Ambroxol hydrochloride sustained-release pellet and preparation method
A technology of ambroxol hydrochloride and sustained-release pellets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, and medical preparations containing active ingredients. Difficult to achieve uniform release and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
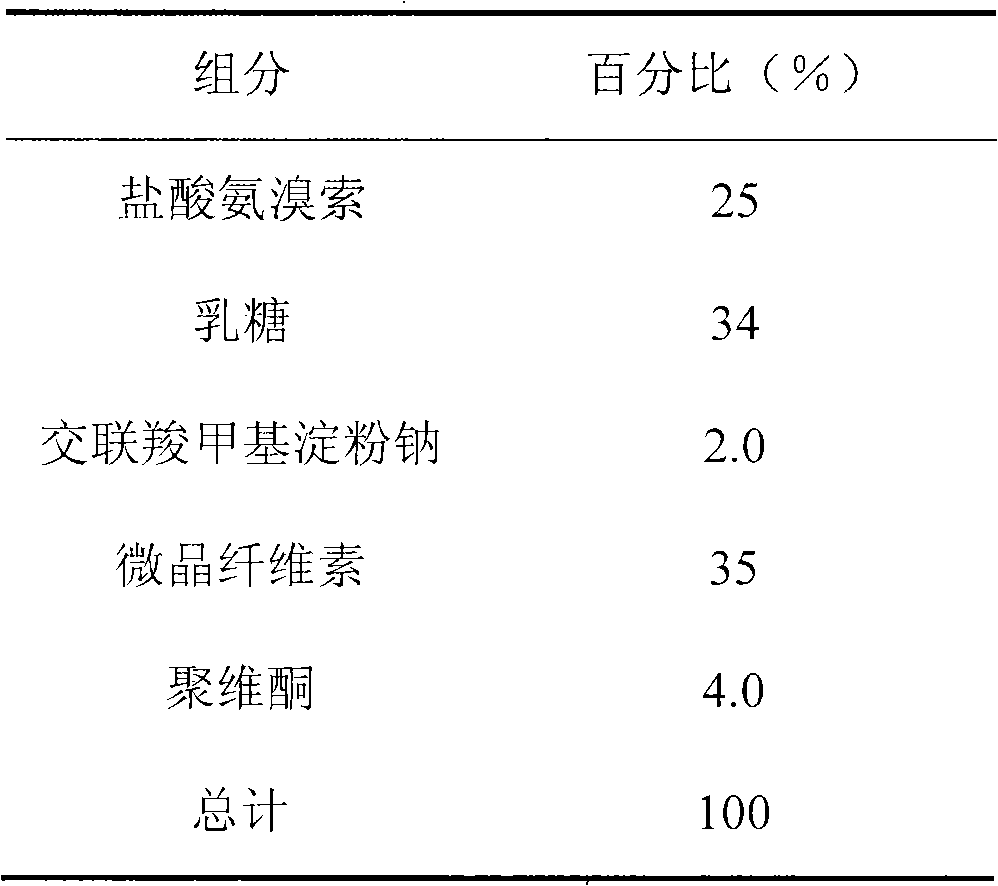
[0020] Preparation of Pill Hearts
[0021]
[0022] Preparation Process:
[0023] Mix ambroxol hydrochloride with lactose, cross-linked sodium carboxymethyl starch, and microcrystalline cellulose, use 10% povidone aqueous solution as a binder to make a soft material, use an extrusion spheronizer to prepare pellets, and dry overnight , and sieve out pellets with a diameter of about 1 mm as the pellet core.
