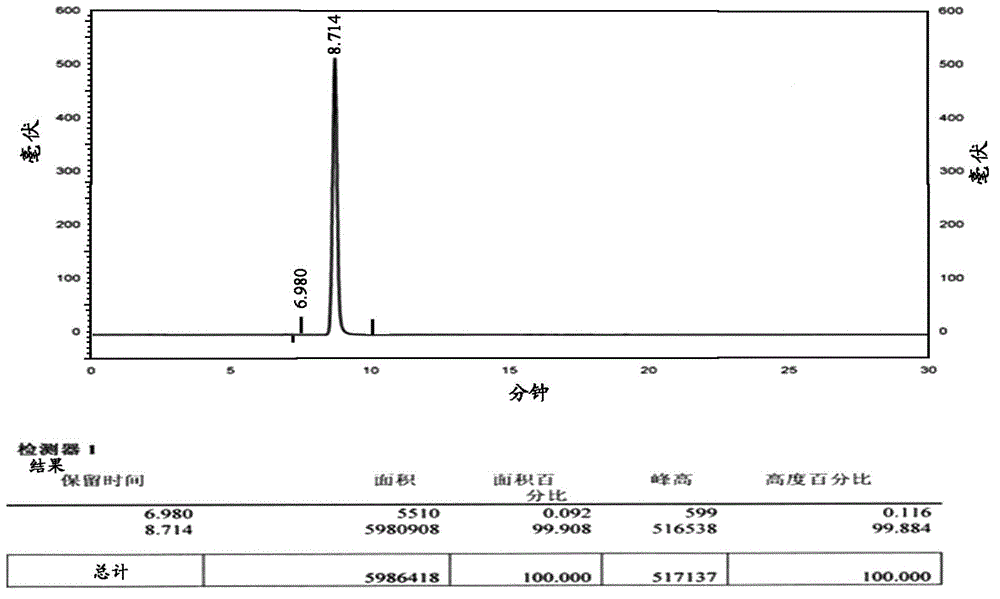
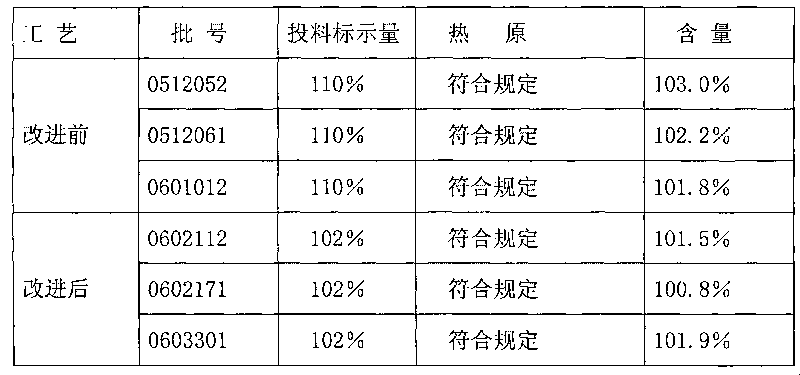
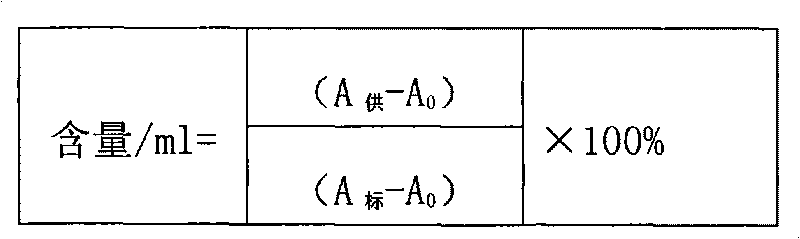
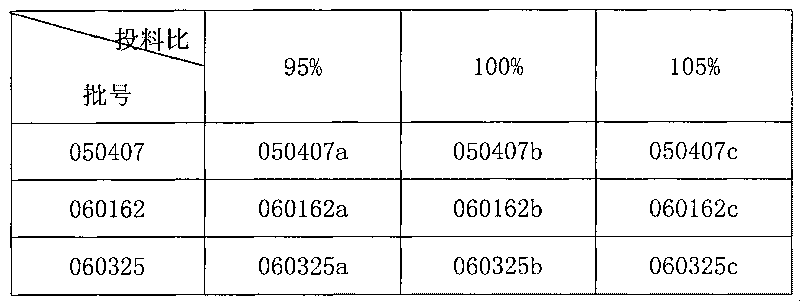
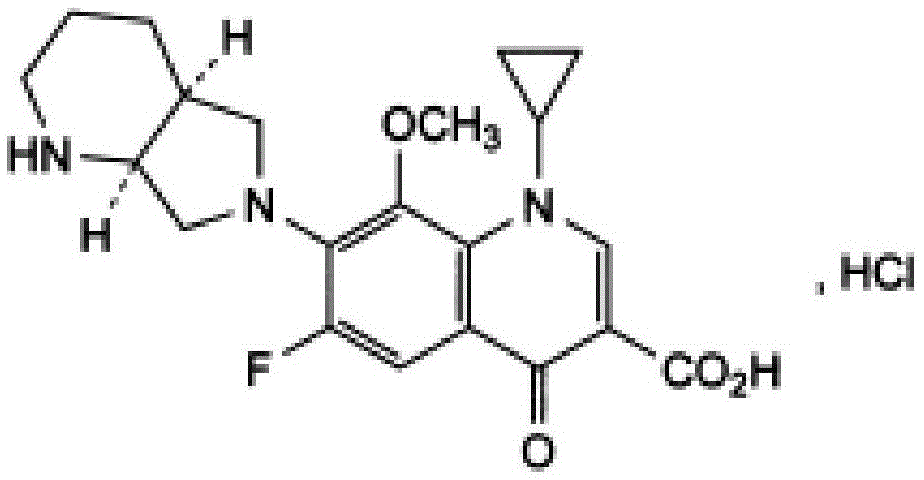
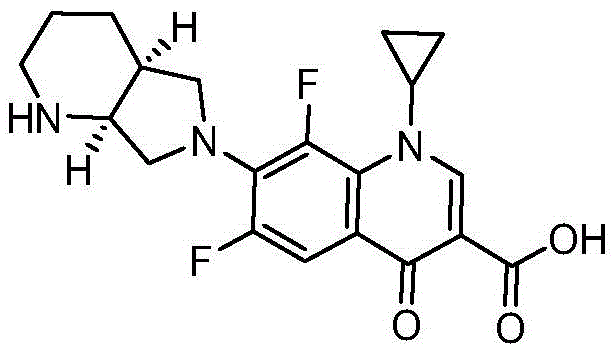
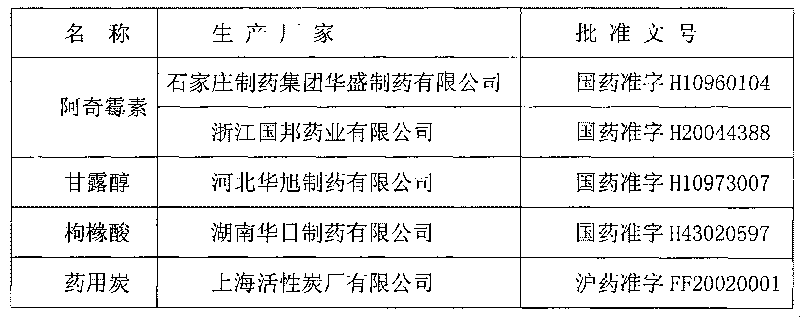
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89 results about "Medicinal carbon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Voriconazole phosphate ester for injection and preparation method thereof
ActiveCN101744778AImprove performanceNo pollution in the processOrganic active ingredientsPowder deliveryPhosphateMedical prescription
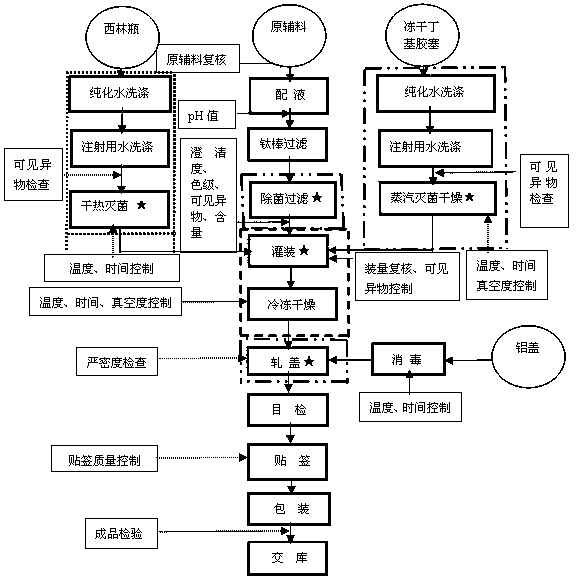
The invention provides voriconazole phosphate ester for injection and a medicinal salt thereof and a preparation method for the voriconazole phosphate ester for injection and the medicinal salt thereof. The preparation method comprises the following steps: adding 5 to 98 percent water for injection in a liquid preparation container; adding 90 to 110 percent of the accurate formula dosage of voriconazole phosphate ester and the medicinal salt thereof in the container; stirring the mixture; slowly dropwise adding a pH value regulator; regulating pH to between 6.0 and 11; supplementing water to the full dosage and then adding 0.01 to 1.0 percent (weight in volume) medicinal carbon into the product; stirring the mixture for 15 to 60 minutes; using a sand filter stick to carry out rough filtration and decarburization on the obtained product, and using a 0.22mum millipore filter to carry out fine filtration on the product until the clarity is qualified; after determining that the content of the midbody is qualified, determining the filling quantity and subpackaging the finished product in the vial; adding the semi-plug; carrying out freezing and drying on the sample; controlling the moisture content between 1 and 8 percent; pressing the plug; and carrying out capping.
Owner:HC SYNTHETIC PHARMA CO LTD
Ambroxol hydrochloride atomization inhalant, and preparation method and application thereof
ActiveCN104027326AImprove stabilityEase of use and flexibilityOrganic active ingredientsAerosol deliveryDiseaseNitrogen gas
The invention provides an ambroxol hydrochloride atomization inhalant and a preparation method and application thereof. The ambroxol hydrochloride atomization inhalant uses injection water as a solvent and contains ambroxol hydrochloride with a concentration of 7.5 mg / ml and a pharmaceutically acceptable carrier with a concentration of 18 to 28 mg / ml; the carrier comprises a pH value stabilizing agent which is one or more selected from the group consisting of sodium pyrosulfite, sodium hydrosulphite and sodium hydrogen sulfite. The preparation method comprises the following steps: weighing ambroxol hydrochloride and the pharmaceutically acceptable carrier, adding a proper amount of injection water to dissolve ambroxol hydrochloride and the pharmaceutically acceptable carrier, carrying out cooling, then adjusting a pH value by using the pH value stabilizing agent containing sodium pyrosulfite, sodium hydrosulphite and / or sodium hydrogen sulfite and adding water until a desired total amount is almost obtained; then carrying out full stirring with medicinal carbon, carrying out filtering to remove carbon and adding water until the desired total amount is obtained; carrying out filtering with a microfiltration membrane; and filling each bottle with 2 ml, 4ml, 10 ml or 20 ml of the atomization inhalant, then introducing nitrogen and carrying out autoclaved sterilization. The invention further relates to application of the atomization inhalant in preparation of drugs used for treating diseases of the respiratory system.
