Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
372 results about "AMBROXOL HYDROCHLORIDE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ambroxol hydrochloride is a medication indicated to alleviate chest congestion associated with conditions that include bronchitis, pneumonia and bronchospasm asthma.
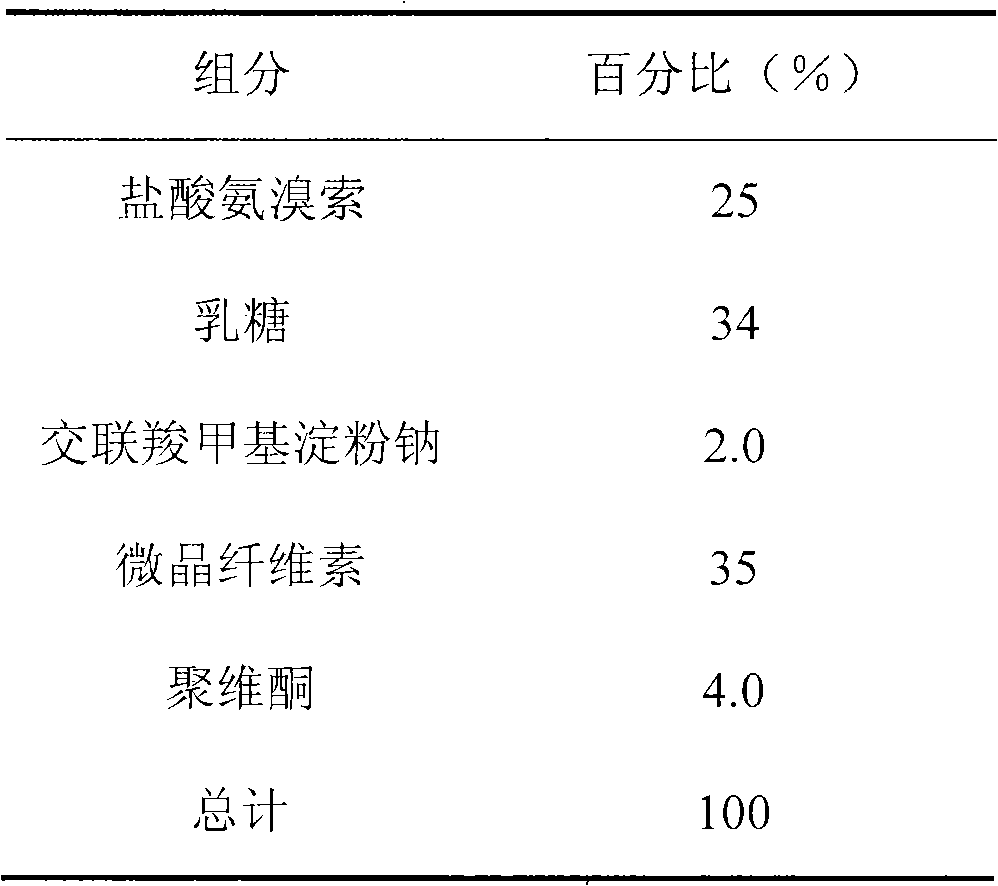
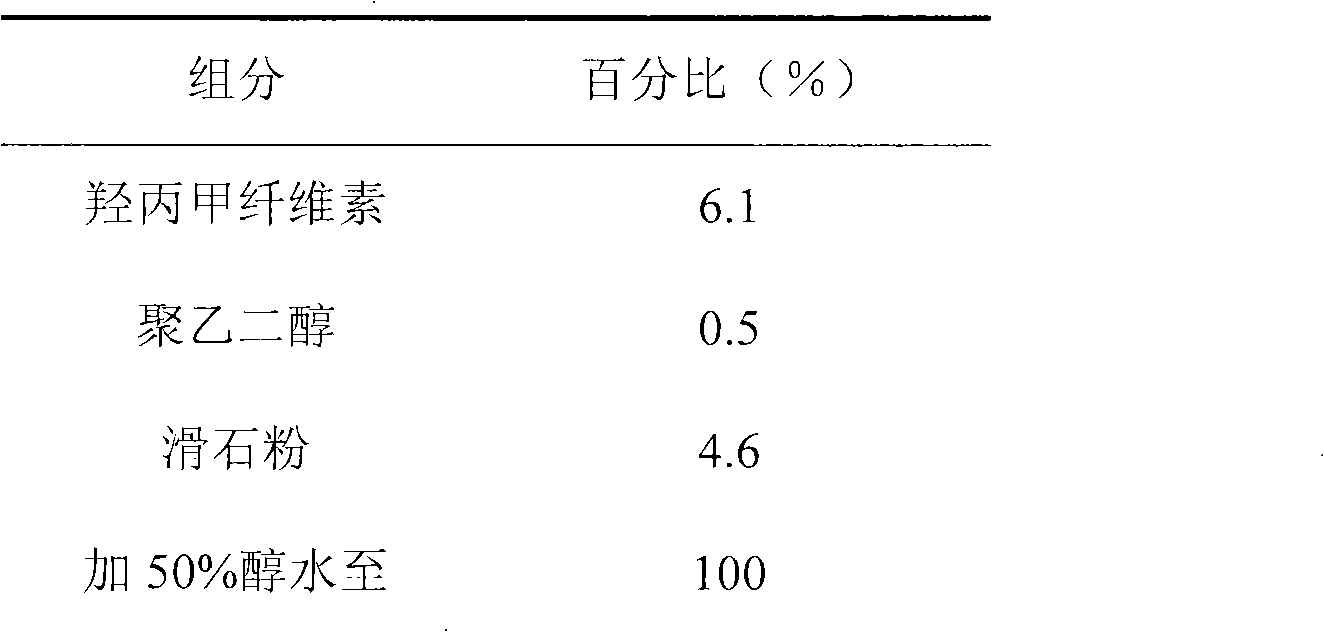
Ambroxol hydrochloride compound and medicine composition thereof
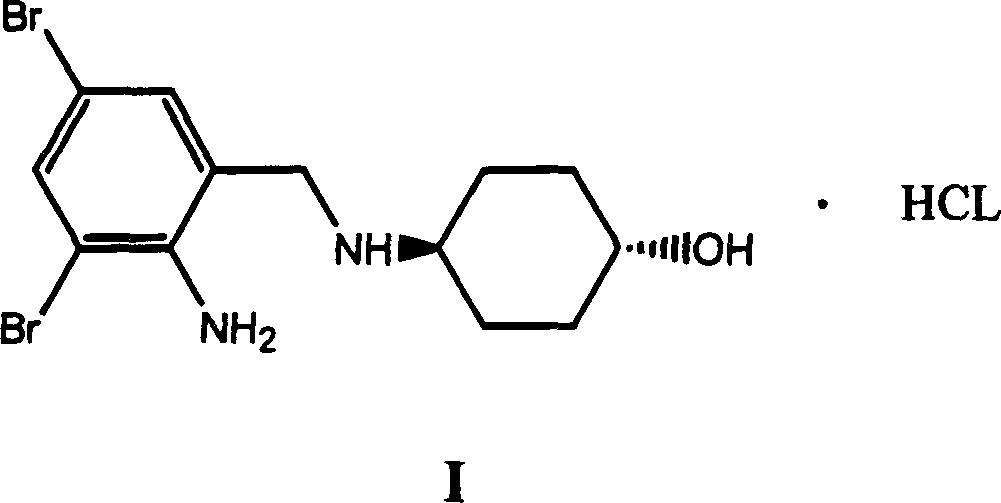
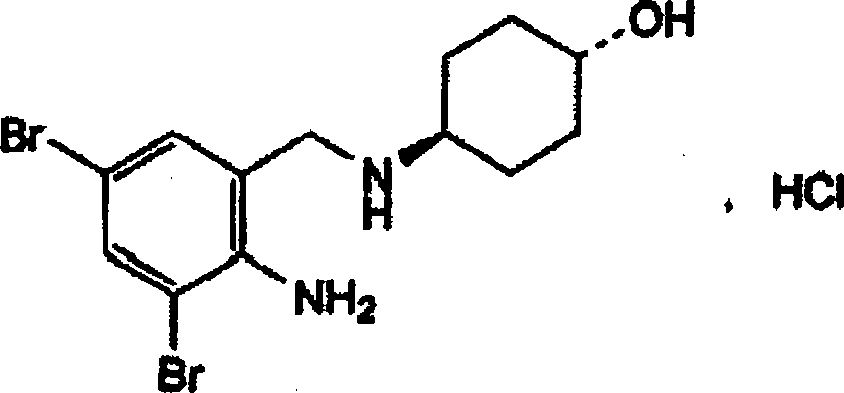
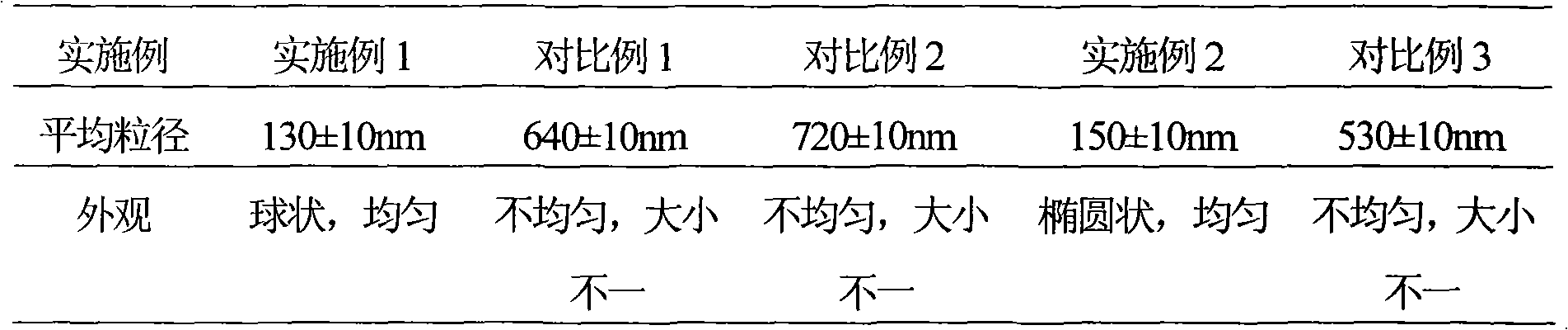
The invention discloses an ambroxol hydrochloride crystal and a medicine composition prepared by the ambroxol hydrochloride crystal. The ambroxol hydrochloride crystal shows specific diffractive peaks in parts of 6.9 degrees, 7.2 degrees, 12.8 degrees, 15.6 degrees, 17.5 degrees, 20 degrees, 21 degrees, 22 degrees and 24 degrees in an X-ray powder diffraction pattern which is shown in form of 2 Theta + / -0.2 degrees. The prepared ambroxol hydrochloride crystal is high in yield and liquidity; and the amount of lubricant in original prescription is halved, so that the weight and uniformity of tablets can meet the specification.
