Long-acting sustained-release pellet and preparation method thereof
A slow-release pellet and long-acting technology, which is applied in the preparation of the preparation, in the field of long-acting trimetazidine hydrochloride sustained-release pellets, can solve the problems of different drug content in the pellets, affecting the stability of the therapeutic effect, etc. Achieve the effect of maintaining stable blood drug concentration, low production cost, and uniform size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0154] The method for preparing the above-mentioned long-acting trimetazidine hydrochloride sustained-release pellets includes preparing a pill-containing core, directly coating the sustained-release coating layer on the pill-containing core, or first coating the isolation coating layer on the pill-containing core, and then The preparation method of coating sustained-release coating layer containing pill core comprises the following steps:
[0155] 1) Sieving: sieve the active ingredients, fillers, binders, and glidants, and set aside;
[0156] 2) Mixing: Accurately weigh the sieved active ingredients, fillers, binders, and glidants and place them in a mixer and mix them evenly;
[0157] 3) Preparation of pellets: extrude-spheronize the mixture and wetting agent to obtain a soft material, dry at 30°C to 80°C, and sieve to obtain a pellet core containing medicine.
[0158] A long-acting trimetazidine hydrochloride sustained-release oral preparation, the preparation is a disper...
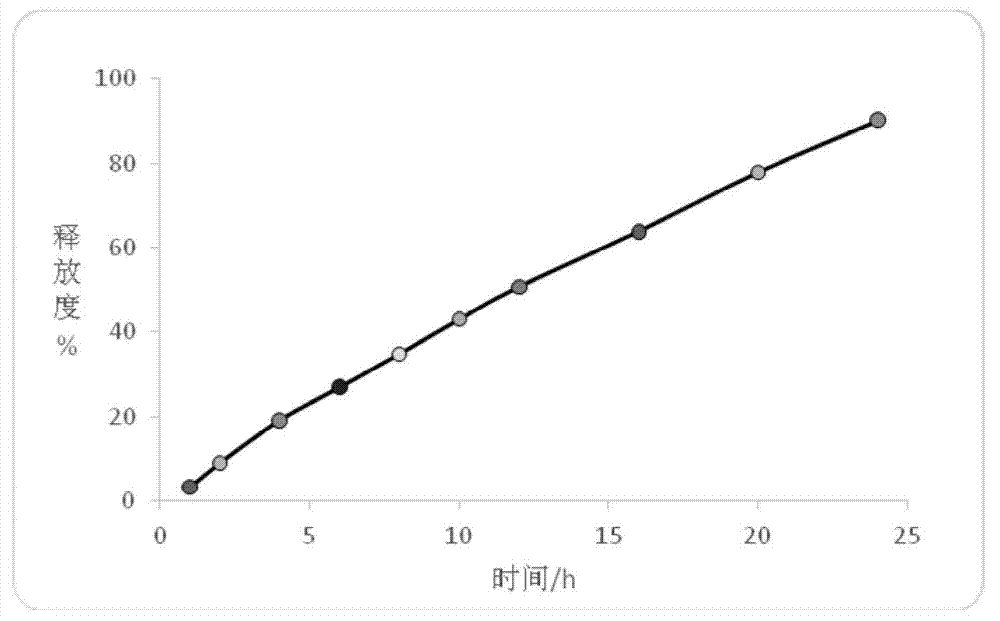
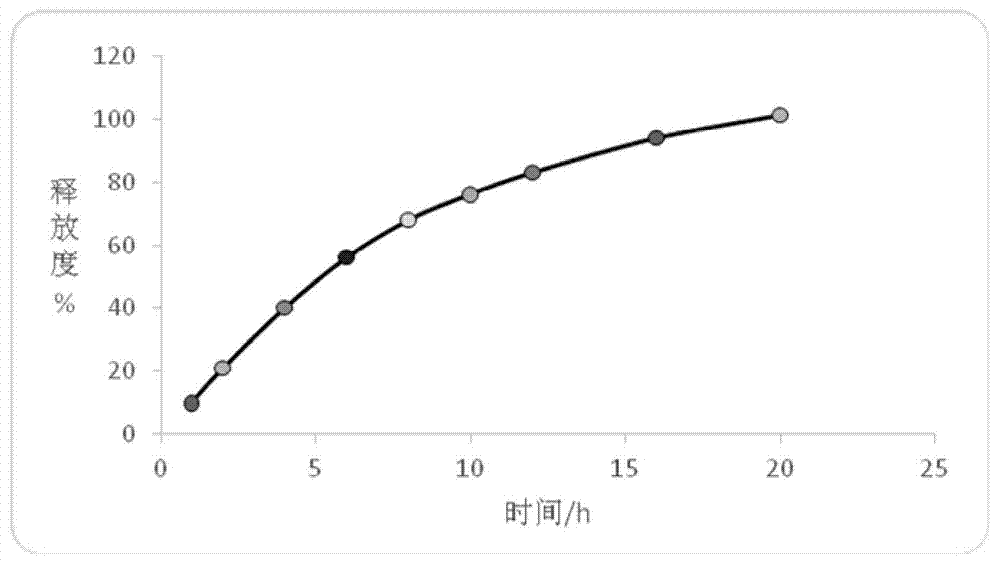
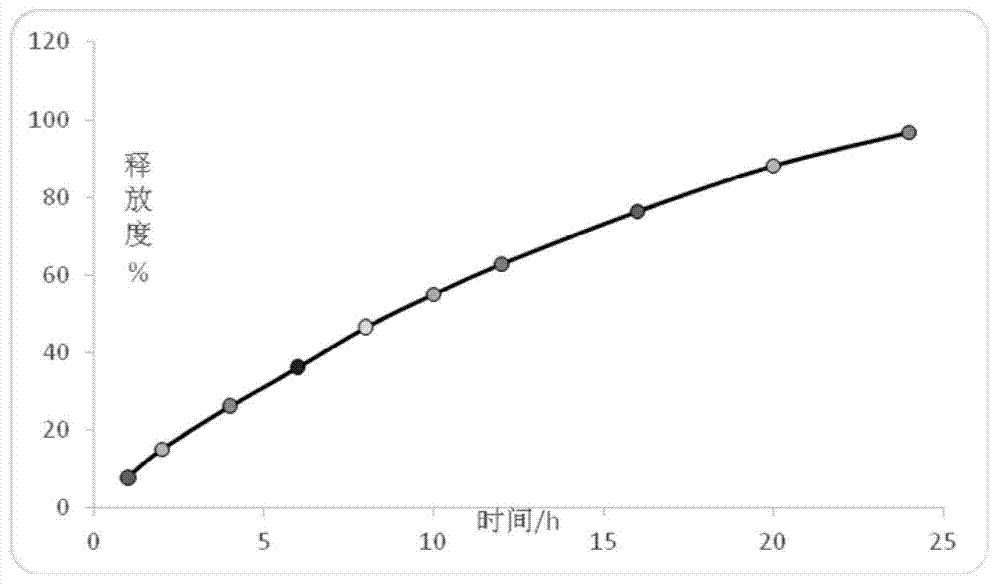
Embodiment 1
[0169] A kind of trimetazidine hydrochloride sustained-release pellets, comprising the following components:
[0170] Contains pill core:
