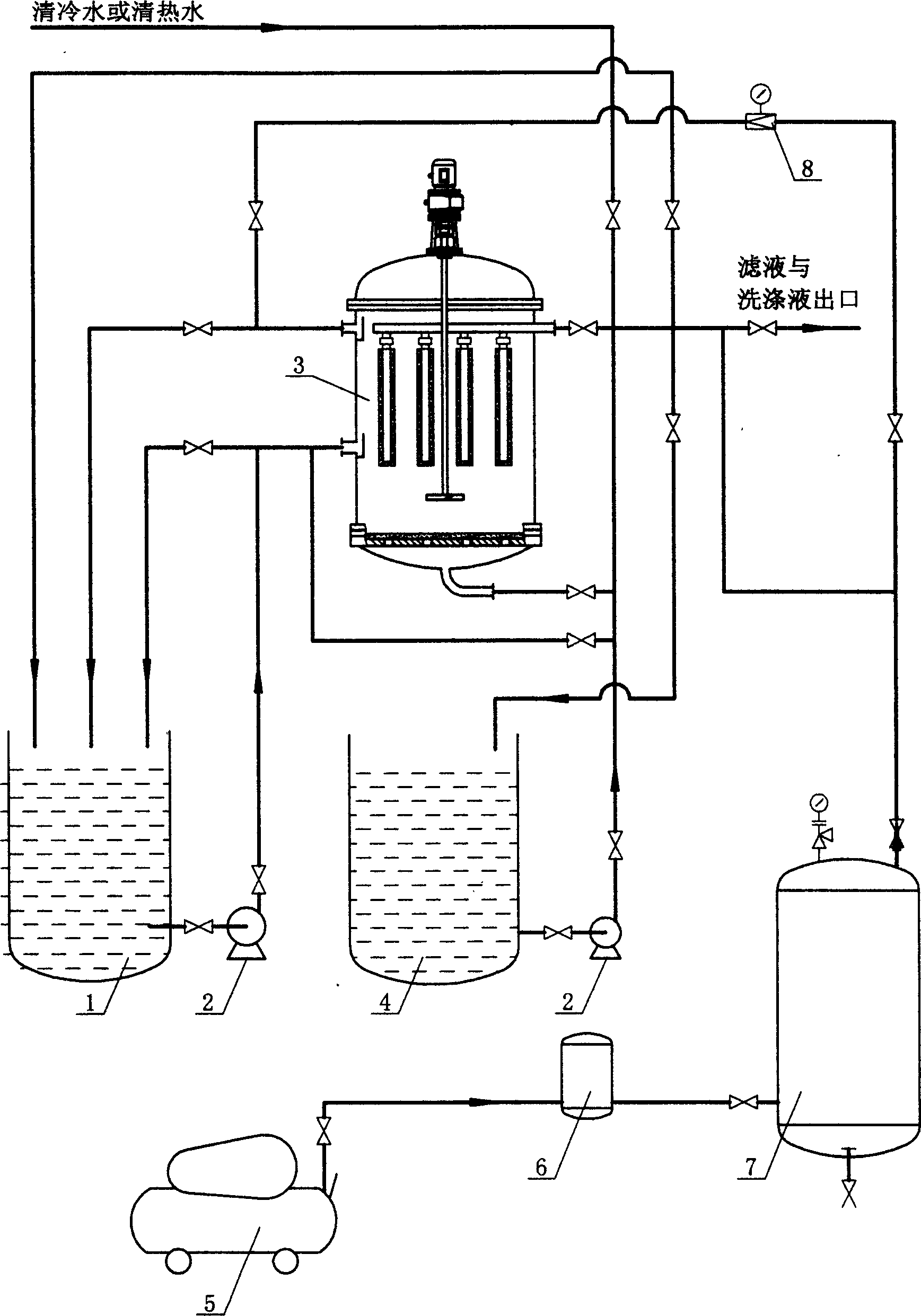
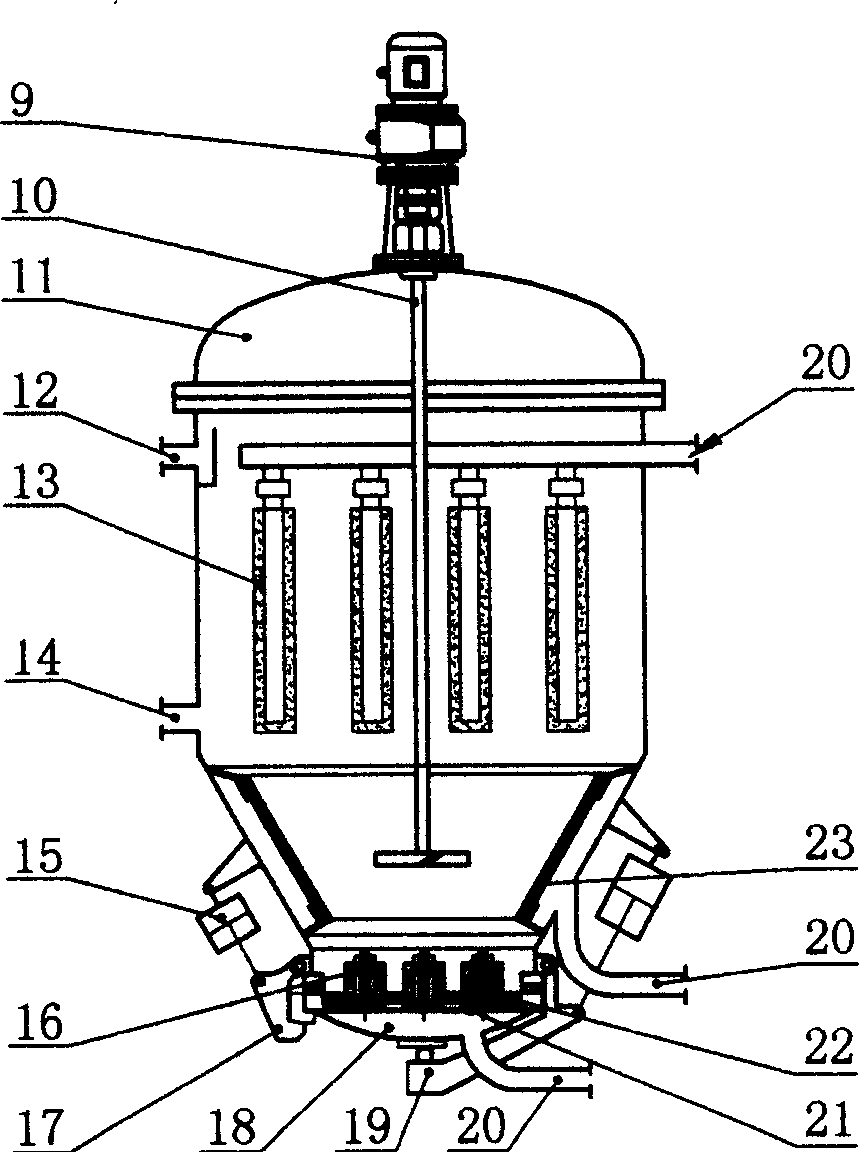
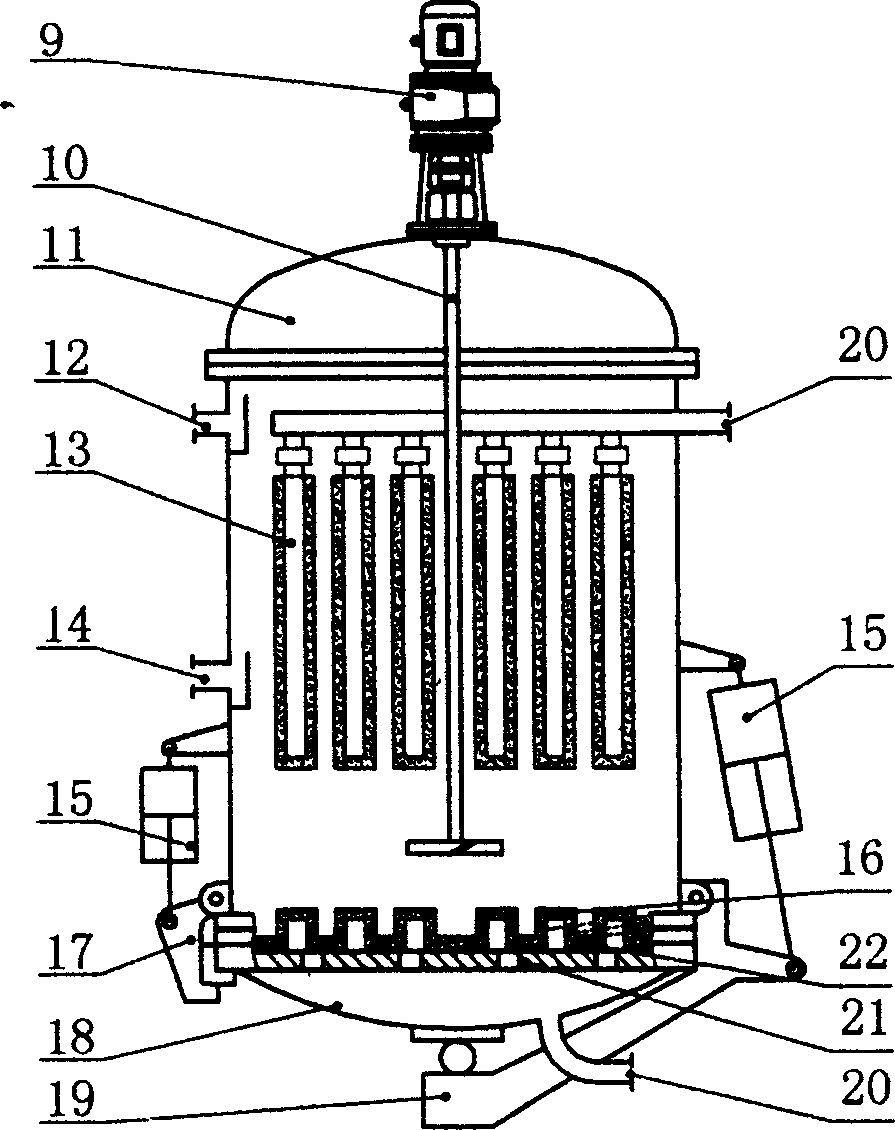
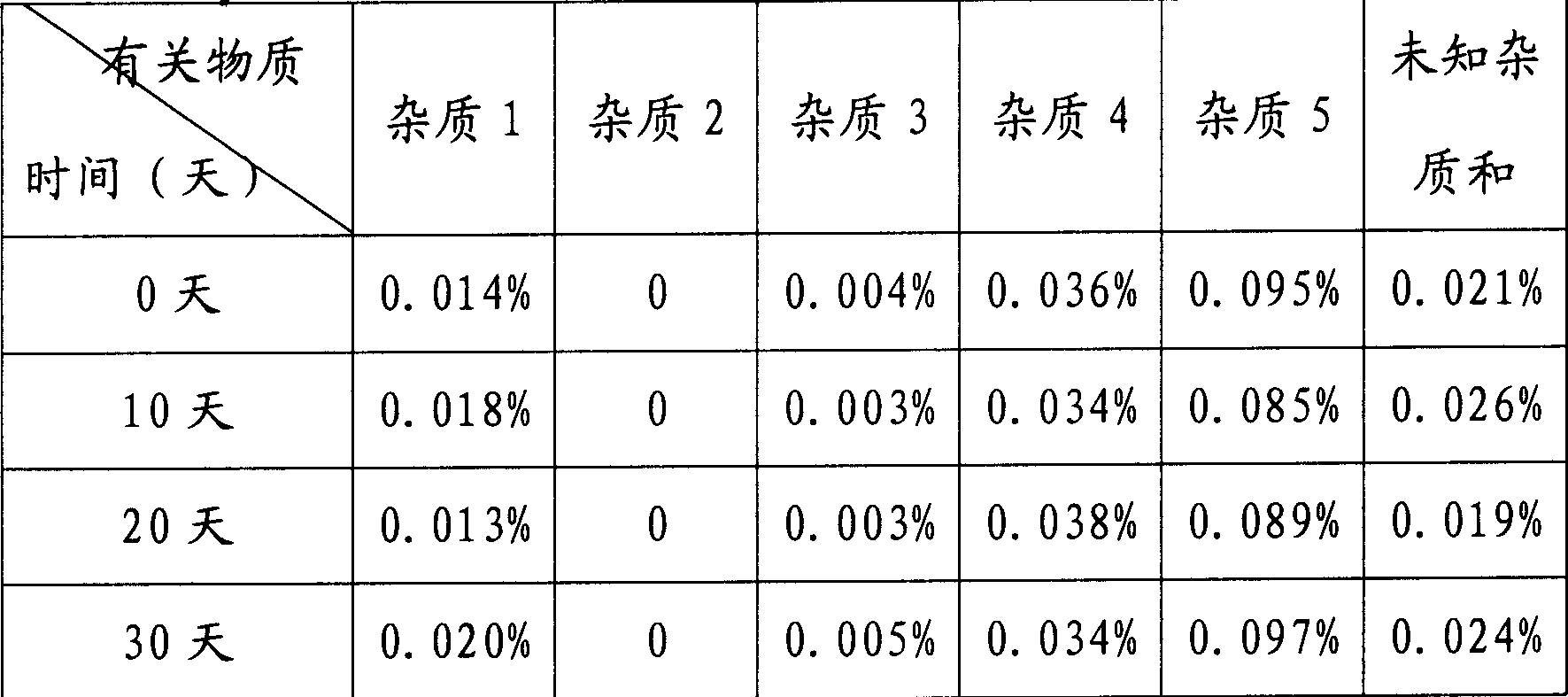
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
229 results about "Millipore Filters" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for fabricating fiber reinforcement type microporous filter membranes of hollow fiber made from polyvinylidene fluoride
InactiveCN1695777AHigh tensile strengthTightly boundSemi-permeable membranesHollow fibrePolymer science
A fibre reinforced millipore filter membrane of hollow polyvinylidene fluoride fibre is prepared through preparing solution from polyvinylidene fluoride, solvent and additive, pretreating the reinforcing fibres, spinning, gelatinizing and post-treating. Said solvent, additive and reinforcing fibre are also disclosed.
Owner:TIANJIN UNIV
Reinforced microporous filter membrane and method and device for preparing the same