Owner:天津药物研究院药业有限责任公司
Famotidine injection and preparation method thereof
InactiveCN101716136ADecrease in the marked amount of feedingHigh yieldOrganic active ingredientsDigestive systemDisodium EdetateUltraviolet lights
The invention discloses a preparation method of a famotidine injection. 1000ml of injection contains the following raw materials: 20.0g of famotidine, 3.5g of aspartic acid, 0.2g of natrium adetate, 0.5g of medicinal carbon and the balance of injection water. The preparation method comprises the following steps: (1) adding fresh injection water which accounts for 80% of the preparation liquid to aspartic acid and natrium adetate and stirring to be thoroughly dissolved; (2) adding a formula amount of medicinal carbon and allowing to stand to absorb for 20 minutes; (3) filtering, feeding 102% of famotidine and stirring to be dissolved; (4) adjusting a pH value to 5.2-5.4 with 10% aspartic acid solution, adding injection water to a full amount and uniformly stirring; (5) sequentially filtering by a filter element of 0.45 microns and a filter element of 0.22 microns until clarity is qualified, obtaining a half finished product of famotidine injection and adopting an ultraviolet light-visible light photometry to control the quality of the half finished product; (6) finally, filtering the half finished product by a filter membrane of 0.22 microns after the half finished product is detected to be qualified, filling 2ml of nitrogen and encapsulating; and (7) sterilizing in flowing steam at 100 DEG C for 30 minutes, detecting leakage and lamps, airing, printing characters and packaging to obtain the famotidine injection.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Moxifloxacin hydrochloride glucose injection and preparation method and use thereof
InactiveCN101836950APrecipitation does not occurReduce solubilityAntibacterial agentsOrganic active ingredientsAdditive ingredientMoxifloxacin hydrochloride
The invention provides moxifloxacin hydrochloride glucose injection and a preparation method and use thereof. The method for preparing the injection comprises the following steps of: adding water for injection accounting for 20 to 98 percent of the batch volume into an ingredient tank, and adding glucose, a metal complexing agent and the moxifloxacin hydrochloride in a ratio; after stirring to fully dissolve the components, regulating the pH value to between 4.0 and 4.5 by using 1mol / L hydrochloric acid solution or 1mol / L sodium hydroxide, adding medicinal carbon accounting for 0.05 percent (W / V) of the total volume, uniformly stirring, maintaining the temperature of between 70 and 80 DEG C for 20 minutes, and performing circular filtering for over 20 minutes; replenishing the water for injection to the batch scale, stirring for 5 to 10 minutes, and detecting the pH value of the prepared solution (controlling to between 4.0 and 4.5); after determining that no residual water is present in an elevated tank and a pipeline, opening a valve of the elevated tank, and sampling liquid medicament at a self-circulation pipeline sampling port after the liquid medicament circulates for 20 minutes through a filter element and the elevated tank; detecting according to the intermediate quality standard, requiring that the content of the moxifloxacin hydrochloride is between 1.52 and 1.68 mg / ml, the glucose content is between 47.5 and 52.5 mg / ml, and the pH value is between 4.0 and 4.5; after the intermediate is detected to be qualified, beginning to fill; and conveying the filled semi-finished products into a sterilizing cabinet for sterilization, wherein the sterilization condition is to sterilize for 8 to 30 minutes at 121 DEG C through thermal pressure steam.