Owner:SHENYANG XINMA PHARMA +2
Stable amorphous ambroxol hydrochloride compound
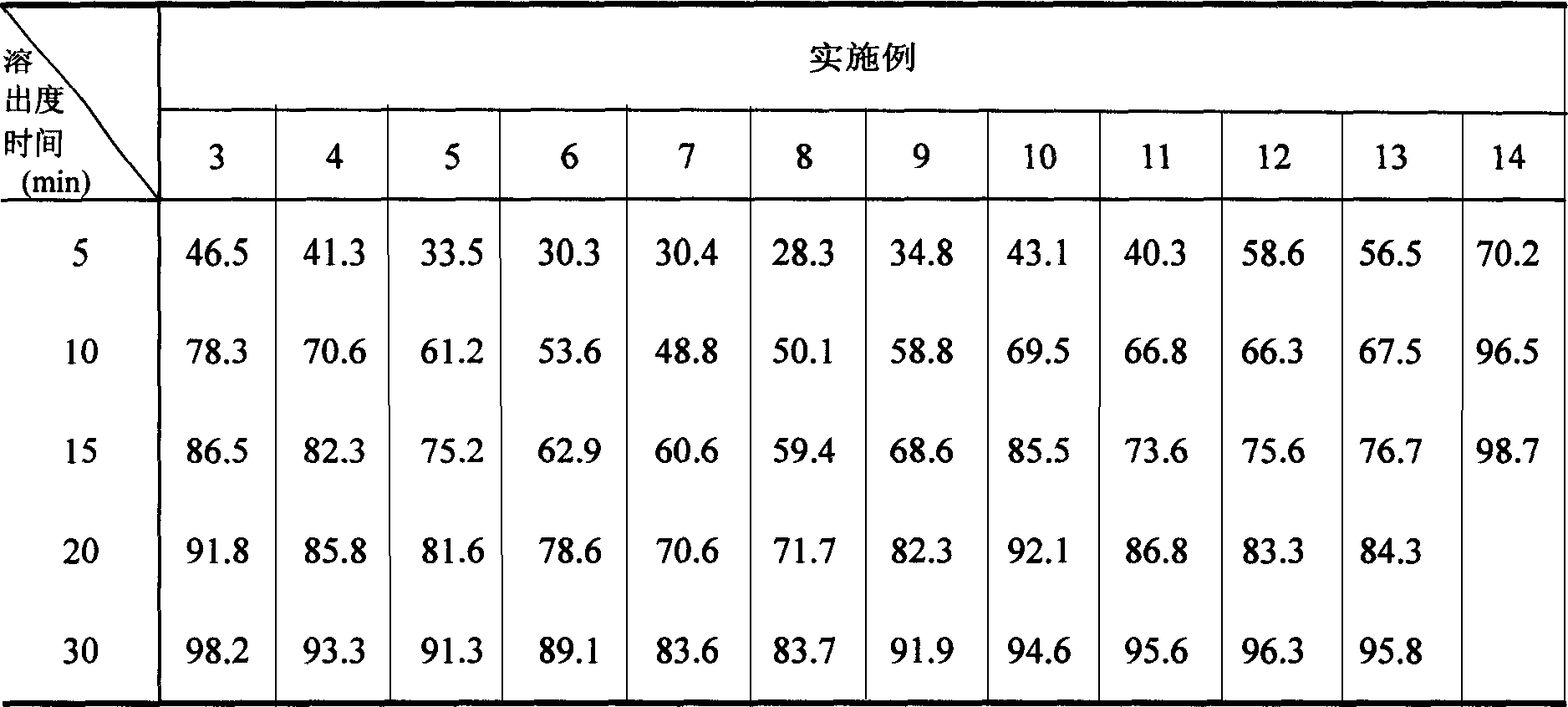
The invention belongs to the technical field of medicine, and particularly relates to an amorphous ambroxol hydrochloride compound and a preparation method thereof. The amorphous ambroxol hydrochloride compound has high purity and stability, does not have obvious moisture absorption and weight increase even under the condition of high humidity, ensures that relative substances do not grow, and has higher dissolution rate than crystalline ambroxol hydrochloride. The invention also relates to an application of the amorphous ambroxol hydrochloride compound in preparation of medicine for the treatment of acute and chronic pulmonary diseases with the symptom of ropy sputum and difficult expectoration, phlegm eliminating treatment of acute exacerbation of chronic bronchitis, asthmatic bronchitis and bronchial asthma, prophylactic treatment of lung complication after an operation, and treatment of infant respiratory distress syndrome (IRDS) of premature infants and neonates.