InactiveCN101239280AHigh strengthSmall filter resistanceSemi-permeable membranesPolymer resinOrganic solvent
The present invention provides an enhanced millipore filtering film and a method, device for preparing, the enhanced millipore filtering film includes a non-woven fabric as substrate, employing casting wipe way, casting film liquid is applied on the front and the back surfaces of the non-woven fabric, two layer film is formed by gelatin, characterized in that the thickness of the non-woven fabric is 30-120 micron, planar density of the non-woven fabric is 30-100g / m2, thickness of each film layer is 5-10 micron; casting film liquid in two film layer is composed by mixing a polymer resin, an organic solvent and an additive; parts by weight of the polymer resin in the casting film liquid, parts by weight of the organic solvent : parts by weight of the additive is 5-40:40-90: 0.5-50. The enhanced millipore filtering film of the invention is applied in the sampling monitor or filter of alpha radioaerosol under the high radon environment.
Owner:BEIJING RADIATION APPL RES CENT
Lung-targeted medicine carrying precursor liposome for injection and method of use thereof
ActiveCN101474155AQuality improvementAvoid strong toxicityPharmaceutical non-active ingredientsRespiratory disorderDiseaseCholesterol
The invention relates to the technical field of medicament delivery by a carrier, in particular to a lung targeting medicament carried proliposome used for injection and an application method thereof. Solid dispersion technology is adopted to obtain solution A by dissolving active medicament, phospholipid liposome, cholesterol liposome, surface active agent and acid type carrier in absolute ethyl alcohol or the mixed solvent of absolute ethyl alcohol and absolute ether; active carbon which is used for injection and has the volume being 0.1-1% of the volume of the solution A is added into the solution A; stirring, standing and filtering of the solution by a millipore filter are carried; therefore, aseptic solution B free of pyrogen is obtained; aseptic additives are added into the solution B under an aseptic condition; organic solvent is removed; and solid granular medicament carried proliposome or powdered medicament carried proliposome are obtained. The invention has the advantages that the preparation process is simple; the cost is low; the quality is controllable; the stability is good; and the invention is suitable for industrial production. The medicament carried proliposome prepared by effervescency dispersion technology has small and uniform particle diameter, the electric charge ranges from -10mv to -30mv, the proliposome can deliver over 90% medicament to a lung, and remarkable lung targeting effect is achieved, and thus being the best preparation delivering medicament by a carrier for treating lung diseases.