Owner:HC SYNTHETIC PHARMA CO LTD
Gabexate mesylate composition for injection and preparation method thereof
ActiveCN103211774ASimple prescriptionEliminate security risksPowder deliveryOrganic active ingredientsCLARITYFiltration
The invention provides a gabexate mesylate composition for injection. The formula is simple. Two-step liquid preparation method is adopted in the preparation process, and the preparation method specifically comprises the following steps: firstly dissolving auxiliary materials, adding medicinal carbon, fully stirring, keeping the temperature at 60 DEG C-80 DEG C to perform adsorption, filtering, then adding a main medicine into filtrate, performing fine filtration, and freeze-drying to prepare a freeze-dried preparation. By adopting the two-step liquid preparation method, the adsorption against the main medicine of the medicinal carbon is reduced, and the stirring is simultaneously performed at higher temperature to fully play the adsorption action of the medicinal carbon and ensure that the content and the pyrogen of a sample are in line with the requirements. The prepared product according to the invention is better than the prior art in the quality evaluation indexes of traits, content, clarity, related substances and the like, and is more suitable for large industrialized production.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD +1
Process for preparing pantoprazole sodium for injection
ActiveCN101961309AIncrease profitReduce manufacturing costOrganic active ingredientsDigestive systemMANNITOL/SORBITOLHigh volume manufacturing
The invention relates to a preparation process (preparation method) for pantoprazole sodium for injection, overcomes the defect of the traditional preparation process for the pantoprazole sodium for injection, and provides a preparation process with low cost, high yield and more stable finished product quality. The process comprises the following steps of: (1) feeding materials according to 100 percent of formula quantity, adding mannitol into injection water in an amount which is about 15 percent of preparation quantity, dissolving the materials with stirring, adding medicinal carbon in an amount which is 0.2 percent (weight / volume) of preparation quantity into the solution, boiling the solution for 30 minutes, removing carbon and filtering the solution, cooling the filtered solution to between 10 and 20 DEG C, and introducing nitrogen (the pressure of the nitrogen is 0.3 to 0.5MPa) into the solution for later use; (2) introducing the nitrogen (the pressure of the nitrogen is 0.3 to 0.5MPa) into the 10-20 DEG C injection water in an amount which is about 60 percent of preparation quantity, adding the medicinal carbon in an amount which is 0.1 percent (weight / volume) of preparation quantity into the solution, stirring the solution uniformly, standing the solution for 20 minutes, removing carbon and filtering the solution; and (3) mixing the filtered solution, introducing the nitrogen (the pressure of the nitrogen is 0.3 to 0.5MPa) into the solution for protecting, adjusting the pH value of the solution to between 10.0 and 10.8 by using 10 percent sodium hydroxide solution, adding 10-20 DEG C injection water to the preparation quantity, stirring the solution uniformly, retesting the pH value which should be between 10.0 and 10.8, filtering the solution by using filters with a 0.45mum filter element and a 0.22mum filter element respectively, and filling the product after the submitted semi-finished products are qualified.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Ribavirin injection and preparation process thereof
InactiveCN102366401AMeet the requirementsOrganic active ingredientsPharmaceutical product form changeOfficinalOrganic chemistry
The invention provides ribavirin injection and a preparation process thereof, belonging to the field of pharmaceutical preparations. The ribavirin injection provided in the invention comprises ribavirin, sodium chloride, medicinal carbon and fresh injection water; each 1000 ml of the ribavirin injection comprises 100 g of ribavirin, 9 g of sodium chloride and 0.5 g of medicinal carbon, with the balance being fresh injection water. The preparation process for the ribavirin injection in the invention is characterized by comprising the following steps: a, adding sodium chloride into injection water to dissolve sodium chloride, then adding ribavirin and dissolving ribavirin with stirring; b, adjusting the pH value of a solution obtained in step a to 5.0 to 5.3, adding medicinal carbon, homogenizing an obtained mixture with stirring and standing the mixture for 15 min; c, carrying out filtration on a medicinal soup obtained in step b by sequential using a titanium rod filter, a 0.45 mu m cylindrical filter and a 0.22 mu m cylindrical filter. According to the invention, the ribavirin injection has a high content; inspection items of the ribavirin injection like related substances and pyrogen accord with standard requirements for ribavirin injections in Chinese Pharmacopeia, the edition of year 2010, the second part.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Preparation process of freeze-dry powder injection containing esomeprazole sodium
InactiveCN102657622AReduce moisture contentLow content of related substancesOrganic active ingredientsPowder deliveryAntioxidantFreeze-drying
The invention relates to a preparation process of a freeze-dry powder injection containing esomeprazole sodium, and also provides the esomeprazole sodium for injection, which has the advantages of safe quality and high stability. The preparation process comprises the following steps of: dissolving the esomeprazole sodium, an excipient, a metal ion complexing agent, an antioxidant and the like in injection water; adding a pH value adjusting agent to adjust the pH of mixed solution; adding medicinal carbon to decolorize; filtering; canning the filtrate; partially plugging; performing freeze-drying; pressing a plug under vacuum; rolling a cover; performing lamp inspection; and packaging to obtain a finished product. The preparation process is rational in prescription design; the defects of instability of the esomeprazole sodium upon heating and poor clarity of solution after redissolution are effectively overcome; and a prepared product has the advantages of high stability, high redissolution and the like and is suitable for industrial production.