[0171] Trimetazidine Hydrochloride (active ingredient) 85.00g
[0172] Hypromellose (binder) 30.00g
[0173] Calcium hydrogen phosphate (filler) 120.00g
[0174] Microcrystalline cellulose (filler) 200.00g
[0175] Talc powder (glidant) 15.00g
[0176] Extended Release Coating:
[0177] Surelease (sustained release coating material) 15.75g
[0178] Talc powder (anti-sticking agent) 2.25g
[0179] Triethyl citrate (plasticizer) 1.13g
[0180] Acryl (porogen) 0.68g
[0181] Carbomer (gastrointestinal adhesion agent) 1.80g
[0182]The method for preparing the above-mentioned trimetazidine hydrochloride sustained-release pellets is as follows:
[0183] A. Preparation of drug-containing pellet cores:
[0184] 1) Screening: Active ingredients, fillers, binders and glidants are passed through a 40-mesh sieve for later use;
[0185] 2)...
Embodiment 2
[0193] A kind of trimetazidine hydrochloride sustained-release pellets, comprising the following components:
[0194] Contains pill core:
[0195] Trimetazidine Hydrochloride 80.00g
[0196] Povidone (Binder) 20.00g
[0197] Pregelatinized starch (filler) 280.00g
[0198] Lactose (filler) 50.00g
[0199] Talc powder (glidant) 20.0g
[0200] Isolation coating:
[0201] Polyvinyl alcohol (isolation coating material) 13.50g
[0202] Talc powder (anti-sticking agent) 6.30g
[0203] Dimethyl phthalate (plasticizer) 2.70g
[0204] Extended Release Coating:
[0205] Surelease (sustained release coating material) 27.00g
[0206] Magnesium stearate (anti-adhesive agent) 2.25g
[0207] Polycarboxyethylene Pentaerythritol Ester (Gastrointestinal Adhesive) 13.50g
[0208] The method for preparing the above-mentioned trimetazidine hydrochloride sustained-release pellets is as follows:
[0209] A. Preparation of drug-containing pellet cores:
[0210] 1) Screening: Active ingred...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com