Owner:CHONGQING MEDICAL UNIVERSITY +1
Method for preparing reduced graphene oxide heat conductive film
The invention discloses a method for preparing a reduced graphene oxide heat conductive film. The method comprises the following steps: adding graphite oxide into the deionized water to perform ultrasonic treatment, centrifuging the obtained suspension solution for 5-20 min at the speed of 2000-3000 rpm, getting a supernate, drying and grinding to obtain the graphene oxide; and adding the obtained graphene oxide into a solvent to perform ultrasonic treatment to obtain a graphene oxide dispersed solution with the concentration of 1-5 mg / ml, performing vacuum filtration by a millipore filter, drying the obtained filter cake with the filter membrane, stripping the filter cake from the filter membrane, exerting the pressure of 0-10 MPa to the obtained graphene oxide, under the atmosphere of nitrogen, argon or helium, and processing for 2-8h at the temperature of 600-1000 DEG C to obtain the reduced graphene oxide heat conductive film with high heat conductivity, high reduction degree, high graphene lamellar surface inner orientation degree, smooth surface, good flexibility, wide curl degree without crack and small interlamellar spacing. The preparation method is simple and the reduced graphene oxide heat conductive film is easy for realization of industrial production.
Owner:SHANGHAI INST OF TECH
Quantitative detection device based on fibrous-membrane gathering and separation and detection method thereof
ActiveCN103323590AEfficient separationAvoid repeated washing operationsMaterial analysisFiberBuffer solution
The invention relates to a quantitative detection device based on fibrous-membrane gathering and separation and a detection method thereof. The quantitative detection device comprises a separation testing cup, a reaction cup, an analysis buffer solution and a cleaning solution; wherein the separation testing cup comprises a receiving tank, a water absorption cup and millipore filters between them. A capture agent and a labeling agent are solidified on the bottom of the reaction cup and the capture agent is fixed on the surface of latex beads. The detection method comprises: conjugation reaction, separation, cleaning and detection. In the invention, not only the characteristics of convenient operation and rapid detection of immune infiltration (IF) and immunochromatography (IC) are maintained, but also precision and sensitivity of the detection are obviously better than that of IF and IC. Therefore, quantitative immunodetection and nucleic acid hybridization analysis can be implemented effectively. The invention can be used for the quantitative detection of substances such as protein, nucleic acid, small molecule and microbe.
Owner:SHANGHAI YUNZE BIOTECH +1
Quercetin nano-micelle preparation and preparation method thereof
InactiveCN101982168AImprove solubilityImprove biological activityOrganic active ingredientsPowder deliveryOrganic solventEvaporation
The invention discloses a quercetin nano-micelle preparation which is made from the following components in parts by weight: 20-40 parts of quercetin as a bulk drug and 220-330 parts of a pluronic surfactant as a drug carrier. A preparation method comprises the following steps: mixing the quercetin with the pluronic surfactant and dissolving in an organic solvent until being fully dissolved, and carrying out rotary evaporation at 15-60 DEG C for removing the organic solvent to obtain a drug-containing film; and drying the film in vacuum, adding a water phase for hydrating at 15-60 DEG C to obtain micelle solution, and filtering with a millipore filter to obtain the quercetin nano-micelle preparation. The obtained nano-micelle preparation has small nano-grade particle size of 20-60nm, a hydrophobic core and a hydrophilic shell, thus ensuring that the preparation has small possibility of being swallowed by a reticuloendothelial system in body, achieving effects such as targeting, long circulating and the like, and being beneficial to clinical application.
Owner:SHANDONG UNIV
Connection type microbial cultivation device with embedded millipore filter and culturing method thereof
InactiveCN101109006AImprove trainabilityLow priceTissue/virus culture apparatusInterior spaceMicroorganism
The invention discloses a built-in microporous filter membrane connection microbe culture device belonging to the microbe culture technology, a culture method thereof, and a microbe culture, isolation and purification and observation technology in various environments by utilizing a culture medium. The culture device is provided with clapboard in an internal space composed of a dish body and a dish body lid, and the clapboard is fixed with a microporous filter membrane which divides the internal space of the culture device into two spaces. The two spaces are respectively used to bear the microbial culture group and provide a simulation external environment medium of a microchemistry matter with a microbial growth necessary factor. The dissolved matters at the two sides of the filter membrane are capable of freely exchange and the cell cannot permeate. Therefore, the culture device is capable of culturing more miceobes that the regular method can not culture. The invention applies the culture device to culture the microbes, which can effectively improve the culturablity of the environmental microbes, facilitate experiment system construction, make the operation easy and time-saving and has stronger industrialized application potention.
Owner:TSINGHUA UNIV
Microporous filter with an antimicrobial source
InactiveUS20100051527A1Improve prior art filtersRisk for releaseMembranesUltrafiltrationFluid filtrationMillipore Filters
A fluid filtration device having a fluid inlet and a fluid outlet and a confined fluid path between the inlet and the outlet through a microporous filter with a pore size adapted for filtering microbes, for example bacteria and virus. The device comprises an antimicrobial source, preferably halogen source, adding antimicrobial substance to the fluid in the confined fluid path between the fluid inlet end the microporous filter in order to prevent biofilm formation in the microporous filter.