Owner:KAMP PHARMA
Calcium folinate for injection and production technology for calcium folinate
InactiveCN102429878ASolve the defect of poor formabilityPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLFreeze-drying
The invention provides calcium folinate for injection and a preparation method for the calcium folinate. The calcium folinate for injection consists of anhydrous calcium folinate, mannitol, medicinal carbon and fresh water for injection. The preparation method comprises the following steps of: dissolving the mannitol and the calcium folinate into the water for injection, adding the medicinal carbon, boiling, standing and decarburizing; and regulating the pH value by using sodium hydroxide, filtering through a cylindrical filter, and thus obtaining the calcium folinate for injection, wherein the prepared calcium folinate for injection is prepared into freeze-dried powder injection by pre-freezing, subliming and drying. Because the mannitol serving as an excipient is added in the calcium folinate for injection, the prepared freeze-dried powder injection is a loose and porous block and is quickly dissolved into the water and the solution is clear, the defect of poor forming property of the calcium folinate freeze-dried powder injection in the prior art is overcome.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Doxycycline hydrochloride freeze-dried power prepn. for injection, and process for preparing same
ActiveCN1868460AEasy to useLittle side effectsPowder deliveryTetracycline active ingredientsGlycineVitamin C
A freeze-dried powder injection of doxycycline hydrochloride is proportionally prepared from doxycycline hydrochloride, VC and glycine through dissolving them in the water for injection, adding the water for injection, stirring, adding medicinal carbon, stirring, coarse filtering to remove carbon, fine filtering by filtering membrane, pouring in container, and freeze-drying.
Owner:GUANGDONG JIANXIN PHARMA
Preparation method of ligustrazine phosphate powder injection
ActiveCN101647781AStable in natureNot easy to crystallizePowder deliveryOrganic active ingredientsFiltrationPhosphate
The invention discloses a preparation method of ligustrazine phosphate powder injection, comprising the following steps: dissolving ligustrazine phosphate into ethanol; adding medicinal carbon for adsorption; obtaining filter liquor through filtration and decarburization; degerming and filtering the filter liquor by a millipore filter membrane, and then crystallizing under the temperature of 0-35DEG C while stirring at the same time; drying crystallized crystals under the temperature of 0-80 DEG C, and then preparing the ligustrazine phosphate for the injection through subpackage, stopper addition and capping. The preparation method has the advantages of easy operation, short production period without special equipment, low cost and high yield coefficient and is suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Ornithine aspartate pharmaceutical composition for injection
InactiveCN103655533AFix stability issuesHigh yieldPowder deliveryOrganic active ingredientsDiseaseMANNITOL/SORBITOL
The invention belongs to the technical field of medicine, and discloses an ornithine aspartate pharmaceutical composition for injection. The ornithine aspartate injection consists of ornithine aspartate, mannitol and anhydrous sodium carbonate; a preparation method of the ornithine aspartate pharmaceutical composition comprises the following steps: fetching water for injection of the prescribed amount, adding the mannitol and the anhydrous sodium carbonate, and stirring for dissolution; adjusting the pH value to 6.2-7.2; adding the ornithine aspartate and stirring till complete dissolution; adding medicinal carbon, and stirring; performing suction filtration, supplementing the water for injection till full dose, and mixing uniformly; and performing fine filtration, filling, freeze-drying, light inspection and warehousing to obtain the ornithine aspartate pharmaceutical composition. The ornithine aspartate pharmaceutical composition has good stability and is mainly used for treating hepatic encephalopathy and chronic hepatitis B related diseases.
Owner:TIANJIN SONGRUI MEDICAL TECH
Ozagrel sodium drug combination for injection
InactiveCN102846561AFix stability issuesHigh yieldPowder deliveryOrganic active ingredientsProduction rateMANNITOL/SORBITOL
The invention discloses an ozagrel sodium drug combination for injection. The ozagrel sodium injection solution consists of ozagrel sodium, mannitol and anhydrous sodium carbonate, wherein each piece contains 20-80 mg of ozagrel sodium, 20-80 mg of mannitol and 5-15 mg of anhydrous sodium carbonate. The drug combination is prepared by a method comprising the following steps: taking a recipe quantity of injection water, adding the mannitol and the anhydrous sodium carbonate and stirring until the mannitol and the anhydrous sodium carbonate are dissolved; adding the ozagrel sodium, and stirring until the ozagrel sodium is fully dissolved; regulating a pH value between 7.7 and 8.7; adding medicinal carbon and stirring; leaching, replenishing the injection water to a full quantity and uniformly mixing; finely filtering; encapsulating; freeze-drying; inspecting with a light; and warehousing to obtain the ozagrel sodium drug combination. The ozagrel sodium drug combination has good stability. The production rate of the product is increased, the cost is lowered, and industrialization is realized. The drug combination can be better applied in clinic and has more remarkable advantages.
Owner:TIANJIN SONGRUI MEDICAL TECH
Refinement technique of hydromorphone acid salt
The invention aims to overcome the defects in the prior art, and provides a new refinement method of hydromorphone acid salt. The method comprises the following steps: a. adding hydromorphone and dilute acid into a reaction kettle, and dissolving by stirring at 30-60 DEG C; cooling to 15--5 DEG C, stirring to precipitate abundant solid, adding alcohol and ether, and stirring to crystallize for 0.5-3 hours; filtering, washing until the pH value of the filtrate is 6-7, and drying in a drying oven to obtain a hydromorphone acid salt crude product; b. adding purified water into a refinement decolorization tank, heating to 30-60 DEG C, adding the hydromorphone acid salt crude product, stirring until the hydromorphone acid salt crude product is completely dissolved, adding medicinal carbon, adding dilute acid, and decolorizing at the constant temperature of 30-60 DEG C for 10-30 minutes; filtering, washing the filter cake with purified water, merging the washing liquid and filtrate, distilling under reduced pressure, refrigerating at 5+ / -5 DEG C to crystallize for more than 8 hours; and filtering, washing the filter cake with 95% ethanol, drying in a drying oven to obtain the hydromorphone acid salt.
