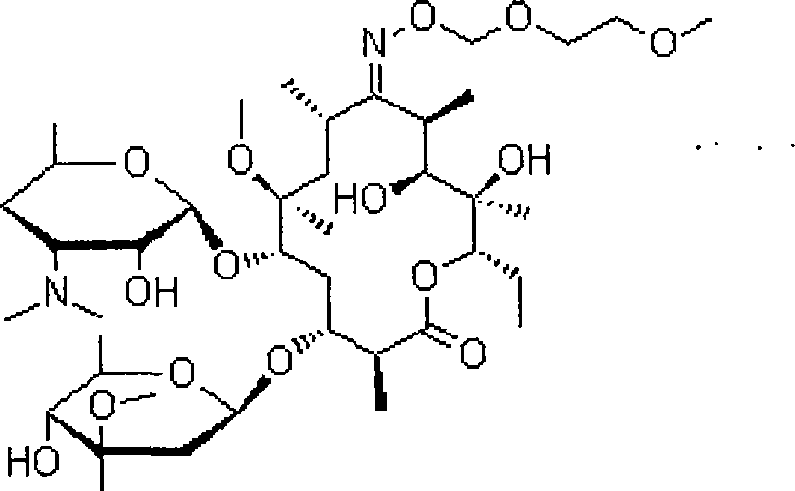
The invention relates to an ambroxol derivative and a method for preparing the same, in particular to the ambroxol derivative of a formula (I) or a pharmaceutically acceptable salt thereof, a pharmaceutical composition thereof and application thereof in medical treatment. In the formula (I), R is as defined as description.
Owner:JIANGSU HANSOH PHARMA CO LTD
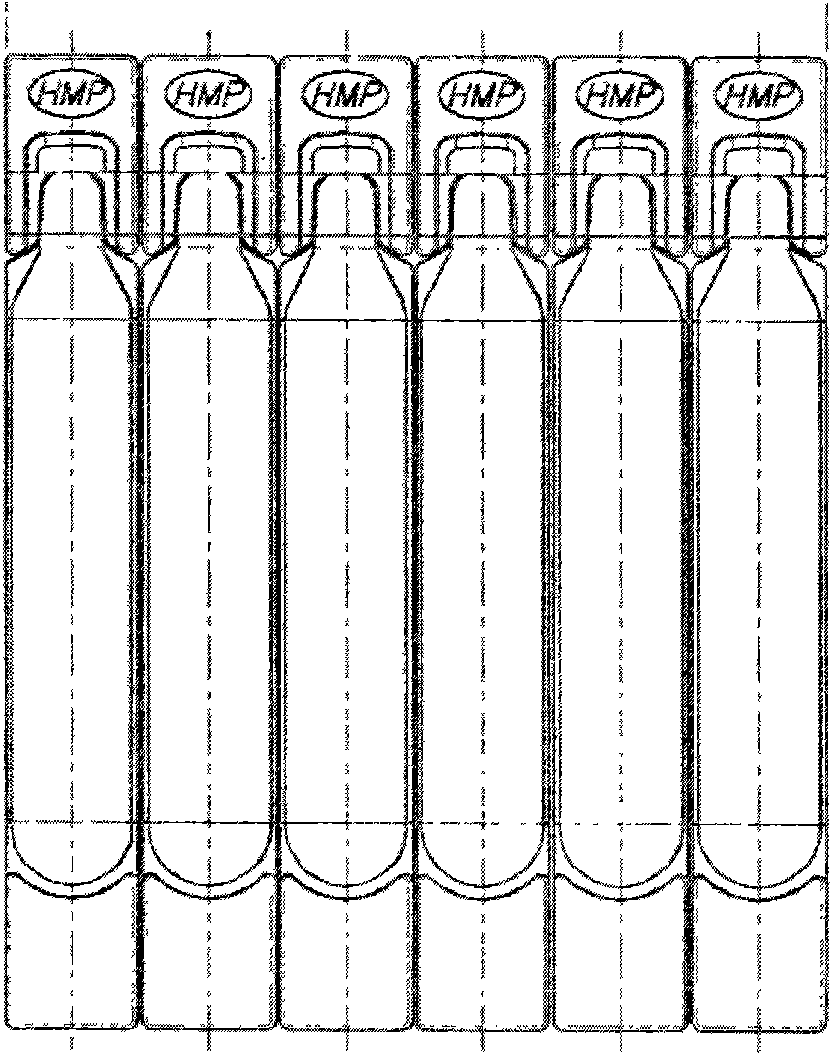
Solution preparation for atomizing and inhaling ambroxol and preparation method thereof
InactiveCN101606903ASafe for clinical useLess irritatingOrganic active ingredientsAerosol deliveryPreservative freePreservative
The invention relates to a solution preparation for atomizing and inhaling ambroxol, and belongs to the field of pharmaceutics. The solution preparation for atomizing and inhaling the ambroxol comprises (1) ambroxol or medicinal salt thereof with single medicinal dosage, (2) isotonic agent and solvent, (3) buffer solution and (4) medicinal package with single dosage; and in terms of the ambroxol, the mass ratio of the medicinal components to the isotonic agent is 1:1.1-1:3.3.The invention provides the solution preparation for atomizing and inhaling the ambroxol with single dosage and the medicinal package adopting blowing-filling-sealing (BFS) integrative full-automatic production, which have the advantages that the medicinal package is designed and manufactured for patients special for atomizing and inhaling; the solution preparation does not contain preservative, and is used with disposable single dosage; the using process is convenient and does not need dilution and preparation; and the microbe pollution, waste and the like during using can be greatly reduced. The medicinal preparation provides a treatment medicament and a treatment proposal with accurate medicinal dosage, good-quality and stable medicament quality, and safe and simple clinical application lacking in the prior art.
Owner:BEIJING HANMI PHARMA CO LTD
Ambroxol hydrochloride injection with small volume and preparation method thereof
InactiveCN101647777AStrong buffer capacityImprove stability and securityOrganic active ingredientsPharmaceutical delivery mechanismChemistryCarbon dioxide