Owner:天津梅花生物医药科技有限公司
Oral solid preparation containing ambroxol hydrochloride and salbutamol active components
InactiveCN101099729APlace stableEasy to carryPowder deliveryOrganic active ingredientsDiseaseRespiratory tract disease
The present invention discloses an oral solid preparation containing ambroxol hydrochloride and salbutamol active components. It is formed from ambroxol hydrochloride, salbutamol and auxiliary material according to a certain mixing ratio. Said oral solid preparation has the obvious synergistic action for remitting the symptoms of dyspnea, etc. due to respiratory tract obstruction diseases of bronchial asthma, chronic bronchitis and pulmonary emphysema, etc.
Owner:GUANGZHOU LIXIN PHARM CO LTD
Preparation method of ambroxol hydrochloride
InactiveCN103012167AAvoid processing powerAvoid the corresponding processOrganic compound preparationAmino-hyroxy compound preparationBiotechnologyBiochemical engineering
The invention discloses a preparation method of ambroxol hydrochloride. According to the preparation method, a 'one-pot reaction' is adopted in the reaction, so that the separation of an intermediate is avoided, the operation is simple, the production period is short, the production cost is reduced, and the method is suitable for industrial production and application; the operation in refining the ambroxol hydrochloride is easy to carry out; and the ambroxol hydrochloride with purity more than 99.9% can be simply obtained by one-time recrystallization, so that the demand on preparation production can be met.
Owner:石药集团中诺药业(石家庄)有限公司
Process for preparing ambroxol, analogue thereof or salts thereof
ActiveCN101337897AOrganic compound preparationAmino-hyroxy compound preparationCombinatorial chemistryAMBROXOL HYDROCHLORIDE
The invention provides a simple method for synthesizing ambroxol hydrochloride, which is characterized in that the ambroxol product is obtained conveniently from amide, the precursor of ambroxol, by a proper reduction method.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Method for refining injection-level ambroxol hydrochloride, product and injection thereof
ActiveCN102153482AQuality improvementImprove stabilityOrganic active ingredientsOrganic compound preparationSolventAqueous solution
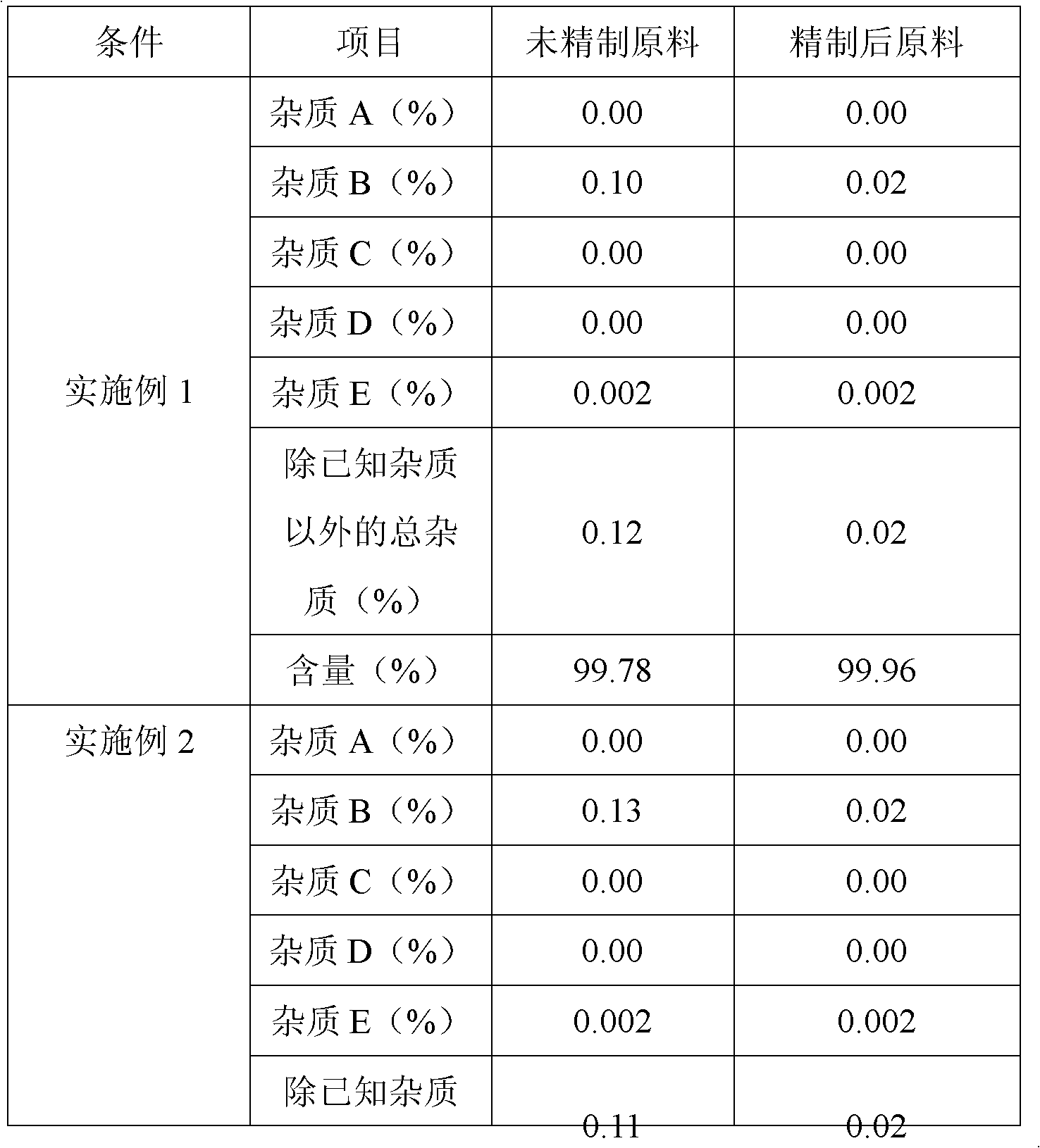
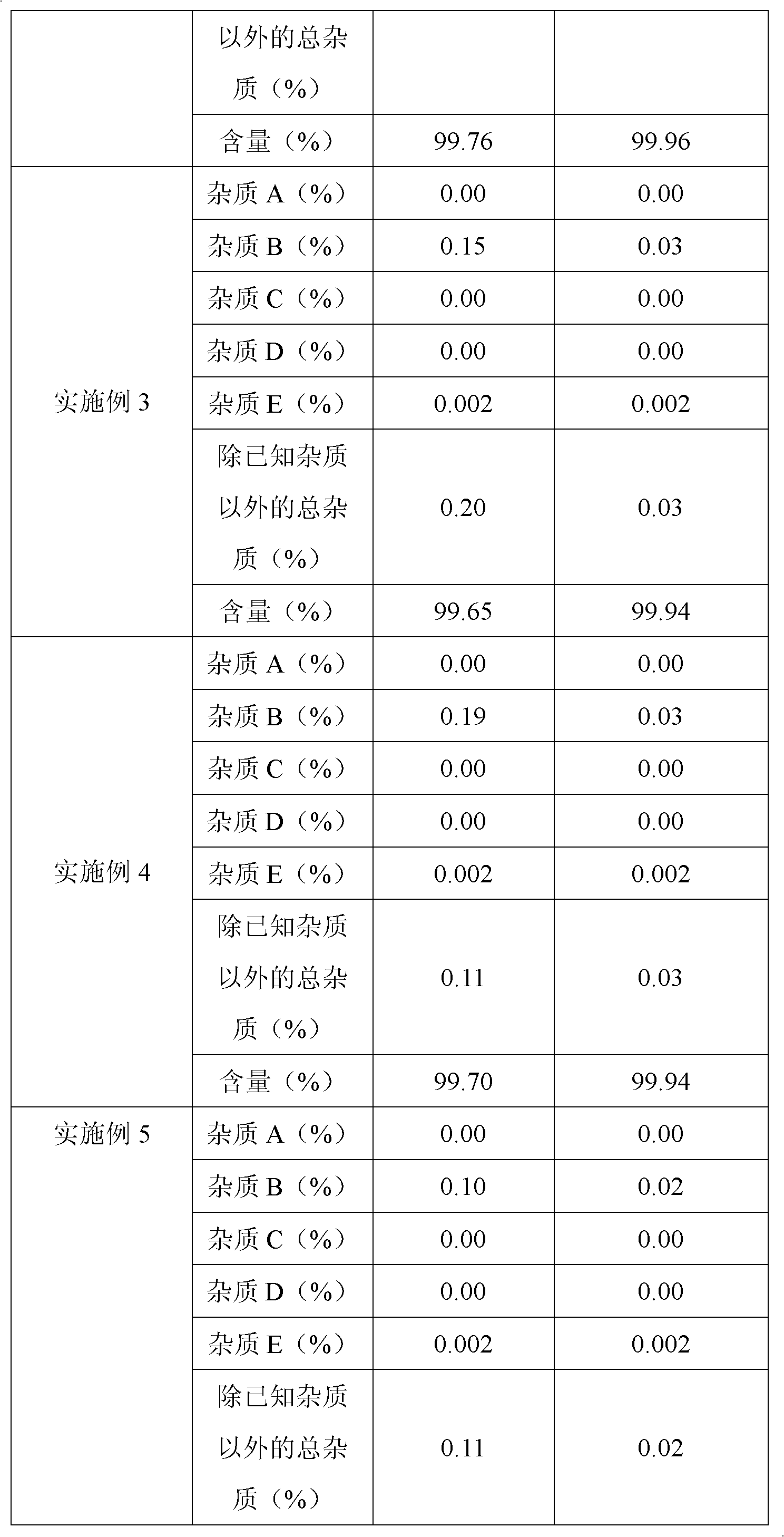
The invention belongs to fields of a method for refining and purifying compounds and products thereof, and particularly relates to a method for refining and purifying an ambroxol hydrochloride Chinese herbal medicine, a product thereof and the field of application. The method for refining the injection-level ambroxol hydrochloride is characterized by comprising the following steps of: adding 70.2to 88 volume percent of aqueous solution of ethanol (g / ml) into an ambroxol hydrochloride raw material (g) of which the purity is over 99.0 percent in a ratio of 1:(5.5-9.2); heating, and distilling until the ambroxol hydrochloride raw material is dissolved fully; stopping heating, cooling and crystallizing to separate ambroxol hydrochloride out; and filtering the solvent to obtain crystals, and drying to obtain the injection-level ambroxol hydrochloride. In the injection-level ambroxol hydrochloride raw material, an impurity B is less than or equal to 0.03 percent, an impurity E less than orequal to 0.002 percent, and total impurities except for the impurities B and E are less than or equal to 0.03 percent; and the purity is over 99.9 percent.
Owner:天津市铭泰医药科技有限公司
Ambroxol hydrochloride compound refining method
ActiveCN103073438APrevent precipitationHigh purityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsThermal insulationBottle
The invention relates to an ambroxol hydrochloride compound refining method, comprising a step of synthesizing an ambroxol hydrochloride crude product and a step of refining ambroxol hydrochloride, wherein the refining step comprises the following steps: 1) adding water and the ambroxol hydrochloride crude product into a reaction bottle, heating to a temperature of 70-75 DEG C and stirring, and dissolving the crude product, so as to obtain an ambroxol hydrochloride crude product solution; adding active carbon into the ambroxol hydrochloride crude product solution, decolorizing and filtering in a thermal insulating manner, and collecting filtrate; 2) cooling the filtrate to a temperature of 55-60 DEG C, carrying out thermal insulation and stirring; 3) cooling the filtrate to a temperature of 45-48 DEG C, controlling a stirring speed of 15 r / min, adding seed crystal, and crystallizing for 1 hour by controlling a temperature and a stirring speed; 4) cooling to a temperature of 20-25 DEG C and crystallizing for 20 minutes, slowly cooling to a temperature of 10 DEG C for 1.5 hours again after a crystallizing process, and obtaining an ambroxol hydrochloride fine product by leaching and drying. With the adoption of the ambroxol hydrochloride compound refining method provided by the invention, the ambroxol hydrochloride only contains an impurity B in five know impurities; and a content of the impurity B is controlled below 0.005%.
