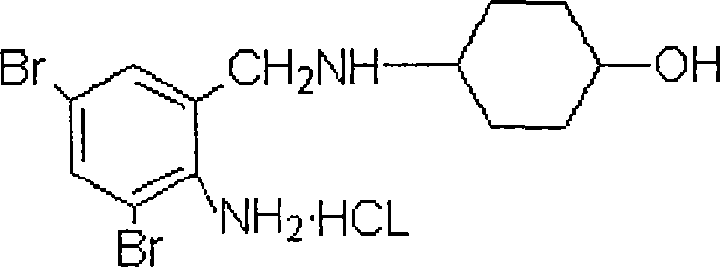
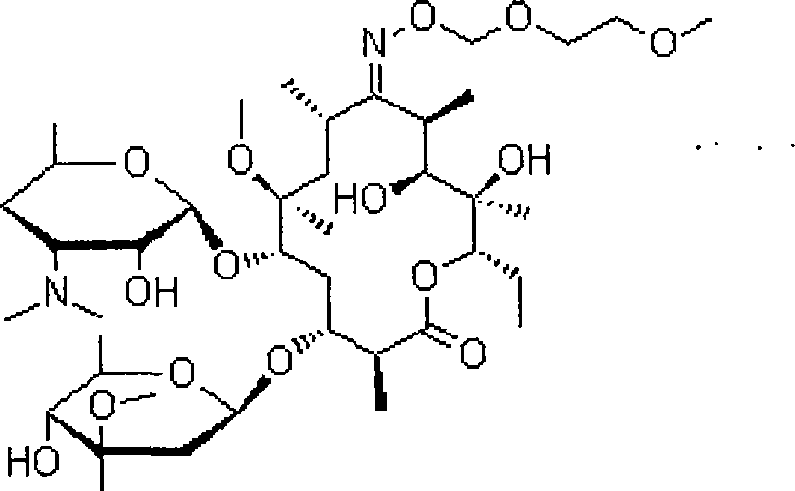
Compound ambroxol hydrochloride sustained-release tablet and preparation method thereof
A technology of ambroxol hydrochloride and sustained-release tablets, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery and other directions, and can solve problems such as no literature reports on sustained-release preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Compound Ambroxol Hydrophilic Gel Matrix Tablet
[0041] prescription:
[0042] Ambroxol Hydrochloride 75g
[0043] Roxithromycin 250g
[0044] HPMC K4M 80g
[0045] CMC-Na 80g
[0046] Tartaric acid 90g
[0047] Lactose 70g
[0048] Starch 100g
[0049] 95% ethanol appropriate amount
[0052] Preparation process: get ambroxol hydrochloride crude drug, pulverize and pass through a 100 mesh sieve, roxithromycin adopts micronized crude drug, mixes with HPMC K4M, CMC-Na, starch, tartaric acid and lactose, moistens with 95% ethanol Granulate after wet, granulate after drying at 50°C, add magnesium stearate and talcum powder, mix evenly and press into tablets.
[0053] For the release test, the release rate of the sample was measured according to the first method of Appendix XC of the Chinese Pharmacopoeia in 2005, Part Two of the Chinese Pharmacopoeia Edition. Use 900ml of dilute hydrochloric acid (6...
Embodiment 2
[0055] Example 2 Compound Ambroxol Hydrochloride Waxy Matrix Tablets
[0056] prescription:
[0057] Ambroxol Hydrochloride 75g
[0058] Roxithromycin 250g
[0059] Octadecanol 100g
[0060] Citric acid 80g
[0061] 160g pregelatinized starch
[0063] Preparation process: take ambroxol hydrochloride and roxithromycin raw materials, mix them with pregelatinized starch and citric acid, add molten stearyl alcohol, granulate after mixing, add magnesium stearate, mix well Tablet.
[0064] The release test is the same as in Example 1, and the results are as follows:
[0065] time (hours)
Embodiment 3
[0066] Embodiment 3 compound ambroxol hydrochloride non-erodible matrix tablet
[0067] prescription:
[0068] Ambroxol Hydrochloride 75g
[0069] Roxithromycin 250g
[0070] Ethylcellulose 110g
[0071] Tartaric acid 100g
[0072] Lactose 80g
[0073] Starch 80g
[0074] 95% ethanol appropriate amount
[0077] Preparation process: Take ambroxol hydrochloride and roxithromycin raw materials, mix them evenly with lactose, starch, tartaric acid, ethyl cellulose, add appropriate amount of 95% ethanol to granulate, add magnesium stearate and talcum powder after drying, mix Press evenly.
[0078] The release test is the same as in Example 1, and the results are as follows:
[0079] time (hours)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com