The invention discloses an ambroxol hydrochloride injection with small volume and high stability and a preparation method thereof. In the ambroxol hydrochloride injection with small volume, the weightpercentage concentration of ambroxol hydrochloride is 0.3-4 percent, the ambroxol hydrochloride solution comprises a component capable of forming acid-base buffer pairs and further comprises carbon dioxide, and a part of space in an ampoule is filled with nitrogen gas. By utilizing carbon dioxide to remove oxygen from the solution, the invention provides the ambroxol hydrochloride injection whichhas small volume and longer stable phase and can be prepared by ambroxol hydrochloride material in different syntheticroutes. The ambroxol hydrochloride injection with small volume can be directly injected into a vein and has good buffering capacity for pH value of blood plasma.
Owner:HEILONGJIANG FUHE HUAXING PHARMA GROUP
Ambroxol for the treatment of chronic nociceptive pain
A method of treating chronic nociceptive pain, osteoarthritis, irritable bowel syndrome, fibromyalgia, visceral pain, or rheumatoid arthritis in a patient in need thereof, the method comprising administering to the patient ambroxol or a pharmacologically acceptable salt thereof.
Owner:BOEHRINGER INGELHEIM INT GMBH
Pharmaceutically acceptable composition containing ambroxol in non-salt form
The invention relates to a medicinal composition of ambroxol in a non-salt form. Particularly, the invention especially relates to a composition comprising ambroxol in a base form and other oral substances with or without physiological activity or a drug container. The inventor surprisingly finds that when ambroxol is prepared into an oral preparation in a non-salt form, and especially a preparation such as a suspension, a dry suspension, a granule and the like needing to be diluted and prepared with water before drinking, the mouthfeel is significantly improved when compared with that of an ambroxol hydrochloride solution, and the ambroxol preparation has significantly slower absorption in vivo than an ambroxol hydrochloride preparation; and the realization of a slow-release effect which requires great effort on preparation process can be easily achieved. It is well known that the slow release of a drug can reduce drug side effects and increase security with the guarantee of curative effects.
