Rifampicin freeze-dried powder injection and preparation process thereof
A technology of freeze-dried powder injection and rifampicin, which is applied in the field of medicine and can solve problems such as poor controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
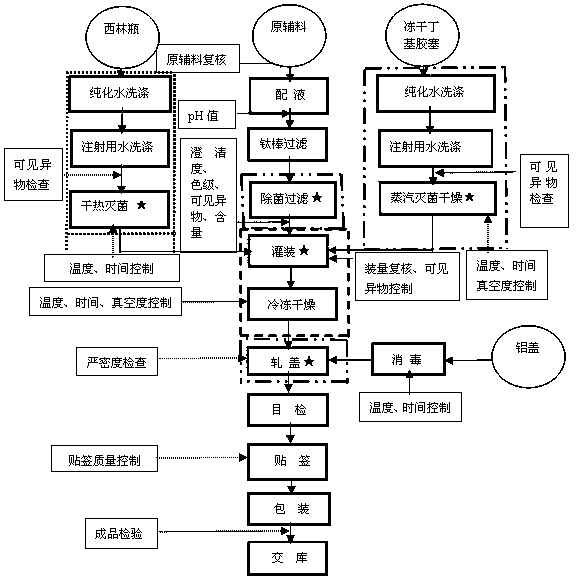
[0009] A best implementation of the present invention, a preparation of rifampicin freeze-dried powder for injection and its preparation process, adopts conventional technology to mix rifampicin and necessary materials into aqueous solution, rifampicin 450g, sodium thiosulfate (Na 2 S 2 o 3 .5H 2 O) 14.1g, sodium hydroxide 15.3g, medicinal charcoal 6g, water for injection 6 L. Among them, rifampicin is fed according to 102.5% after conversion according to the moisture and content (used to offset the residual loss when the material is transferred to the liquid mixing tank after weighing, the adsorption of medicinal charcoal and fine filtration adsorption), and the content of the intermediate is used for filling. The calculation is based on 100% filling. Sodium thiosulfate 14.1g is equivalent to 9g Na 2 S 2 o 3 , the concentration is 0.15%, and the dosage of 6g of medicinal charcoal is 0.1% of the volume of the liquid medicine, which is finally removed. The preliminary pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com