Owner:YICHANG HUMANWELL PHARMA
Calcium gluconate injection and preparation method thereof
InactiveCN107115279AGood solubilization effectPrevent crystallizationOrganic active ingredientsMetabolism disorderForeign matterNitrogen
The invention discloses a calcium gluconate injection and a preparation method thereof. The method comprises (1) adding 40% water for injection into a concentrated preparation tank, heating to slightly boiling, adding calcium gluconate, and replenishing the water for injection to the full amount 70% and stir to dissolve; (2) add calcium lactate, boil for 30 minutes, then add medicinal charcoal, boil for 30 minutes; (3) decarbonize with Suzhou sand filter stick until clear; (4) add water for injection to the full amount and stir; (5) filter back to clarity with Suzhou sand filter rods, 0.45 μm and 0.22 μm polyethersulfone filter elements; (6) test the intermediate content and pH value, filter the liquid medicine after passing the test, and fill it with nitrogen; (7) After filtering through a 0.22 μm polyethersulfone filter element, check for visible foreign matter, and pass it into a liquid storage container; (8) potting; (9) sterilize at a temperature of 121° C. for 30 minutes. The injection of the invention avoids the crystallization of calcium gluconate, and the product quality is stable and reliable.
Owner:SHANGHAI XINYI JINZHU PHARMA
Medicine composition containing docetaxel and preparation method thereof
InactiveCN101708177AShort dissolution and dispersion timeThe main drug is stableOrganic active ingredientsPharmaceutical non-active ingredientsAlcoholDocetaxel
The invention relates to a medicine composition containing docetaxel and a preparation method thereof. The medicine composition is characterized by comprising the following components according to the mixture ratio (in parts by weight): 10-100 parts of docetaxel, 260-2600 parts of polysorbate 80, 1-20 parts of stabilizing agent and 0-11 parts of anhydrous alcohol; The preparation method comprises the following steps: (1) adding the stabilizing agent which regulates the pH into the polysorbate 80, dispersing, dissolving and adding medicinal carbon for refining the polysorbate 80; (2) taking part of the refined polysorbate 80, adding the docetaxel and the anhydrous alcohol, dissolving the medicine by the dissolution-aiding means of ultrasonic, stirring and the like and stirring the mixture evenly; (3) after removing the anhydrous alcohol, adding the remanent refined polysorbate 80, stirring and mixing the mixture evenly; and (4) after sterilizing and filtering, filling the obtained solution into a sterile container by filling equipment.
Owner:ZHEJIANG WAN SHENG PHARMA CO LTD
Potassium dehydroandrogrpholide succinate injection and preparation method thereof
InactiveCN101721359AAvoid adsorptionLow costAntibacterial agentsOrganic active ingredientsSodium bicarbonateUltrafiltration
The invention discloses a potassium dehydroandrogrpholide succinate injection which contains the following components in 2,000ml of injection: 40.0g of potassium dehydroandrogrpholide succinate, 7.6g of sodium hydrogen carbonate, 7.0g of sodium dihydrogen phosphate anhydrous, 2.8g of disodium hydrogen phosphate, 2.8g of Beta-mercaptoethanol, 2.0g of medicinal carbon and the balance of injection water. A preparation method comprises the following steps of: after dissolving the sodium hydrogen carbonate by using the injection water with 50-60 DEG C, adding the potassium dehydroandrogrpholide succinate, stirring until the potassium dehydroandrogrpholide succinate is dissolved completely; and sequentially adding buffer solutions of the sodium dihydrogen phosphate anhydrous and the disodium hydrogen phosphate and the Beta-mercaptoethanol, uniformly stirring for constant volume, adding the active carbon for absorption, decarburizing and filtering, nitrogenizing and encapsulating after detecting that the semi-finished product is qualified, wherein the filling quality of the injection is 2ml. The preparation method is characterized in that: 1, a hollow fiber ultrafiltration device is used for replacing the original active carbon absorbed filtering system during the production; and 2, the quality of the semi-finished product of the potassium dehydroandrogrpholide succinate injection is controlled by using a high performance liquid chromatography.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Stable fasudil hydrochloride injection and preparation method thereof
ActiveCN105919931AStable lightingHigh clarityOrganic active ingredientsNervous disorderMass ratioFiltration
The invention belongs to the field of pharmaceutical preparation, and particularly relates to stable fasudil hydrochloride injection and a preparation method thereof. The fasudil hydrochloride injection comprises fasudil hydrochloride, stabilizers, anhydrous citric acid, sodium citrate dihydrate, sodium chloride and water for injection, wherein the stabilizers comprise N-carboxymethyl chitosan and reduced glutathione according to the mass ratio of 1:(0.05-0.35). The preparation method includes the steps: dissolving components in the formula by the water for injection after pyrogen removal; adjusting pH (potential of hydrogen); adding medicinal carbon for stirring adsorption to further remove pyrogen and perform discoloration; performing filtration and decarburization; performing filtration, sterilization, sub-package, potting and sterilization to obtain the fasudil hydrochloride injection. The fasudil hydrochloride injection has fine light stability and simple in formula, insoluble particles are effectively suppressed, the preparation process is easily operated, and industrialization is facilitated.
Owner:中润药业有限公司
Method for preparing cefathiamidine freeze-dried sterile raw material medicine
InactiveCN1615881AQuality improvementEasy to transportAntibacterial agentsOrganic active ingredientsOrganic solventFreeze-drying
The present invention relates to preparation process of freeze dried sterile cefathiamidine as raw medicine material, and solves the problems of raising cefathiamidine yield and eliminating organic solvent residue. The technological scheme includes dissolving cefathiamidine in proper amount of water for injection at room temperature, adding proper amount of medicinal carbon to decolor for 20-30 min, sterile filtering and freeze drying to water content 0.1-3 wt% and to obtain product. The weight ratio between cefathiamidine and water for injection is 1 to 1-10, and the weight ratio between cefathiamidine and active carbon is 1 to 0.01-0.1. The present invention has simple production process, low production cost, low organic solvent residue, high yield over 97 %, stable medicine quality, and convenient transportation and storing.