Owner:沈阳华泰药物研究有限公司
Ambroxol hydrochloride liquid preparation and preparation method thereof
ActiveCN101627967AImprove solubilityOvercome the defect of being slightly soluble in waterOrganic active ingredientsPharmaceutical delivery mechanismWater useBULK ACTIVE INGREDIENT
The invention discloses an ambroxol hydrochloride liquid preparation and a preparation method thereof. The method comprises the steps: dissolving ambroxol hydrochloride, stabilizing agent and osmotic pressure regulator into water used for injection, and evenly mixing together to obtain solution I; then, filtering the solution I, and obtaining the ambroxol hydrochloride liquid preparation. The preparation method does not introduce active carbon, so as to avoid the danger of hurting human body since active carbon particle is introduced into the preparation; meanwhile, the active ingredients in the preparation is ensured to be stable, and the safety (namely, the chemical stability of the ambroxol hydrochloride can be effectively improved, the particle content in the preparation is reduced, and the purity of the preparation is improved) of the finished product can be guaranteed.
Owner:上海华源药业(宁夏)沙赛制药有限公司
Ambroxol hydrochloride dry powder inhalant and preparation thereof
InactiveCN101214227APurpose of safe treatmentEasy to usePowder deliveryOrganic active ingredientsMaterials scienceAMBROXOL HYDROCHLORIDE
The present invention belongs to the medication technical field and discloses hydrochloric ambroxol dry powder inhalant and a preparation method thereof. The hydrochloric ambroxol dry powder inhalant consists of hydrochloric ambroxol, dispersion flow aid and thinner, the weight percentage of which is 60 percent to 90 percent of the hydrochloric ambroxol, 10 percent to 30 percent of the dispersionflow aid and 0 percent to 40 percent of the thinner. The present invention comprises the following steps that the hydrochloric ambroxol or the hydrochloric ambroxol and the dispersion flow aid or thehydrochloric ambroxol, the dispersion flow aid and the thinner are processed for spray drying to obtain powder body which is collected into a glutin or plastic capsule or into an aluminum-plastic bubble cap or is contained inside a large dosage dry powder absorbing device as the storehouse form. The present invention adopts the dry powder inhalant to ensure that the hydrochloric ambroxol is directly absorbed into respiratory tract and lung by a medication device to act directly inside the respiratory tract, so as to achieve the purpose of the safe and targeting treatment with quick result andhigh efficiency.
Owner:SHENYANG PHARMA UNIVERSITY
Ambroxol cysteine analogs and their preparation process and use thereof
The invention relates to an ambroxol cysteine analogs and their preparation , the medicinal composition containing the compound, and the use in preparing apophlegmatic medicaments. The method for preparation comprises reacting ambroxol and sulfo-aminolactic acid analogue in solvent, the crystallizing, wherein n=1, 2, A is defined in the specification.
Owner:JIANGSU HANSOH PHARMA CO LTD
Loratadine-ambroxol pharmaceutical composite and liposome solid preparation thereof
InactiveCN101627998AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical product form changeYolkMedicine
The invention relates to a loratadine-ambroxol pharmaceutical composite and a liposome solid preparation thereof and a preparation method thereof; the liposome comprises the following components according to the parts by weight percent: 1 part of loratadine, 5 parts of ambroxol hydrochloride, 3-30 parts of yolk lecithin, 1-14 of cholesterol, 1.2-10 parts of sodium deoxycholate and 3-18 parts of poloxamer 188.
