Implantation of micronized allograft tissue over a microfractured defect
a technology of allograft tissue and microfracture, which is applied in the field of surgery, can solve the problems of blood clot over the defect, limited hyaline repair tissue, and insufficient data on its long-term
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
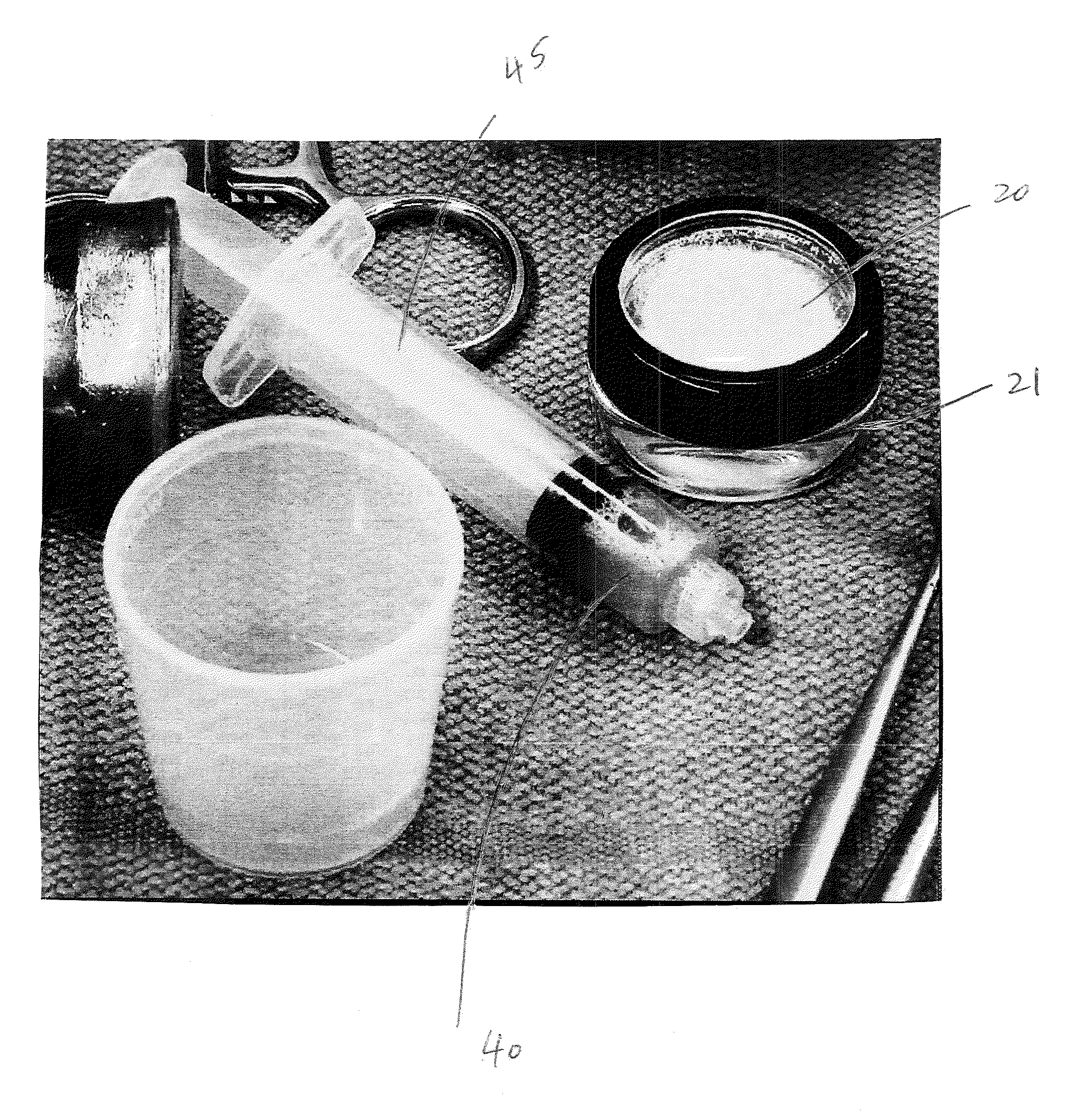
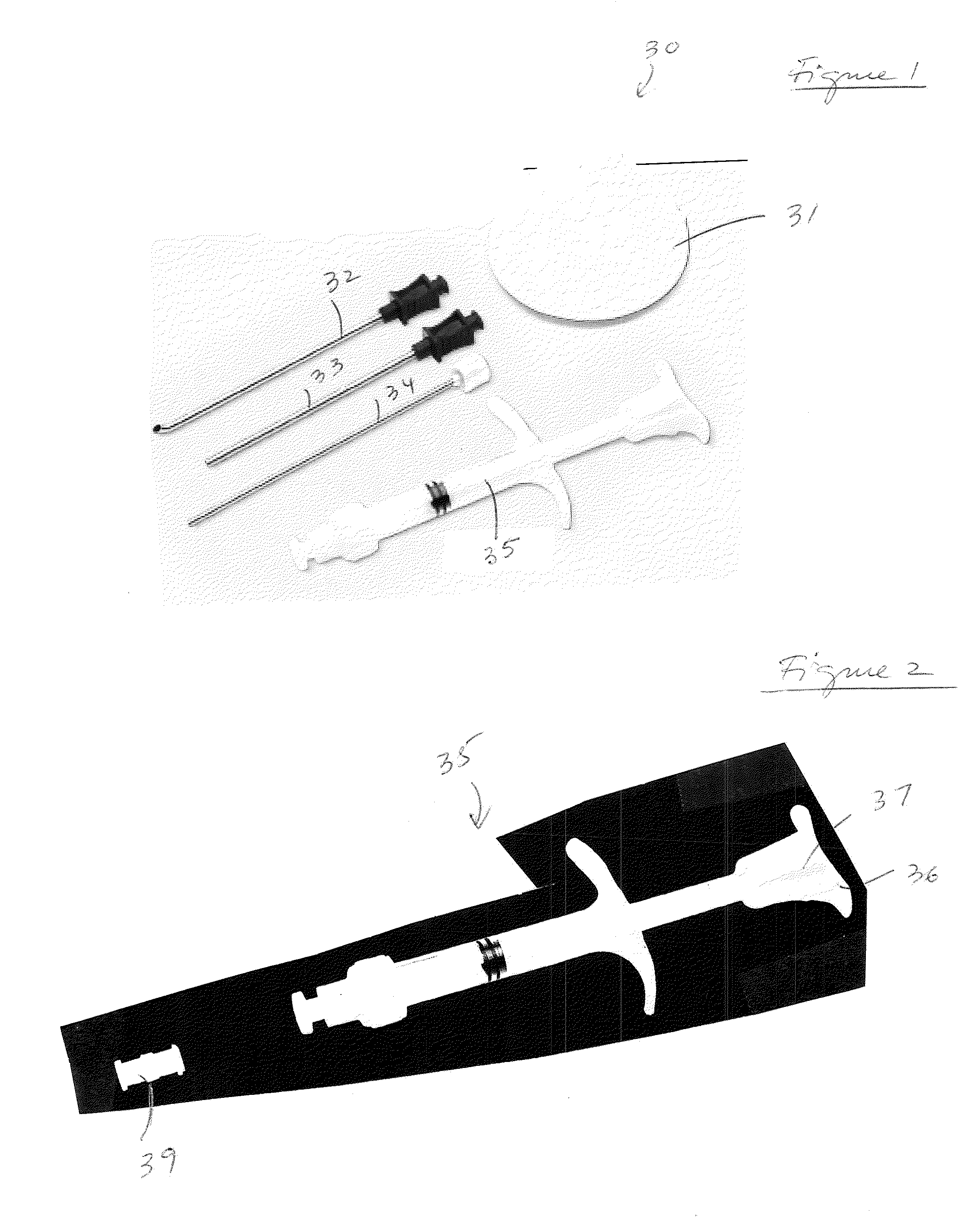
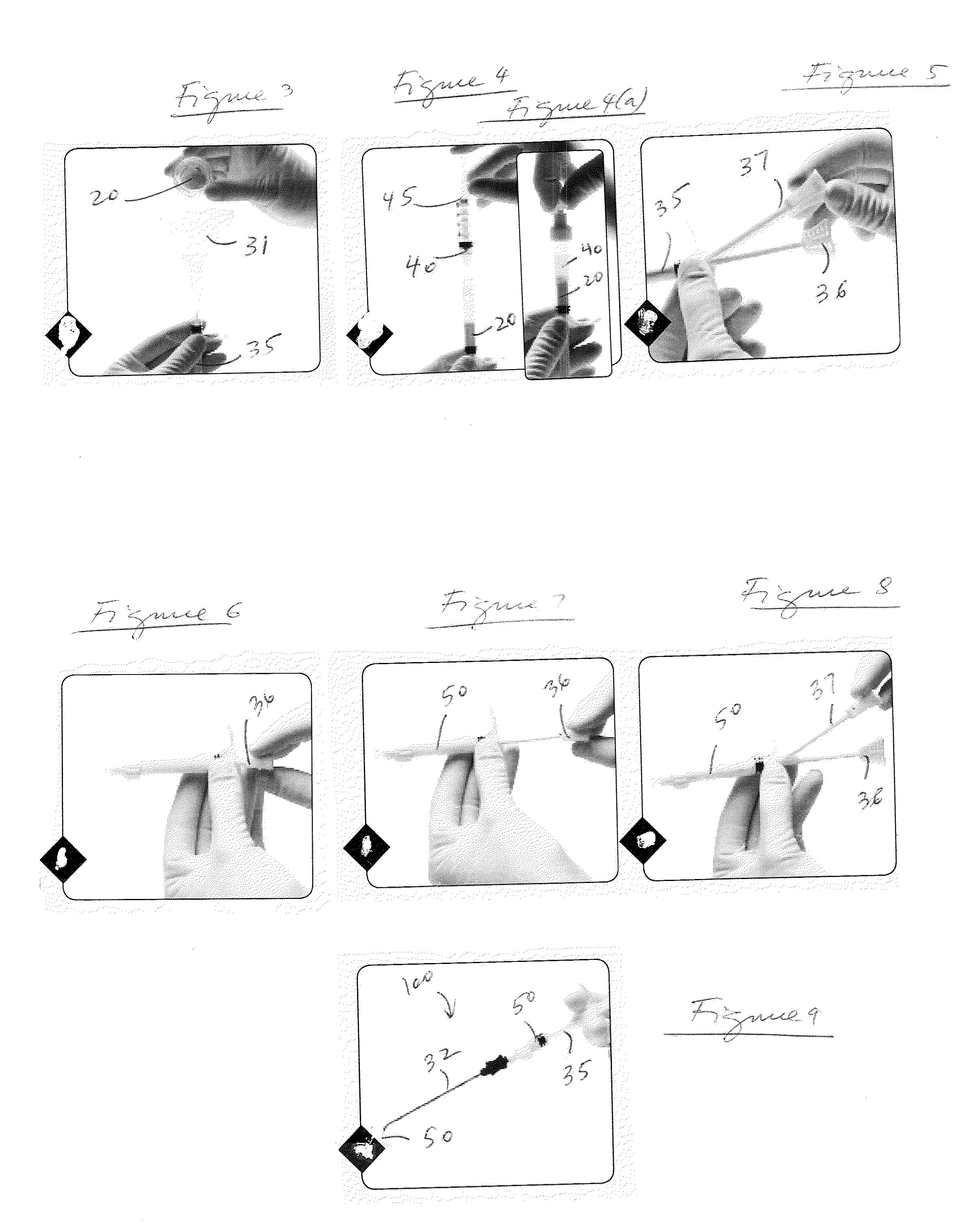
[0020]The present invention provides mixing and delivery techniques for micronized allograft tissue over a microfractured defect. The present invention also provides techniques for implantation of such micronized allograft tissue at a microfracture site.
[0021]Allograft tissue is delivered over a cartilage defect that has been debrided and microfractured without the need for a periosteal covering or separate type of patch sewn over the top. The allograft tissue may be allograft cartilage in the form of micronized cartilage particulates which may be cartilage delivered in its native form, dehydrated via lyophilization, dehydrated via desiccation, or dehydrated by any other method, among others. The micronized cartilage particulates may have a size of about 0-300 microns.
[0022]In an exemplary embodiment only and as detailed below, cartilage with particles of about 0-300 microns is employed to form a moldable allograft paste (mixture or composition). Preferably, the moldable allograft p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com