Owner:济南永曜医药科技有限公司
Application of medicinal carbon in preparation of medicaments for curing hyperphosphatemia
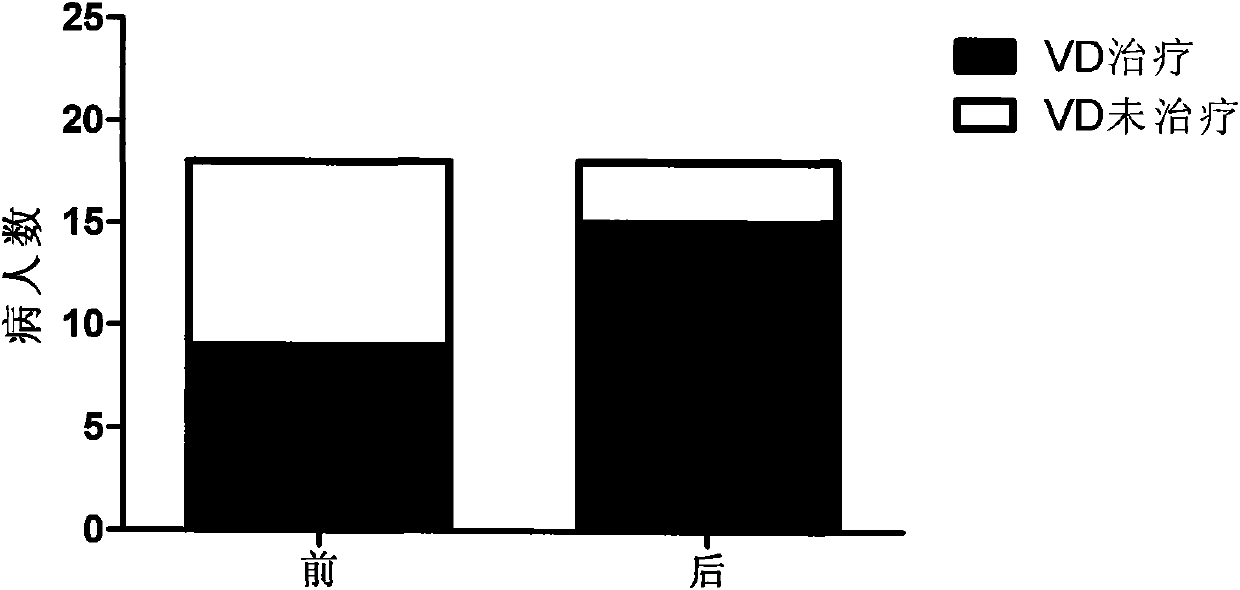
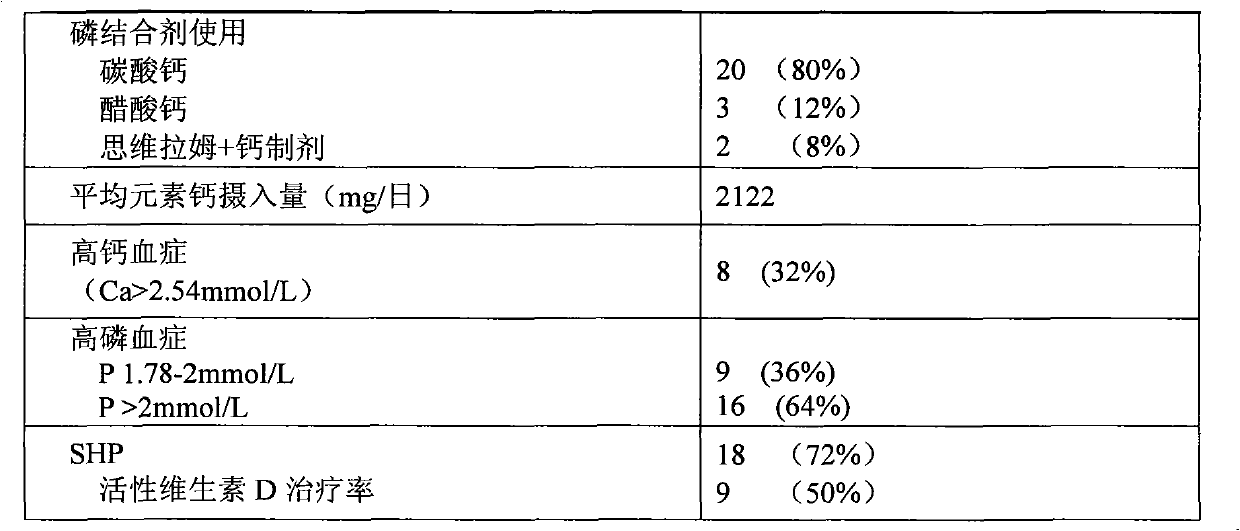
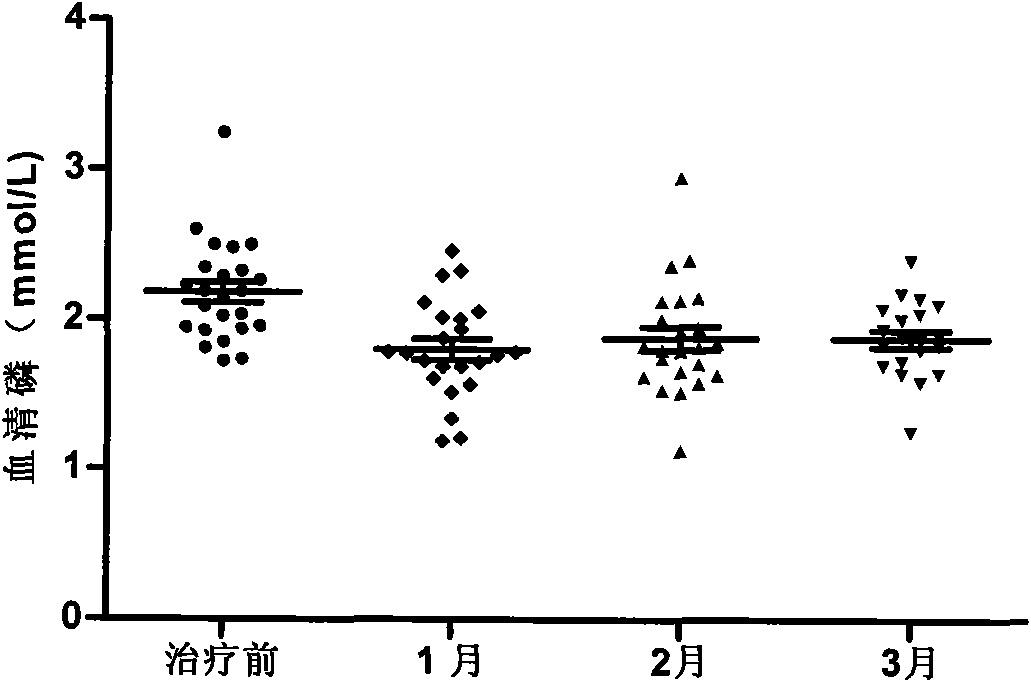
InactiveCN101904868AReduce deathReduce productionMetabolism disorderCarbon active ingredientsPeritoneal dialysisSecondary hyperparathyroidism
The invention relates to novel medicinal application of medical carbon, in particular to the application of the medicinal carbon in the preparation of medicaments for curing hyperphosphatemia. In the invention, clinical tests prove that the medicinal carbon, when taken orally, can reduce the serium inorganic phosphorus level and the product of calcium and phosphorus of patients who have received hemodialysis and peritoneal dialysis but do not have the hyperphosphatemia controlled after receiving the treatment by the calcium-containing phosphate binder, and provides a voltage detection (VD) therapy opportunity for patients with secondary hyperparathyroidism which cannot be treated by the VD therapy because of over-high product of calcium and phosphorus. The medical carbon is favorable for reducing and preventing angiocardiopathy development death, caused by chronic kidney disease-mineral and bone disorder (CKD-MBD), of the dialysis patients. Thus, the medicinal carbon can be used as novel medicaments for curing the hyperphosphatemia of patients with dialysis.