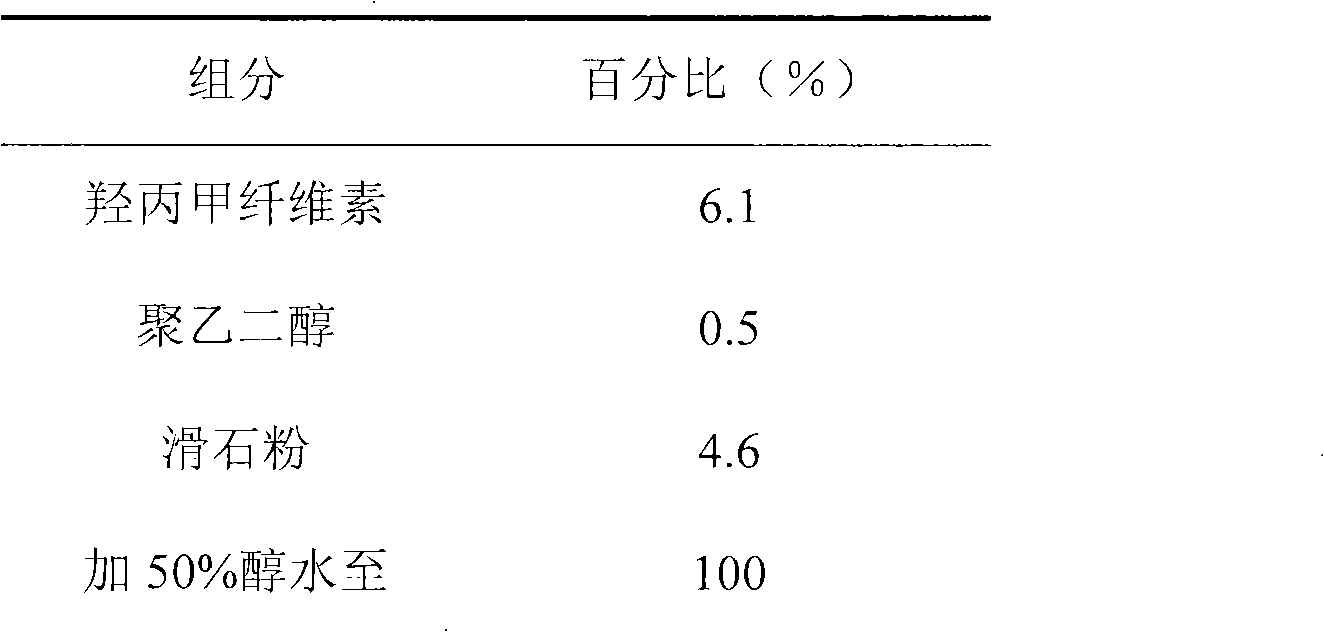
[0024] Isolation gown
[0025]
[0026] The hypromellose, polyethylene glycol and talcum powder of the prescribed amount are placed in 50% alcohol water, stirred to make it fully dissolved or dispersed, coated, and the weight increased to 2%.
[0027] slow release coating
[0028]
[0029]
[0030] Place Eudragit, triethyl citrate and talcum powder in 95% ethanol in the prescribed amount, stir to make it fully dissolve or disperse, coat, and increase the weight to 8%;
[0031] The coated micropills are filled into capsules to obtain.
Embodiment 2
[0033] Preparation of Pill Hearts
[0034]
[0035] Preparation Process:
[0036] Mix ambroxol hydrochloride with mannitol, croscarmellose sodium, and microcrystalline cellulose, use 8% hypromellose aqueous solution as a binder to make soft materials, and use an extrusion spheronizer to prepare pellets , dried overnight, and sieve out pellets with a diameter of about 1 mm as pellet cores.
[0037] Isolation gown
[0038]
[0039]
[0040] The hypromellose, polyethylene glycol and talcum powder of the prescribed amount are placed in 50% alcohol water, stirred to make it fully dissolved or dispersed, coated, and the weight increased to 4%.
[0041] slow release coating
[0042]
[0043] Put Eudragit, dibutyl phthalate and talcum powder in the prescription amount in 95% ethanol, stir to make it fully dissolve or disperse, coat, and increase the weight to 15%;
[0044] The coated micropills are filled into capsules to obtain.
Embodiment 3
[0046] Preparation of Pill Hearts
[0047]
[0048] Preparation Process:
[0049] Mix ambroxol hydrochloride with starch, crospovidone, and microcrystalline cellulose, use 10% starch slurry as a binder to make a soft material, use an extrusion spheronizer to prepare pellets, dry overnight, and sieve out Pellets with a diameter of about 1mm are used as pellet cores;
[0050] Isolation gown
[0051]
[0052] The hypromellose, polyethylene glycol and talcum powder of the prescribed amount are placed in 50% alcohol water, stirred to make it fully dissolved or dispersed, coated, and the weight increased to 6%.
[0053] slow release coating
[0054]
[0055] Put Eudragit, dioctyl phthalate and talcum powder in the prescription amount in 95% ethanol, stir to make it fully dissolve or disperse, coat, and increase the weight to 15%;
[0056] The coated micropills are filled into capsules to obtain.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com