Owner:VESTERGAARD FRANDSEN AS
Filtration and washing technology in preparing metal or non metal fine granular powder
ActiveCN1686593AOvercome the disadvantages of filtrationHigh yieldFiltration circuitsEngineeringSpherical form
A filtering and washing process for preparing the metallic or non-metal fine particles includes filtering, washing, blow-drying of filtered cake, discharging and cleaning filter. Said filtering step features use of the precise millipore filter with at least two filter regions for respective filtering. Said washing step includes reversely blowing the washing liquid to drop the filtered cake onto filter plate, instantaneously reversely blowing compressed air several times, and stirring the mixture of filtered cake and washing liquid.
Owner:ZHEJIANG DONGOU FILTERING MASCH MFG CO LTD
Method for determining size distribution of solid particles in catalytic cracking oil slurry
InactiveCN105004643AAvoid the problem of incomplete enrichmentFully reflect the particle size distributionParticle size analysisFiltrationSolid particle
The invention discloses a method for determining size distribution of solid particles in catalytic cracking oil slurry, and the method comprises the following steps: (1) carrying out a pretreatment for filter membranes of different apertures, and weighing for standby application; (2) carrying out a pretreatment for catalytic cracking oil slurry; (3) carrying out dilution for the oil slurry treated in the step (2), and carrying out ultrasonic concussion for the diluted oil slurry for standby; (4) filtering the oil slurry diluted in the step (3) with filter membranes of different apertures successively according to an application sequence from the ones with large apertures to the ones with small apertures; 5) carrying out a constant weight treatment of the filter membranes obtained after pumping filtration, and weighing after the constant weight treatment; (6) obtaining the mass of solid particles on each filter membrane according to mass differences of millipore filter membranes of different apertures before and after pumping filtration, acquiring the total mass of solid particles in the oil slurry by adding the additive operation, and calculating mass fractions of solid particles on millipore filter membranes of different apertures in total solid particles. The invention effectively solves the problem of incomplete gathering of solid particles in oil slurry, and can comprehensively reflect particle size distribution of solid particles in oil slurry.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Ropivacaine freeze-dried powder and injection preparation in use for injection and preparation method
InactiveCN1626081AAvoid decompositionReduce moisture contentPowder deliveryAnaestheticsFreeze-dryingDextran
A freeze-dried injection of ropivacaine is prepared from the ropivacaine methanesulfonate (or hydrochloride), diluent chosen from mannitol, lactose, sodium chloride, dextran, glucose, glycine, hydrolytic gelatin and povidone, isotonic regulator and pH regulator through dissolving them in the water for injection, stirring, cooling, adding the water for injection, adding activated carbon, adsorption, filtering for removing carbon, fitlering by millipore filter and freeze drying.
Owner:BEIJING BOERDA BIO TECH DEV
N(2)-L-alanyl-L-glutamine aseptic powdery preparation and process for prepairing same
InactiveCN1679531AOvercoming demandsOvercome can not be used directly as injection powderOrganic active ingredientsPowder deliveryL-alanyl-l-glutamineVacuum drying
An aseptic powder of N(2)-L-alanyl-L-glutamine is prepared through adding N(2)-L-alanyl-L-glutamine to distilled water in aseptic condition, stirring, heating for dissolving, adding medical activated carbon, stirring, filtering for removing activated carbon, millipore filtering for removing bacteria, adding aseptic alcohol to educe out crystals, filtering, washing with cold alcohol, and vacuum drying. It has high optical and thermal stability.
Owner:哈尔滨智诚医药科技研究院
Method for preparing cation polystyrene template based on porous materials
The invention discloses a method for preparing a cation polystyrene template based on porous materials, which comprises the following steps: by utilizing soap-free emulsion polymerization, after styrene monomer polymerization is carried out for 1.5 hours, starting to inject the mixture of cation DMC and deionized water (with volume ration being 2:1) into a reaction system by a micro sample injector. The first three times of injections are respectively carried out with the interval of half an hour, and the later two times of injections are carries out with the interval of one hour. Subsequently, a plurality of times of DMC mixtures can be continuously injected every three minutes, 50 Mul of the DMC mixture is injected each time, and the highest content of the injected DMC can reach up to 30-50% (compared with the mass of St). Aqueous millipore filter membrane (0.2 mu m) is then used for centrifuging and separating the prepared cation template, and finally a cation nano polystyrene template with monodispersity is obtained. The template can be applied to the synthesis of the porous materials, is extremely easy to be dissolved in alcohol and little dichloromethane solvent, and is beneficial to removing the template of the porous materials.