Owner:河北长天药业有限公司
Rifampicin freeze-dried powder injection and preparation process thereof
The invention discloses a rifampicin freeze-dried powder injection and a preparation process thereof, and the preparation process is as follows: weighing rifampicin raw material drug and water for injection for standby use, adding sodium thiosulfate, stirring for dissolving, adding into a liquid mixing tank; filling nitrogen into the liquid mixing tank, adding rifampicin, stirring into a suspension, adding a sodium hydroxide solution, mixing evenly, taking medicinal carbon, adding the water for injection to prepare into paste to add into the material mixing tank, stirring evenly, and standing for adsorption; using a titanium stick to filter for remove carbon; opening a sterile liquid storage tank for circulating mixing in a filling material liquid circulating pump, sampling and testing the pH value, using filtered and sterilized nitrogen for pressure maintaining; filling the drug liquid in an injection bottle, pressing until a plug is semi-closed, placing in a lyophilizer for freeze drying. The rifampicin freeze-dried powder injection has the advantages of longer shelf life, higher quality stability and safety, more convenient transportation and storage, and can be widely used in rifampicin freeze-dried powder injections and the preparation process thereof.
Owner:REYOUNG PHARMA
Preparation method for injection phosphorus propofol sodium
InactiveCN101756913AReduce the number of recipesPowder deliveryOrganic active ingredientsMedicineBottle
The invention relates to a preparation method for injection phosphorus propofol sodium. The preparation obtained by the method needs no filing auxiliary materials and stabilizes, which reduces qantity of formulation of transfusion preparations. The preparation method for injection phosphorus propofol sodium comprises the following steps of: adding 5% to 98% of water for injection to a solution preparing container; adding 90% to 110% of phosphorus propofol sodium of accurate medical prescription quantity; dissolving the materials by stirring; slowly dripping pH value regulator to regulate the pH value to be 5.0 to 8.5; supplementing water to the full dose; then adding 0.01% to 1.0% (W / V) of medicinal carbon; coarsely filtering and decarbonizing with a sand filtering stick; fine filtering with a 0.22 mum millipore filter unitl the clarity is qualified; determining charge quantity and filling the preparation to cillin bottles after content of midbody is qualified through measurement; blanking the cillin bottles with half-plugs; controlling water in samples to 0.1% to 8% after freezing and drying; plugging by press; and rolling covers.
Owner:HC SYNTHETIC PHARMA CO LTD
Medicine composition for treating multiple kinds of diarrhea of animals
InactiveCN103301448AEnhance phagocytosisEnhance immune functionHeavy metal active ingredientsOrganic active ingredientsProtozoaSide effect
The invention discloses a medicine composition for treating the multiple kinds of diarrhea of animals and belongs to the human living need department, the veterinary medicine department, and the technical field of the curative activity of compounds or medicine preparations, divided according to the International Patent Classification (IPC). The invention aims at providing a medicine composition which is free of toxic and side effects, simple to produce, and capable of treating multiple kinds of diarrhea of animals. The medicine composition is composed of a traditional Chinese medicine, namely berberine, a Western medicine, namely bismuth subcarbonate, tannalbin, medicinal carbon and pepsase in an optimized proportioning ratio. The medicine composition is characterized by having a wide antibacterial spectrum, the effects of inhibiting protozoa, improving leukocyte phagocytosis, enhancing a body immune function, converging, relieving diarrhea, detoxicating, enhancing digestion and the like, and the special effect of treating the various diarrhea of animals. According to the novel scheme, the medicine composition disclosed by the invention is wide in applications in the technical field of the curative activity of veterinary medicine preparations. The pharmaceutically acceptable auxiliary materials of the medicine composition disclosed by the invention are medicinal carriers, excipients and other additives for conventional preparations.
Owner:陶箭
Colored medicinal charcoal film coated tablet preparation method
ActiveCN101103997AEasy to acceptImprove clinical complianceDigestive systemCarbon active ingredientsColoredExcipient
The invention discloses a preparation method of painted medicinal carbon film-coating tablet produced by the raw material of medicinal carbon and medicinal excipients. The invention is characterized in that painted medicinal carbon film coating tablets are produced in the preparation method, thus the visual defect of black medicinal carbon is overcome and the painted tablets are easy to be accepted by patients. The smooth and round form of the painted medicinal carbon film coating tablet can help effectively guarantee the tablet quality and improve the clinical compliance of patients.