Owner:SHANDONG YUXIN PHARMA CO LTD
High dose Ambroxol hydrochloride freeze-dried preparation and preparation method
InactiveCN1954808AGood solubilization effectInhibition of precipitation and crystallizationOrganic active ingredientsPowder deliveryFreeze-dryingHigh doses
A freeze-dried powder injection of high-dosage (500mg) ambroxol hydrochloride and its preparing process are disclosed.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Ambroxol hydrochloride freeze-dried powder injection and preparing method thereof
ActiveCN101224196AAvoid interactionImprove stabilityOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLFreeze-drying
The invention relates to an ambroxol hydrochloride freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection comprises ambroxol hydrochloride and mannitol. The ambroxol hydrochloride freeze-dried powder injection prepared by a freeze-drying technology has good stability and high dissolution velocity while all other indexes accord with regulation.
Owner:SHANDONG YUXIN PHARMA CO LTD
Ambroxol hydrochloride composition injection
ActiveCN101836953AImprove stabilityInjection fitOrganic active ingredientsPharmaceutical delivery mechanismCitric acidInjection solution
The invention provides an ambroxol hydrochloride composition, which comprises the following components in parts by weight: 14-16 parts of ambroxol hydrochloride, 2.6-3.2 parts of citric acid, 4.8-6.0 parts of disodium hydrogen phosphate and 13-16 parts of sodium chloride. The components of the ambroxol hydrochloride injection prepared by the composition are simple. Under the condition with higher PH (5.5-7.0), the stability of ambroxol hydrochloride of the active medicine is higher, so the invention is suitable for the injecting of the human body, can reduce the uncomfortableness of a patient when the patient is injected.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Ambroxol hydrochloride oral cavity disintegrating tablet and method of producing the same
The invention discloses an oral disintegration tablet of alcaine aminobromisuo, which contains alcaine aminobromisuo with effective dosage and pharmacological findings, wherein the alcaine aminobromisuo is processed by metarchon with rate of aminobromisuo and metarchon at 1: 0. 1-1. 5, which is blended with pharmacologically acceptable findings; the metarchon can be medically inorganic oxide or / and medicinal organic acid and salt; the acceptable findings is filler, corrigent, disintegration agent, adhesive and lubricant; the oral disintegration tablet of alcaine aminobromisuo hasn't bitterness and numb sense to disintegrate within one minute with stripping degree not less than 90%. The invention is convenient and simple to control with high yield and low energy consumption, which widens the resource of metarchon to save cost.
Owner:CHONGQING CONQUER PHARML
Ambroxol hydrochloride oral solution and preparation method thereof
InactiveCN101352417AWilling to takeImprove complianceOrganic active ingredientsPharmaceutical delivery mechanismSucroseAcute bronchitis
The invention discloses an ambroxol hydrochloride oral solution and a preparation method thereof, and relates to a preparation method of western medicine, in particular to a medical production formula used for curing bronchitis and a preparation method thereof; the preparation method comprises the following steps: sucrose, preservatives and purified water are dissolved in a mixing mode so as to prepare simple syrup; the ambroxol hydrochloride, corrigents and preservatives are dissolved by the purified water so as to prepare ambroxol hydrochloride solution; the ambroxol hydrochloride solution and the simple syrup are stirred and mixed evenly, the corrigents and the preservatives are added successively and discontinuously and then the purified water is added to adjust the concentration. The invention has simple and reasonable process, and is easy to operate practically and the produced products have good stability; the products can used as common phlegm-eliminating drugs, has functions of increasing the liquid level of the respiratory tract, reducing mucus secretion, enhancing the secretion of pulmonary surfactant and the movement of cilium and preventing cough to a certain extent, and is applicable to acute respiratory disease and chronic respiratory disease, such as ropy sputum and difficult cough caused by acute bronchitis and chronic bronchitis, bronchial asthma and pulmonary tuberculosis, etc.