Owner:JIANGSU UNIV
Voriconazole phosphate ester for injection and preparation method thereof
ActiveCN101744778AImprove performanceNo pollution in the processOrganic active ingredientsPowder deliveryPhosphateMedical prescription
The invention provides voriconazole phosphate ester for injection and a medicinal salt thereof and a preparation method for the voriconazole phosphate ester for injection and the medicinal salt thereof. The preparation method comprises the following steps: adding 5 to 98 percent water for injection in a liquid preparation container; adding 90 to 110 percent of the accurate formula dosage of voriconazole phosphate ester and the medicinal salt thereof in the container; stirring the mixture; slowly dropwise adding a pH value regulator; regulating pH to between 6.0 and 11; supplementing water to the full dosage and then adding 0.01 to 1.0 percent (weight in volume) medicinal carbon into the product; stirring the mixture for 15 to 60 minutes; using a sand filter stick to carry out rough filtration and decarburization on the obtained product, and using a 0.22mum millipore filter to carry out fine filtration on the product until the clarity is qualified; after determining that the content of the midbody is qualified, determining the filling quantity and subpackaging the finished product in the vial; adding the semi-plug; carrying out freezing and drying on the sample; controlling the moisture content between 1 and 8 percent; pressing the plug; and carrying out capping.
Owner:HC SYNTHETIC PHARMA CO LTD
Nano particle intercalation graphene oxide thin film and preparation method and application
ActiveCN107720886AIncreasing interlamellar spacingSmall sizeCarbon compoundsGeneral water supply conservationSolventCvd graphene
Th invention discloses a nano particle intercalation graphene oxide thin film and a preparation method and application. The thin film is formed by spraying graphene oxide onto a millipore filter in anelectrostatic spraying mode to be stacked, nano particles are inserted between graphene oxide sheets, the particle size of the nano particles ranges from 10 nm to 40 nm, and the nano particles are hydrophilic nano particles. The preparation method comprises the steps that graphene oxide and the namo particles are added into a solvent to be dispersed uniformly, a mixed solution is obtained, and the nano particle intercalation graphene oxide thin film can be obtained by spraying the mixed solution to the millipore filter in the electrostatic spraying mode. The nano particle intercalation graphene oxide thin film can effectively increase the space between the graphene oxide sheets, the pure water flux is increased, and meanwhile the high retention rate is kept for organic dyestuff.
Owner:SHANDONG UNIV
Method and device for killing algae and bacteria in pipeline transportation of marine ballast water
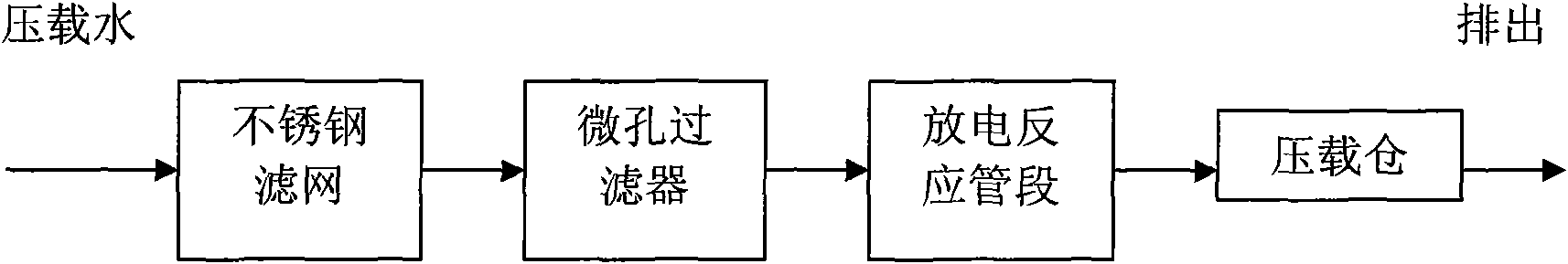
InactiveCN101781053AIncrease contact areaImprove utilization efficiencyMultistage water/sewage treatmentEnergy based chemical/physical/physico-chemical processesPipeline transportFiltration
The invention provides a method and a device for killing algae and bacteria in the pipeline transportation of marine ballast water. A millipore filter and a tubular high-voltage discharge reaction unit are connected on a transportation pipeline of the marine ballast water in series, the marine ballast water passes through the millipore filter, the diameter of filtration pores is 20 to 50 microns, and larger suspended matters and aquatic organisms entering a pipeline system of the marine ballast water are removed; and the filtered water enters the tubular high-voltage discharge reaction unit, pulse high voltage of 10 to 60 kilovolts is applied between a high-voltage electrode and a ground electrode of the tubular high-voltage discharge reaction unit, high-voltage pulse discharge is generated, the detention time of the ballast water in the tubular high-voltage discharge reaction unit is 0.1 to 2 hours, and the ballast water passing through the tubular high-voltage discharge reaction unit enters a ballast tank. The invention can kill or inactivate foreign pathogens and pests in the ballast water in a pipeline transportation system and lowers or eliminates the marine pollution caused after the ballast water is discharged.