Owner:河北长天药业有限公司
Preparation process of moxifloxacin hydrochloride sodium chloride injection
The invention aims at providing a preparation process of a moxifloxacin sodium chloride injection with better quality, good stability and small side effect. The preparation method of the moxifloxacin hydrochloride injection provided by the invention comprises the following steps: step 1, weighing: respectively weighing moxifloxacin hydrochloride and sodium chloride by formula dosage and preparing for later use; step 2: preparing a solution (1): taking water for injection, adding moxifloxacin hydrochloride, stirring until full dissolution, adding medicinal carbon, homogenizing, stirring for absorption, removing carbon and filtering by a filter membrane, washing the carbon layer with a proper amount of water for injection and reserving the filtrate for later use; step 3: preparing a solution (2): taking water for injection, adding full dose of sodium chloride by formula dosage, stirring until full dissolution, cooling, adding medicinal carbon, homogenizing, stirring for absorption, removing carbon and filtering by a filter membrane, then washing the carbon layer with water for injection, combining the filtrates, adding water for injection to full dose, regulating the pH value and homogenizing for later filling; and step 4: filling: filtering by a microporous filter membrane for liquid medicine, filling the filtrate into glass infusion bottles, charging nitrogen, plugging and capping for later sterilization.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Ozagrel sodium medicinal composition for injection
InactiveCN102429903AFix stability issuesHigh yieldOrganic active ingredientsPharmaceutical delivery mechanismMedicineSodium calcium edetate
The invention discloses an ozagrel sodium medicinal composition for injection. Ozagrel sodium injection consists of ozagrel sodium, sodium citrate and calcium disodium edetate, wherein each piece of injection contains 40-100 mg of ozagrel sodium, 4-10 mg of sodium citrate and 0.02-0.05 mg of calcium disodium edentate. A preparation method of the composition comprises the following steps of: adding a prescription dose of sodium citrate and calcium disodium edentate into a prescription dose of 90 percent injection water at the temperature of 55-65 DEG C and stirring to dissolve; adding a prescription dose of ozagrel sodium and stirring until the ozagrel sodium is fully dissolved; measuring an initial pH value, and adjusting the pH value to be within a range of 7.5-9.5 by using 4 percent sodium hydroxide solution and 10 percent sodium citrate solution according to the initial pH value; adding medicinal carbon and stirring; performing extraction filtration, replenishing injection water to full amount and uniformly mixing; performing fine filtration; canning; sterilizing; performing light inspection; and warehousing to obtain the ozagrel sodium injection. The ozagrel sodium medicinal composition has high light stability, clarity and stability and does not produce crystals, and has the more obvious advantages of improving the product yield, reducing the cost, realizing industrialization and guaranteeing better application to clinical use.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Rifamycin sodium injection and preparation method thereof
The invention discloses a preparation method of rifamycin sodium injection. In 1,000ml injection, the rifamycin sodium injection comprises the flowing components: 62.5g of rifamycin sodium counted by C37H47NO12, 7.5g of vitamin C, 4g of sodium metabisulfite, 170 ml of propanediol, 1g of medicinal carbon and the balance of water for injection. The preparation method comprises the following concrete steps of: (1) adding the propanediol into the water for injection, evenly stirring, introducing nitrogen, stirring, adding the vitamin C to completely dissolve and adjusting the pH value to 6.2-6.4; (2) adding the sodium metabisulfite for completely dissolving, then adding the rifamycin sodium raw materia, stirring to completely dissolve, adjusting the pH value to 7.2-7.2 and additionally adding the water for injection to full dose; (3) adding active carbon, introducing nitrogen under the condition of 50 DEG C, stirring for 10 minutes, standing, decarburizing and filtering, adjusting the pH value to 7.2-7.3 and sequentially filtering through filter elements with 0.45 micrometer and 0.22 micrometer; (4) after qualifying semi-finished products, filtering the qualifying semi-finished products by the filter element with 0.22 micrometer and introducing nitrogent for protection; and (5) filtering stock solution by the filter element with 0.22 micrometers, introducing nitrogen for encapsulation, sterilizing the solution by 100 DEG C flowing steam for 30 minutes, and carrying leak detection, lamp test, drying, lettering and packaging to finally obtain the rifamycin sodium injection.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Stable moxifloxacin hydrochloride glucose injection
InactiveCN102727432AImprove stabilityAntibacterial agentsOrganic active ingredientsD-GlucoseGlucose polymers
The invention provides a stable moxifloxacin hydrochloride glucose injection. The preparation method of the injection is as follows: weighing glucose and sodium citrate, then dissolving in the water for injection, mixing and dissolving, adjusting the pH value of the solution to 3.5 to 4.5 with diluted hydrochloric acid, and then adding moxifloxacin hydrochloride, mixing to dissolve completely, adding the medicinal carbon to mix for 10 to 30min, adding the water for injection to dilute to the total amount, and stirring well, wherein the concentration of moxifloxacin hydrochloride in the injection by moxifloxacin is 0.1mg / ml to 10mg / ml; the concentration of glucose in the injection is 40mg / ml to 60mg / ml; the concentration of sodium citrate in the injection is 0.01mg / ml to 0.2mg / ml; and the concentration of the added medicinal carbon is 0.05 to 0.2mg / ml. The sodium citrate with the concentration added into the agent provided by the invention does not have adverse effects on the human body, can improve the stability of moxifloxacin hydrochloride glucose injection greatly, and therefore the generation of precipitation is prevented even at a low temperature.
Owner:XINAN PHARMA
Edaravone sodium chloride injection and preparation technology
InactiveCN110478315AGood treatment effectImprove the bactericidal effectOrganic active ingredientsNervous disorderSodium Chloride InjectionPhosphoric acid
The invention provides an edaravone sodium chloride injection and a preparation technology. According to matching, edaravone, sodium bisulfite, cysteine hydrochloride, sodium chloride, sodium hydroxide and phosphoric acid are added to water for injection. The preparation technology comprises the steps that the cysteine hydrochloride, sodium chloride and sodium bisulfite are added into the water for injection, and the mixture is stirred and completely dissolved; medicinal carbon is added for heat preservation and stirring, the solution in a mixing tank is decarburized and filtered, the edaravone with a prescription amount is pre-dissolved with the water for injection, the mixed solution is added into the mixing tank, the water for injection is supplemented to the total amount, the pH is adjusted, the whole solution is conveyed into a dilution tank, and bagging and filling are conducted. The invention provides novel matching components of the edaravone sodium chloride injection. The invention provides the new production preparation technology of the edaravone sodium chloride injection. The production preparation technology has a better bacterium-killing sterilization effect.
Owner:SICHUAN TAIPINGYANG PHARMA
Azithromycin injection and preparation method thereof
InactiveCN101703465AAntibacterial agentsPharmaceutical delivery mechanismMANNITOL/SORBITOLFreeze-drying
The invention discloses an azithromycin injection, which comprises the following components by weight in 3000 ml injection: 250.0 g or 500.0 g of azithromycin, 1.0 g of citric acid, 120.0 g of mannitol, 18.0 g of medicinal carbon and the balance of water for injection. The preparation method comprises the following steps of: (1) adding the mannitol into the water for injection, heating for dissolving, adding the medicinal carbon, boiling for 30 minutes, decarburizing and filtrating; (2) preparing a suspension by adding the azithromycin to the water for injection; (3) after dissolving the citric acid, adding the dissolved citric acid into the azithromycin suspension for fully dissolving the azithromycin with the pH value controlled to be 5.7-5.9, adding the medicinal carbon, stirring, standing, decarburizing and filtrating; (4) adding the mannitole filtrate into the azithromycin solution with the pH value controlled to be 5.7-5.9, adding the water for injection till a prepared full dose, filtrating through microfiltration membranes with 0.45 micrometers and 0.22 micrometers; (5) measuring the content of a semi-finished product by adopting an optical rotation test method; (6) if the semi-finished product is tested to be qualified, transmitting the medicinal solution to a bottling department, wherein the bottled injection holding volume is 3 ml; (7) freeze-drying, totally pressing pistons, pressing covers, and light-inspecting, and if the semi-finished product is qualified after the quality test, label-sticking, packaging and obtaining the product.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com