Owner:HAINAN YONGTIAN PHARMA INST
Ambroxol hydrochloride detection method
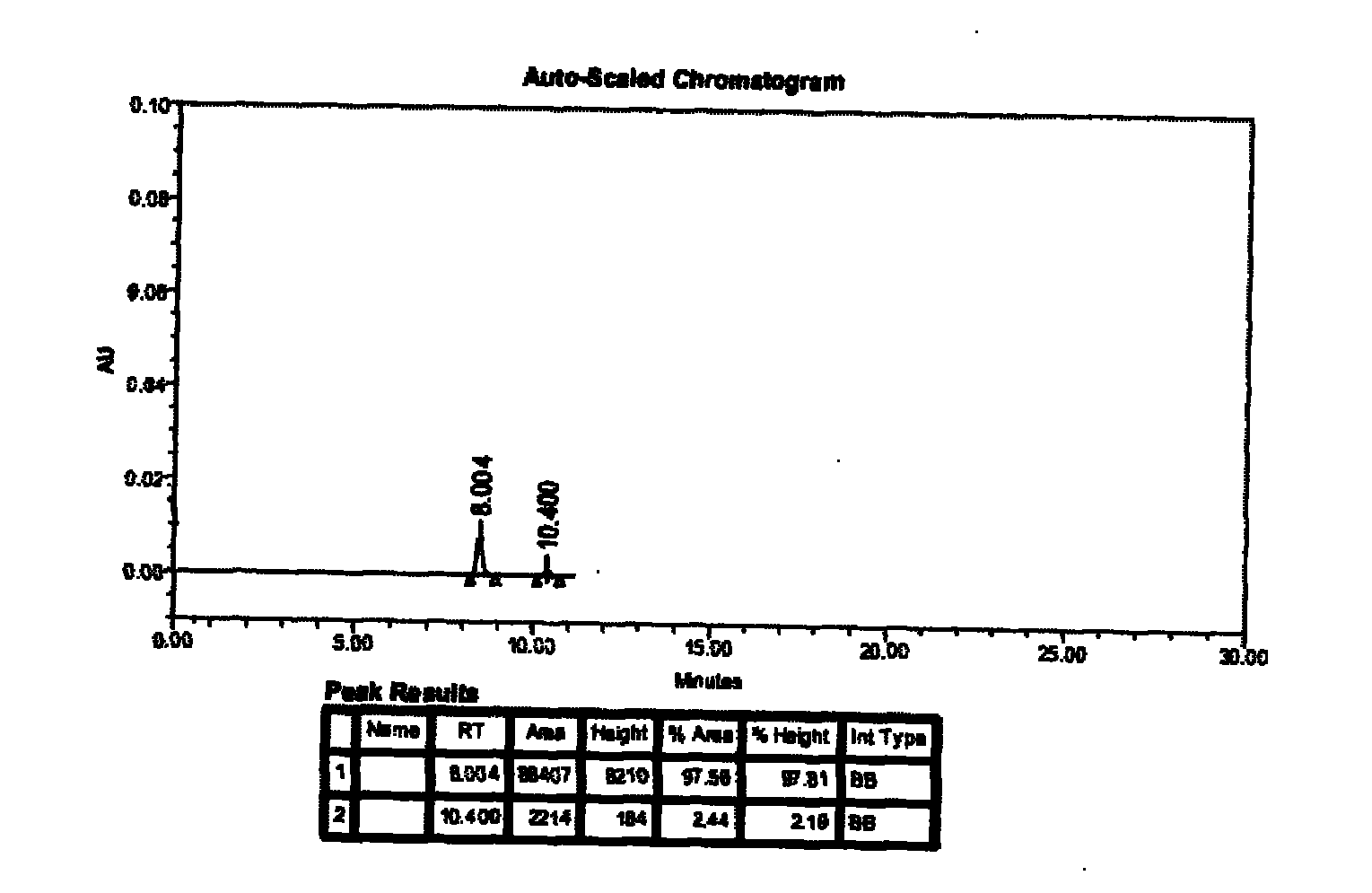
The invention provides a method for measuring ambroxol and related substances trans-4-[6,8-dibromo-1,4-orexin-3(H)] cyclohexanol (impurity B) and 2-amino-3,5-dibromo-benzaldehyde (impurity A) in a preparation of the ambroxol, which is characterized in that: in the measurement of the trans-4-[6,8-dibromo-1,4-orexin-3(H)] cyclohexanol, an adopted detection wavelength is 258 nm and a chromatogram is recorded for a time that is 3 times the retention time of the main component; and the method for measuring the 2-amino-3,5-dibromo-benzaldehyde is characterized in that: the adopted detection wavelength is 238 nm and the chromatogram is recorded for the time that is 3 times the retention time of the main component.