Owner:扬州市三药制药有限公司
Liquid composition containing loratadine and ambroxol hydrochloride
InactiveCN101152181AOrganic active ingredientsPharmaceutical delivery mechanismMedicineActive component
The invention discloses a drug combination with loratadine and ambroxol hydrochloride as the active components and with the treating functions of allergy, relieving cough and expelling phlegm. The drug combination contains stabilizer, pH regulator, preservative, taste masking agent and metalion complexing agent, etc, and the existing forms of the product are syrups and oral liquid.
Owner:BEIJING D VENTUREPHARM TECH DEV
Injection-grade ambroxol hydrochloride and solution for inhalation of injection-grade ambroxol hydrochloride
InactiveCN102924302AImprove stabilityQuality improvementOrganic active ingredientsOrganic compound preparationInhalationAqueous ethanol
The invention relates to a method for refining injection-grade ambroxol hydrochloride. The method is characterized by comprising the steps of adding oral-taking-grade ambroxol hydrochloride with the proportion smaller than or equal to 20:1 into ethanol water solution with the volume ratio of 5-20%; performing heating to completely dissolve the oral-taking-grade ambroxol hydrochloride; stopping heating and performing cooling to seed out ambroxol hydrochloride; and filtering solvent to obtain crystals and drying the crystals to obtain the injection-grade ambroxol hydrochloride. The invention further relates to ambroxol hydrochloride solution for inhalation prepared by utilizing the prepared injection-grade ambroxol hydrochloride as the raw material. The atomization inhalation solution comprises the ambroxol hydrochloride, a stabilizer, a pH conditioning agent and an osmotic pressure conditioning agent. By means of the scientific preparation adopting the carrier, fewest degradation products of the atomization inhalation preparation can be produced under the high temperature of 121 DEG C for 15 minutes, the atomization inhalation preparation is stable, and the service life of the atomization inhalation preparation is prolonged to 5 years.
Owner:HC SYNTHETIC PHARMA CO LTD
Preparation method of ambroxol hydrochloride
InactiveCN102557967AAvoid splittingInhibit synthesisOrganic compound preparationAmino-hyroxy compound preparationCyclohexanolCyclohexadienes
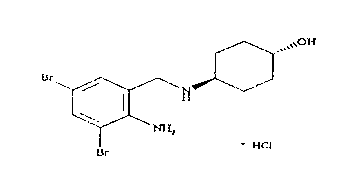
The invention relates to a preparation method of ambroxol hydrochloride. The preparation method comprises the steps of: directly obtaining a compound in formula (2) from simple intermediate 1,3-cyclohexadiene under the catalysis of palladium acetate, reacting with a compound in formula (3) to obtain a compound in formula (4), hydrolyzing the compound in the formula (4) in the presence of potassium carbonate, reducing by sodium borohydride, and carrying out salt forming with hydrochloric acid to obtain ambroxol hydrochloride. Therefore, synthesis of key intermediate trans-4-amino cyclohexanol in a common process is avoided and the separation of racemic products is avoided. The method is simple and feasible in operation, mild in conditions, mature in process and high in product yield and purity, and is suitable for industrialized mass production.
Owner:合肥科尚医药科技有限公司
Pharmaceutically acceptable composition containing ambroxol in non-salt form
The invention relates to a medicinal composition of ambroxol in a non-salt form. Particularly, the invention especially relates to a composition comprising ambroxol in a base form and other oral substances with or without physiological activity or a drug container. The inventor surprisingly finds that when ambroxol is prepared into an oral preparation in a non-salt form, and especially a preparation such as a suspension, a dry suspension, a granule and the like needing to be diluted and prepared with water before drinking, the mouthfeel is significantly improved when compared with that of an ambroxol hydrochloride solution, and the ambroxol preparation has significantly slower absorption in vivo than an ambroxol hydrochloride preparation; and the realization of a slow-release effect which requires great effort on preparation process can be easily achieved. It is well known that the slow release of a drug can reduce drug side effects and increase security with the guarantee of curative effects.
Owner:沈阳华泰药物研究有限公司
Ambroxol hydrochloride granule
InactiveCN1628647AStrong bitternessNot easy to agglomerateOrganic active ingredientsGranular deliverySucroseAdditive ingredient
The invention discloses an ambroxol hydrochloride granule, whose ingredients (by weight ratio) include ambroxol hydrochloride 10-17 parts, lactose 170-670 parts, sucrose 170-670 parts, stevioside 3-27 parts, Firmenich and fragrant citrus essence 3-138 parts, and right amount of binding agent. The invention relates to an ambroxol hydrochloride pelletized granule has the advantages of convenience in carrying, accurate quantitative determination, good taste, high biological availability, thus is especially suitable for children.
Owner:北京金康驰医药投资有限公司
Oral liquid for treating respiratory disease and preparation method thereof
ActiveCN101961307AGrowth inhibitionInhibit growthOrganic active ingredientsPharmaceutical delivery mechanismDiseaseAntioxidant
The invention discloses an oral liquid for treating a respiratory disease. The oral liquid is aqueous solution, does not comprise a preservative but comprises the following components: 1-8g / L ambroxol hydrochloride and 250 to 350 g / L xylitol. A preparation method for the oral liquid comprises the following steps of: 1) adding the xylitol in the amount into water, and stirring until dissolving completely; 2) adding the ambroxol hydrochloride, or adding the ambroxol hydrochloride and clenbuterol hydrochloride into the solution obtained in the step 1) in turn, and stirring until dissolving; and 3) adding a pH regulator into the solution obtained in the step 2), regulating the pH of the oral liquid to be between 4.0 and 6.0; adding one or a combination of a sweetening agent, an antioxidant and an aromatizer; and stirring uniformly to obtain the oral liquid. The oral liquid obtained by the preparation method does not comprise the preservative but has adequate chemical stability and microbial stability, avoids a series of toxic and harmful effects caused by using the preservative, and ensures the safety of medication.
Owner:武汉人福药业有限责任公司
A solid dispersion of ambroxol hydrochloride and composition thereof
ActiveCN1864672AStable in natureRaise quality standardsOrganic active ingredientsPill deliveryAMBROXOL HYDROCHLORIDECarrier material
The present invention relates to one kind of ambroxol hydrochloride dispersion and its preparation process and medicine composition. The ambroxol hydrochloride dispersion contains ambroxol hydrochloride and carrier material in the weight ratio of 1 to 0.3-10. The present invention aims at masking the bad smell of ambroxol hydrochloride.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Ambroxol hydrochloride atomization inhalant, and preparation method and application thereof
ActiveCN104027326AImprove stabilityEase of use and flexibilityOrganic active ingredientsAerosol deliveryDiseaseNitrogen gas
The invention provides an ambroxol hydrochloride atomization inhalant and a preparation method and application thereof. The ambroxol hydrochloride atomization inhalant uses injection water as a solvent and contains ambroxol hydrochloride with a concentration of 7.5 mg / ml and a pharmaceutically acceptable carrier with a concentration of 18 to 28 mg / ml; the carrier comprises a pH value stabilizing agent which is one or more selected from the group consisting of sodium pyrosulfite, sodium hydrosulphite and sodium hydrogen sulfite. The preparation method comprises the following steps: weighing ambroxol hydrochloride and the pharmaceutically acceptable carrier, adding a proper amount of injection water to dissolve ambroxol hydrochloride and the pharmaceutically acceptable carrier, carrying out cooling, then adjusting a pH value by using the pH value stabilizing agent containing sodium pyrosulfite, sodium hydrosulphite and / or sodium hydrogen sulfite and adding water until a desired total amount is almost obtained; then carrying out full stirring with medicinal carbon, carrying out filtering to remove carbon and adding water until the desired total amount is obtained; carrying out filtering with a microfiltration membrane; and filling each bottle with 2 ml, 4ml, 10 ml or 20 ml of the atomization inhalant, then introducing nitrogen and carrying out autoclaved sterilization. The invention further relates to application of the atomization inhalant in preparation of drugs used for treating diseases of the respiratory system.