Owner:云南龙海天然植物药业有限公司
Compound ambroxol hydrochloride sustained-release tablet and preparation method thereof
InactiveCN101084912AImprove complianceReduce the number of dosesAntibacterial agentsOrganic active ingredientsSustained Release TabletAdjuvant
The invention provides a preparation method of compound ambroxol hydrochloride sustained release matrix tablet. The inventive ambroxol hydrochloride sustained release tablet specifically comprises (g / g) ambroxol hydrochloride 5-20%, roxithromycin 15-60%, skeletal material 5-40%, diluent 5-50%, pH regulator 0-20%, proper amount of binding agent, and proper amount of lubricant. The preparation method comprises mixing the materials and tabletting directly; or granulating part of the materials, mixing with the rest materials, mixing, and preparing into sustained release tablet; or mixing part of the raw materials with adjuvant, preparing into quick-release part, mixing the rest raw material, skeletal material, pH regulator and diluent as slow-released part, and preparing into double layer tablet with quick-release layer and sustained release layer. The method for preparing granulate comprises dry method, wet method, melting or fusion.
Owner:SHANDONG INST OF PHARMA IND
Composition of ambroxol, salt thereof and anti-infective drug
InactiveCN1989953AMeet urgent clinical needsStable and uniform qualityOrganic active ingredientsRespiratory disorderDiseaseSuccinic acid
Owner:JIANGSU UNIV OF TECH
Injection of ambroxol hydrochloride and preparation method thereof
InactiveCN102988281AReduce dosageLow costOrganic active ingredientsPharmaceutical delivery mechanismDrugs solutionPolyethylene glycol
The invention provides an injection of ambroxol hydrochloride and a preparation method thereof; the injection comprises 15 parts by weight of ambroxol hydrochloride, 0.01-0.03 part by weight of citric acid, 2-20 parts by weight of polyethylene glycol 400, 16-18 parts by weight of sodium chloride, and 2000 parts by weight of injection water, and preferably comprises 15 parts by weight of ambroxol hydrochloride, 0.02 part by weight of citric acid, 5 parts by weight of polyethylene glycol 400, 17 parts by weight of sodium chloride, and 2000 parts by weight of injection water. The injection of ambroxol hydrochloride provided by the invention can tolerate hot pressurized sterilization at 121 DEG C for 15 min, is compatible with drug solutions with a pH of more than 10, has good stability, and can better guarantee medication safety for human body.
Owner:辽宁科泰生物基因制药股份有限公司
Ambroxol hydrochloride oral solution and preparation method thereof
InactiveCN105193707AImprove securityExtended shelf lifeOrganic active ingredientsPharmaceutical delivery mechanismExpiration dateIsopropylene glycol
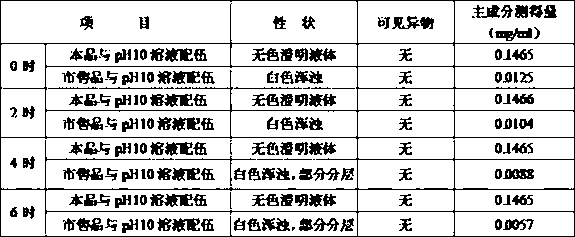
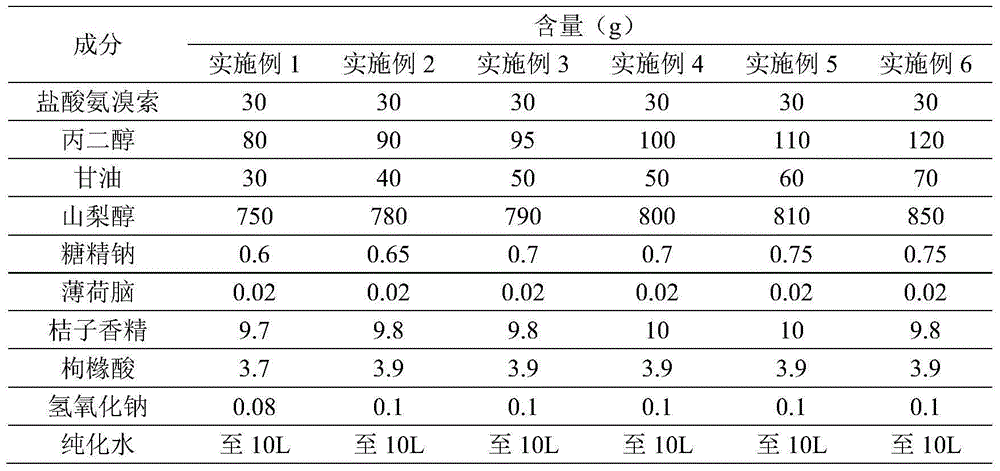
The invention discloses ambroxol hydrochloride oral solution. Each 10 ml of the ambroxol hydrochloride oral solution comprises the followings: 30 mg of ambroxol hydrochloride, 80-120 mg of propylene glycol, 30-70 mg of a wetting agent, 750-850 mg of a corrigent, 3.5-4.1 mg of a pH buffer pair and the balance of purified water; the wetting agent is glycerinum; the corrigent is a composite of sorbitol, saccharin sodium, menthol and flavoring orange essence; the pH value of the ambroxol hydrochloride oral solution is 4.8-5.2. The invention further discloses a preparation method of the ambroxol hydrochloride oral solution. Through adjusting the formula and the pH value of the oral solution, and under the premise of not adding a preservative, the stability of the oral solution is greatly improved, the expiration date is prolonged, and the taste is better.
Owner:NANJING REAL PHARMA
Ambroxol for the treatment of painful conditions in the mouth and pharyngeal cavity
The invention relates to the use of ambroxol and the pharmacologically acceptable salts thereof for preparing a pharmaceutical composition for the treatment of painful conditions in the oral and pharyngeal cavity.
Owner:BOEHRINGER INGELHEIM PHARM KG
Oral disintegration tablet of Loratadine ambroxol, and prepartion method
InactiveCN1915226AImprove bioavailabilityIncrease blood concentrationOrganic active ingredientsPill deliveryLoratadineAMBROXOL HYDROCHLORIDE
An oral disintegrating tablet of compound clarityne-ambroxol for treating cough and allergic rhinitis is prepared from clarityne and ambroxol hydrochloride. Its preparing is also disclosed.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Stable ambroxol hydrochloride taste-masking granule and preparation method thereof
ActiveCN106420625AGreat tasteImprove complianceOrganic active ingredientsPharmaceutical non-active ingredientsPillAMBROXOL HYDROCHLORIDE
The invention provides an ambroxol hydrochloride taste-masking granule, which is prepared from drug-loaded pellets coated by a taste-masking layer and a taste modifying material. Specifically, the drug-loaded pellets coated by a taste-masking layer consist of three layers from the inside out: a drug-loaded pellet core, an isolation layer and a taste-masking layer. The drug-loaded pellets provided by the invention are prepared by a fluidized bed suspension drug-feeding way, the drug-loaded pellet core is externally coated with the isolation layer and the taste-masking layer in order, each layer is coated with a fluidized bed, the taste modifying material is prepared by wet granulation, and the pellets and the taste modifying material are mixed to prepare the granule. The ambroxol hydrochloride taste-masking granule provided by the invention completely covers up the peculiar smell of drug itself, improves the compliance of medication for children, and is added with a sweetener and sugar-containing granules suitable for children to take so as to remove the bitter taste and peculiar smell of chemical drugs, in addition to sugar, the ambroxol hydrochloride taste-masking granule is also added with some fruit essence to greatly improve the mouthfeel of drug, so that children patients can easily accept or actively require to accept the drug, thus enhancing compliance of medication for children. By controlling the particle size of the bulk drugs, the drug feeding yield is increased, the content of drug-loaded pellets is improved, and the particle number of pellets in unit preparation can be reduced, thus reducing the weight increment range of the taste-masking layer, and reaching the purposes of saving labor-hour and cost, and increasing yield.
Owner:山东科成医药科技有限公司
Ambroxol hydrochloride containing hydroxypropyl beta-cyclodextrin and its preparation
InactiveCN1424026ASolve the problem of water solubilityGood for clinical useAmine active ingredientsRespiratory disorderFreeze-dryingWater soluble
An ambroxol hydrochloride injection containing hydroxypropyl beta-dextrin in the form of "liquid injection", "freeze dried powder injection", "aseptic powder injection", etc is composed of the ambroxol hydrochloride and 2-hydroxypropyl beta-dextrin in Wt ratio of 1:(3-50). Its advantage is high solubility in water, especially in high-pH water.