Owner:天津药物研究院药业有限责任公司
Ambroxol hydrochloride dry powder inhalant and preparation thereof
InactiveCN101214227APurpose of safe treatmentEasy to usePowder deliveryOrganic active ingredientsMaterials scienceAMBROXOL HYDROCHLORIDE
The present invention belongs to the medication technical field and discloses hydrochloric ambroxol dry powder inhalant and a preparation method thereof. The hydrochloric ambroxol dry powder inhalant consists of hydrochloric ambroxol, dispersion flow aid and thinner, the weight percentage of which is 60 percent to 90 percent of the hydrochloric ambroxol, 10 percent to 30 percent of the dispersionflow aid and 0 percent to 40 percent of the thinner. The present invention comprises the following steps that the hydrochloric ambroxol or the hydrochloric ambroxol and the dispersion flow aid or thehydrochloric ambroxol, the dispersion flow aid and the thinner are processed for spray drying to obtain powder body which is collected into a glutin or plastic capsule or into an aluminum-plastic bubble cap or is contained inside a large dosage dry powder absorbing device as the storehouse form. The present invention adopts the dry powder inhalant to ensure that the hydrochloric ambroxol is directly absorbed into respiratory tract and lung by a medication device to act directly inside the respiratory tract, so as to achieve the purpose of the safe and targeting treatment with quick result andhigh efficiency.
Owner:SHENYANG PHARMA UNIVERSITY
Ambroxol hydrochloride liquid preparation and preparation method thereof
ActiveCN101627967AImprove solubilityOvercome the defect of being slightly soluble in waterOrganic active ingredientsPharmaceutical delivery mechanismWater useBULK ACTIVE INGREDIENT
The invention discloses an ambroxol hydrochloride liquid preparation and a preparation method thereof. The method comprises the steps: dissolving ambroxol hydrochloride, stabilizing agent and osmotic pressure regulator into water used for injection, and evenly mixing together to obtain solution I; then, filtering the solution I, and obtaining the ambroxol hydrochloride liquid preparation. The preparation method does not introduce active carbon, so as to avoid the danger of hurting human body since active carbon particle is introduced into the preparation; meanwhile, the active ingredients in the preparation is ensured to be stable, and the safety (namely, the chemical stability of the ambroxol hydrochloride can be effectively improved, the particle content in the preparation is reduced, and the purity of the preparation is improved) of the finished product can be guaranteed.
Owner:上海华源药业(宁夏)沙赛制药有限公司
Ambroxol hydrochloride orally disintegrating tablet and preparation method thereof
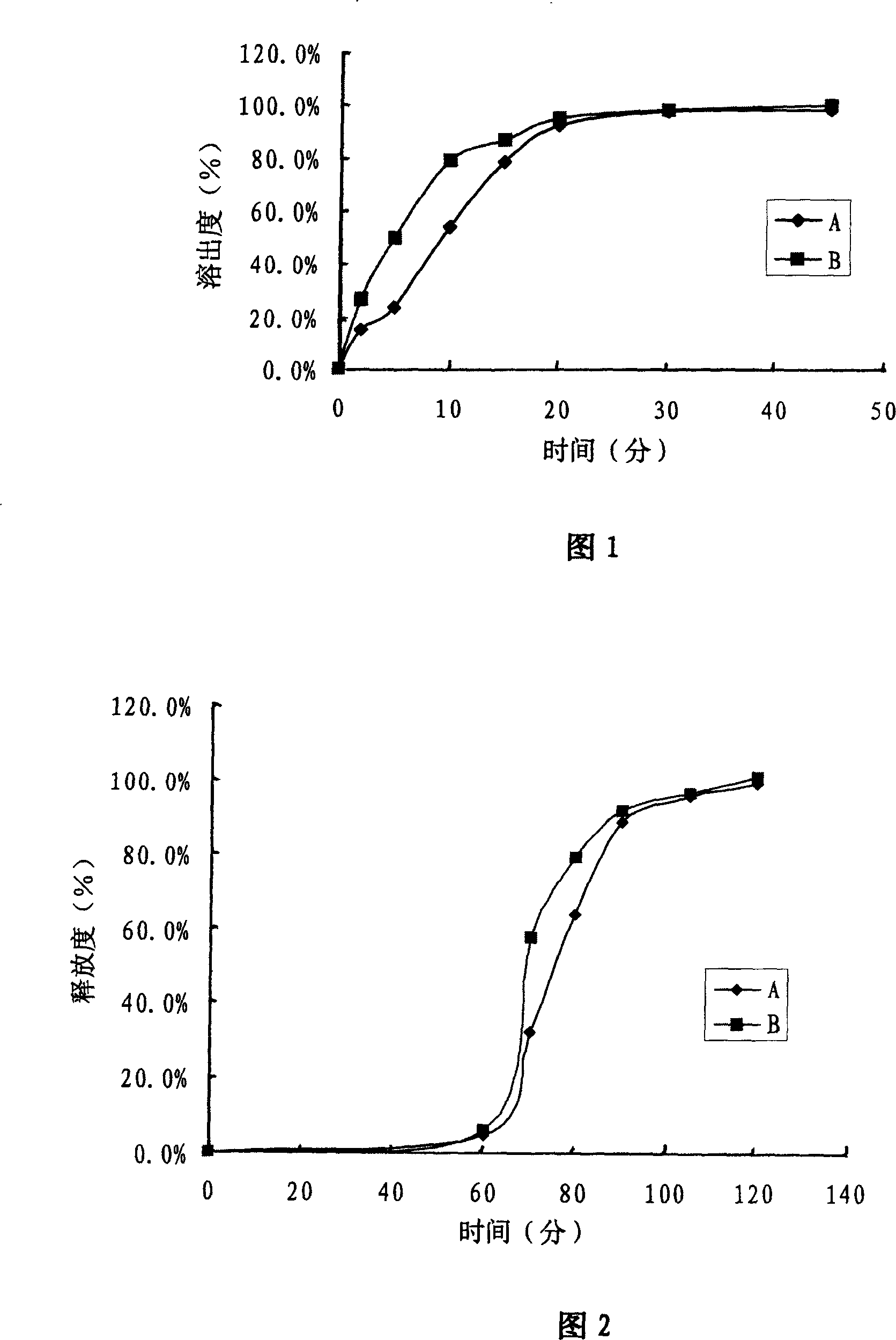
ActiveCN102327244AGood disintegrationHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsThroatOrally disintegrating tablet
The invention discloses an ambroxol hydrochloride orally disintegrating tablet. The orally disintegrating tablet comprises ambroxol hydrochloride and is prepared by proportioning the following raw auxiliary materials in part by weight: ambroxol hydrochloride flavor masking particles are prepared by coating and contain 1 part of ambroxol hydrochloride agents, 1-2 parts of filling agents, 0.1-0.25 part of disintegrating agents, 0.5-1 part of flavoring agents, 0.01-0.05 part of mouth cooling agents, 0.029-0.05 part of lubricating agents and 0.03-0.05 part of flow agents. The ambroxol hydrochloride orally disintegrating tablet has a good disintegrating effect and good dissolution rate. As a certain proportion of mouth cooling agents are added in the auxiliary materials, the discomfort generated to an oral cavity and a throat part is effectively alleviated after drug taking. The ambroxol hydrochloride orally disintegrating tablet has good mouthfeel and short duration of bad mouthfeel, can bring good drug taking process to a patient after being taken and is suitable for industrialization production.