Owner:SHENYANG PHARMA UNIVERSITY
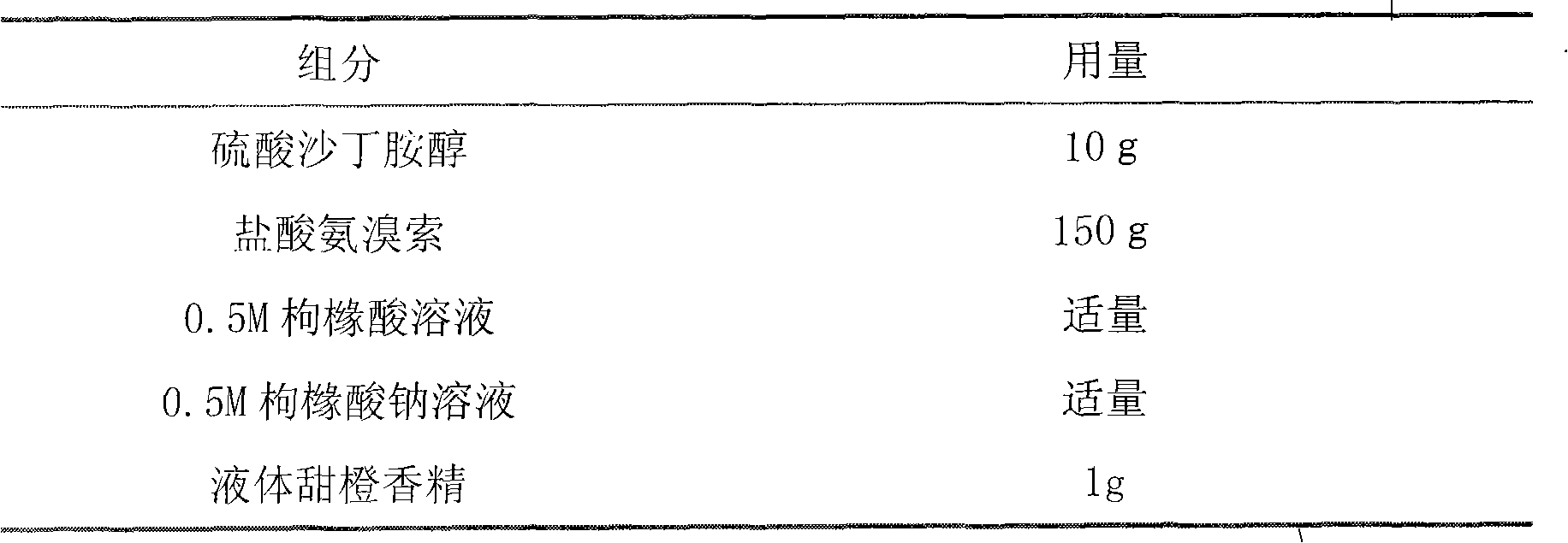
Spray taking salbutamol and ambroxol as active ingredients
The invention relates to a spray taking salbutamol and ambroxol as active ingredients, which is prepared from salbutamol and ambroxol or salts thereof, or purified hydrates thereof serving as medicinal active ingredients and pharmaceutically acceptable auxiliary materials. The spray is prepared and developed by adding some specific types of auxiliary materials in certain proportions into the salbutamol and ambroxol or the salts thereof, or the purified hydrates thereof serving as raw materials according to the technological measure stated by the invention.
Owner:北京利乐生制药科技有限公司
Compound orally disintegrating tablet containing loratadine and ambroxol and preparation process thereof
InactiveCN1994304AImprove bioavailabilityIncrease blood concentrationOrganic active ingredientsPill deliveryOrally disintegrating tabletLoratadine
The invention relates to a luleitadingmione oral calving tablet which can treat allergic rhinitis, etc. wherein, it has quick adsorption, high biological utilization, and simple intake method without water. It uses Loratadine and bromamine acid as materials, with some findings.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com