Owner:HANGZHOU CONBA PHARMA
Orally disintegrating tablet of ambroxol hydrochloride and preparation method thereof
InactiveCN101904827AGreat tasteImprove Medication AdherenceOrganic active ingredientsPharmaceutical non-active ingredientsDiseaseSide effect
The invention discloses an orally disintegrating tablet of ambroxol hydrochloride and a preparation method thereof, which can effectively treat acute and chronic respiratory diseases (such as thick sputum and expectoration difficulty due to acute and chronic bronchitis, bronchial asthma, bronchiectasis, tuberculosis and the like.) The invention aims to provide an orally disintegrating tablet of ambroxol hydrochloride for wide patients and medical staff, i.e. a new preparation of a clinical medicine, which has rapid absorption, high bioavailability, no water for taking, little side effect and more convenience and is prepared by using ambroxol hydrochloride as a raw material, adding some special kinds of accessories in a certain proportion according to a technical measure disclosed in the invention. The product of the invention has sweet and fragrant taste, rapid effect and high bioavailability and is particularly easy to improve the drug compliance of patients.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Loratadine-ambroxol pharmaceutical composite and liposome solid preparation thereof
InactiveCN101627998AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical product form changeYolkMedicine
The invention relates to a loratadine-ambroxol pharmaceutical composite and a liposome solid preparation thereof and a preparation method thereof; the liposome comprises the following components according to the parts by weight percent: 1 part of loratadine, 5 parts of ambroxol hydrochloride, 3-30 parts of yolk lecithin, 1-14 of cholesterol, 1.2-10 parts of sodium deoxycholate and 3-18 parts of poloxamer 188.
Owner:HAINAN YONGTIAN PHARMA INST
Synthesis method of ambroxol hydrochloride compound
ActiveCN103073439AMild conditionsReduce pollutionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsSolvent
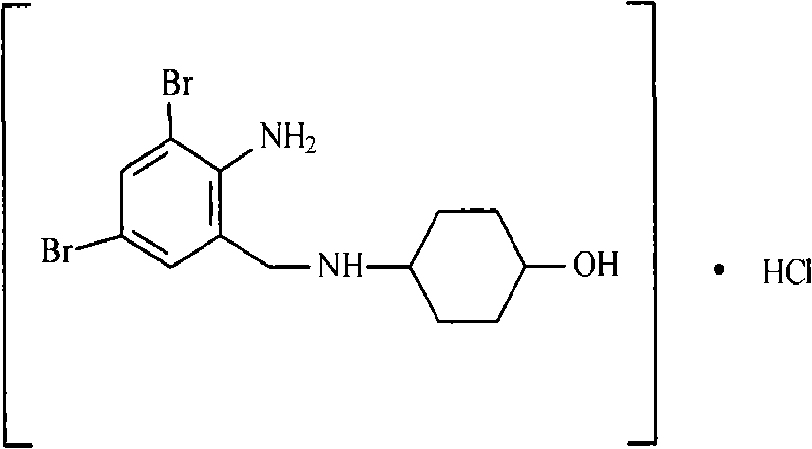
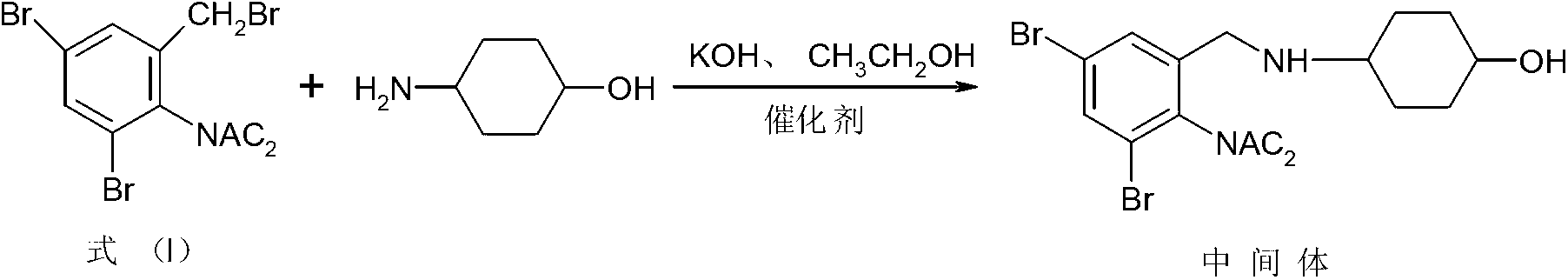
The invention relates to a synthesis method of an ambroxol hydrochloride compound, comprising the following steps of: 1) taking a compound of a formula (I) as a starting raw material; taking DMAP (Dimethylaminopyridine) and 4-dimethylamino cyclohexanol as catalysts to react with p-amino cyclohexanol under the condition of the presence of absolute methanol and anhydrous potassium carbonate to obtain an intermediate; and 2) under the condition of the presence of a solvent ethanol, water and glacial acetic acid, taking the intermediate and concentrated hydrochloric acid to react to generate ambroxol hydrochloride. According to the synthesis method disclosed by the invention, diacetylamido is used for carrying out complete protection on active nitrogen atoms of the starting raw material to avoid a benzene ring 2-substituents amino secondary reaction; the difficulties of the prior art that more condensation secondary reactions, low yield, high catalyst cost, harsh process conditions and the like are overcome to form a novel process with a short reaction line, moderate conditions and small pollution; the yield is higher than 67%; impurity types and contents are obviously reduced; and only one of five known impurities exists and the product purity is more than 99.9%. The formula (I) is shown as the specification.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
Oral solution containing ambroxol hydrochloride and salbutamol sulfate
InactiveCN104622855ASimple prescriptionGuaranteed stabilityOrganic active ingredientsPharmaceutical delivery mechanismDiseaseMedicine
The invention provides an oral solution containing ambroxol hydrochloride and salbutamol sulfate, and belongs to the technical field of medicines. An auxiliary material of the oral solution mainly comprises preservatives and corrigents. The oral solution is used for treating respiratory system diseases such as acute and chronic bronchitis, asthmatoid bronchitis and bronchial asthma, has a simple prescription, a good medication effect and a good taste, and is quick in response.
Owner:CP PHARMA QINGDAO CO LTD
Ambroxol hydrochloride sustained-release pellet and preparation method
ActiveCN101862305AOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsPulmonary circulation diseases
The invention relates to the composition of an ambroxol HCl-containing sustained-release pellet and a preparation method. The sustained-release pellet consists of two parts which are a quick-release pellet core and a sustained-release coating film respectively, can achieve the effect of long-term release approaching to zero-order release, is mainly used for treating acute and chronic respiratory diseases, and is a medicament of first choice for dispelling phlegm.
Owner:AVENTIS PHARMA HAINAN
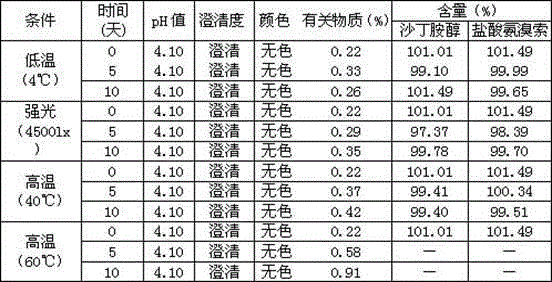
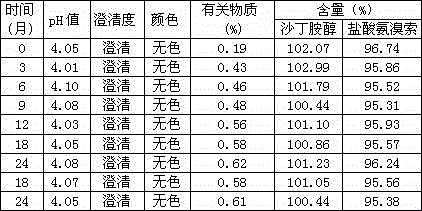
A method for improving the stability of ambroxol hydrochloride
InactiveCN102258462AImprove stabilityIncrease contentOrganic active ingredientsSolution deliveryGlycerolAmmonia
The present invention relates to a method for improving the stability of ambroxol hydrochloride. The main component ambroxol hydrochloride is slightly soluble and unstable, and can be effectively solubilized using glycerin and propylene glycol. The glycerin formula and preparation method have good effects on the stability of the main drug. Stability has a greater impact, and the product obtained by the propylene glycol formula has good stability. Accelerated stability and long-term stability tests have proven that this formula and preparation method have better stability than the same product on the market. stability.
Owner:江西川奇药业有限公司
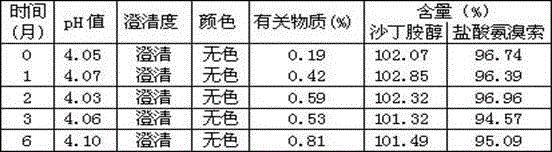
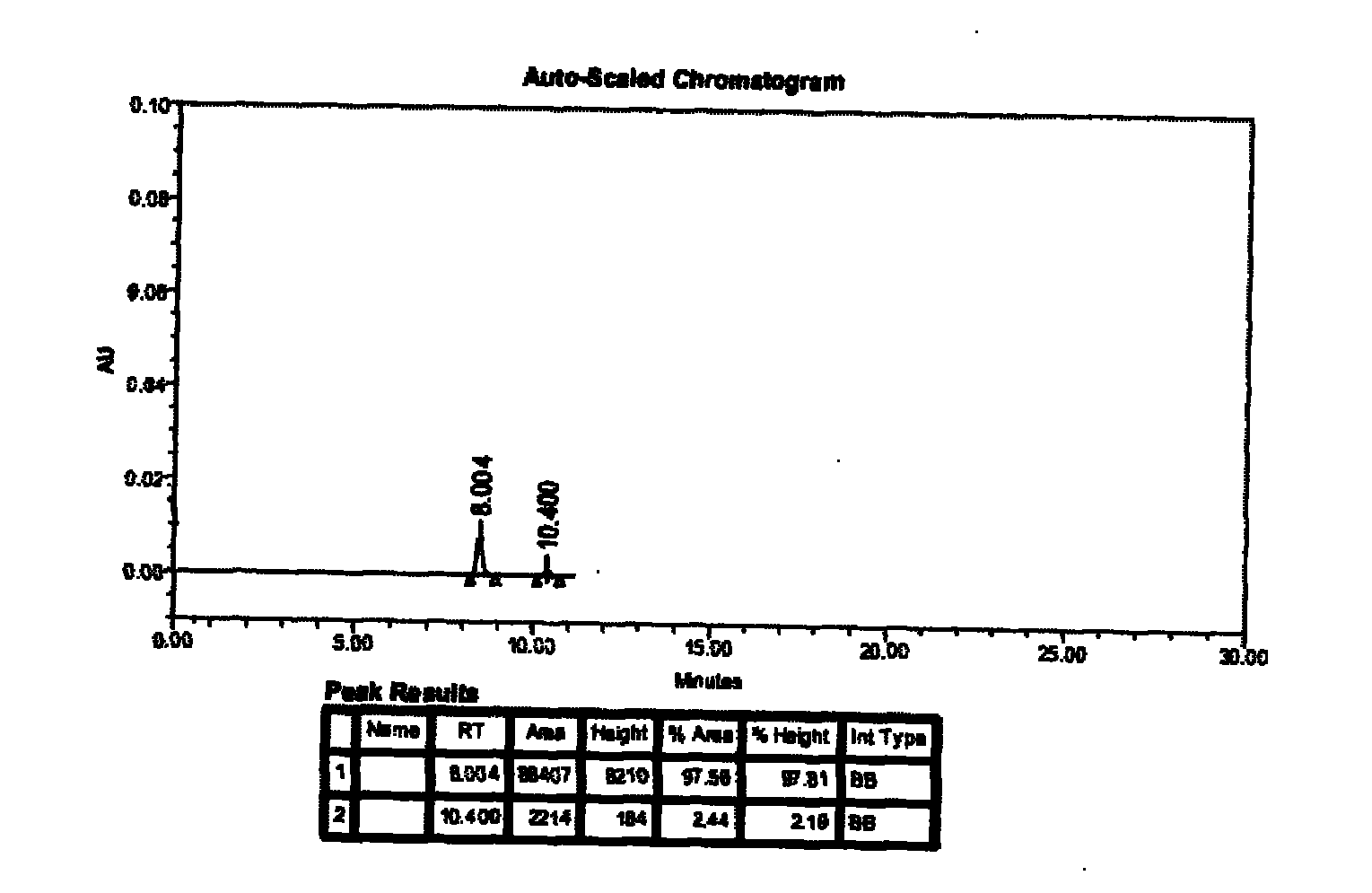
Ambroxol hydrochloride detection method
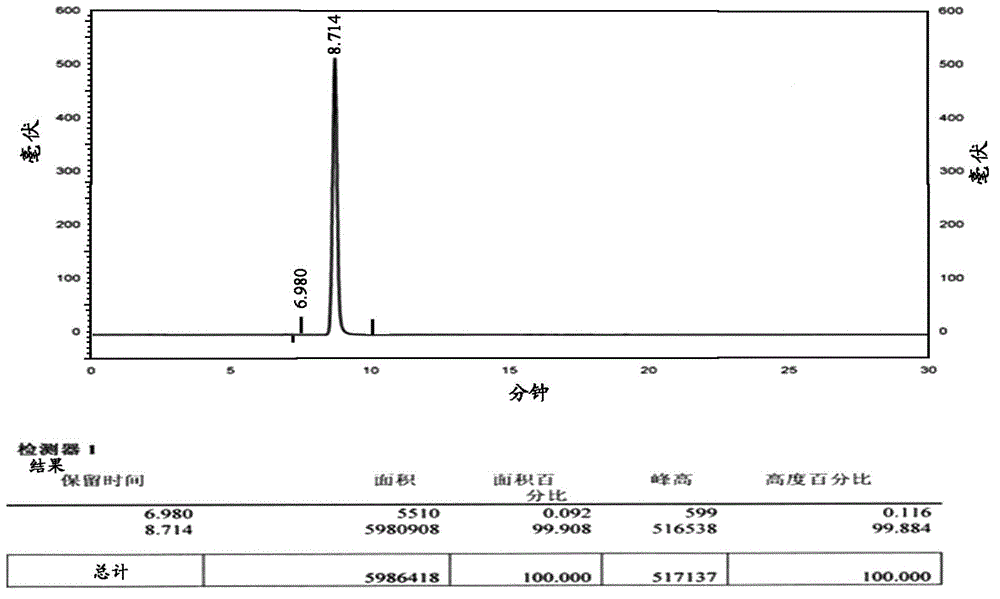
The invention provides a method for measuring ambroxol and related substances trans-4-[6,8-dibromo-1,4-orexin-3(H)] cyclohexanol (impurity B) and 2-amino-3,5-dibromo-benzaldehyde (impurity A) in a preparation of the ambroxol, which is characterized in that: in the measurement of the trans-4-[6,8-dibromo-1,4-orexin-3(H)] cyclohexanol, an adopted detection wavelength is 258 nm and a chromatogram is recorded for a time that is 3 times the retention time of the main component; and the method for measuring the 2-amino-3,5-dibromo-benzaldehyde is characterized in that: the adopted detection wavelength is 238 nm and the chromatogram is recorded for the time that is 3 times the retention time of the main component.
Owner:云南龙海天然植物药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com