Owner:HARBIN ENG UNIV
Preparation method of borate ion crosslinked conductive graphene paper
The invention discloses a preparation method of borate ion crosslinked conductive graphene paper. The preparation method comprises the following steps: preparing graphite oxide by adopting an improved Hummers method and ultrasonically stripping in deionized water to obtain graphite oxide hydrosol; then, adding a sodium hydroxide solution into the graphite oxide hydrosol to adjust the pH to 10-12; then adding boric acid; heating to 80-90 DEG C under a stirring condition; insulating heat for 3-5 hours; then naturally cooling; separating; washing; ultrasonically dispersing in deionized water; and finally filtering by a millipore filter and self-assembling layer by layer to obtain the borate ion crosslinked conductive graphene paper. The preparation method disclosed by the invention is simple, convenient to operate and low in cost, does not need special equipment and is easy for batch production. The obtained graphene paper has an excellent conductive performance and possibly can be used as an ideal conductive material for optoelectronic devices.
Owner:SOUTHEAST UNIV
Tea saponine nanocapsule, preparation method and application thereof
The invention discloses a tea saponine nanocapsule, a preparation method and application thereof, i.e. tea saponin is dissolved with water and is extracted with n-butyl alcohol, 1-5% of sodium hydroxide is added, the mixture is refluxed for 2-5h at 80-100 DEG C, and is decompressed to recycle the n-butyl alcohol, a solvent is used to dissolve the sediment, then crystallization and drying are carried out, so the tea saponine is obtained; and a capsule material is produced into 1-10% of capsule material solution with the solvent, 0.5-5% of emulsifier and 1-10% of tea saponine are added to be stirred and emulsified for 0.5-2h, the emulsion is slowly added into aqueous solution with the pH of 2-6 and 0.1-1% of emulsifier to stir for 1-3h, the mixture passes a millipore filter and is stood for layering, the sediment is dried, i.e. the tea saponine nanocapsule is obtained. The nanocapsule can be produced into sterilizing sterilized, anti-inflammatory and immunity supplement preparation. The nanocapsule improves the stability of the tea saponine, and obviously improves the sterilizing, anti-inflammatory and immunity supplement effects.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing ornithine aspartate powder injection for injection
InactiveCN101843587AReduce manufacturing costSimple production equipmentOrganic active ingredientsPowder deliveryDepyrogenationBarium hydroxide
The invention discloses a method for preparing ornithine aspartate powder injection for injection. The method comprises the following steps of: taking arginine, adding the solution of barium hydroxide, stirring to dissolve the arginine, and heating to hydrolyze so as to completely transform the arginine into ornithine; adding aspartate, uniformly stirring, adding a precipitator, filtering to remove barium ion precipitate, and regulating the pH value of the solution to neutrality; adding an inert organic solvent for crystallization, filtering and pumping, adding water for injection to dissolve the crystals, adding active carbon for needle for decoloration and depyrogenation, coarsely filtering to remove carbon, and filtering to make the solution sterile and clarified by using a 0.22 mu m millipore filter; and adding ethanol for crystallization, obtaining ornithine aspartate sterile powder, packaging the powder into a sterilized vial, capping, and rolling an aluminum cover to obtain the ornithine aspartate sterile powder injection for injection. The method has the advantages of increasing the yield, preparing the ornithine aspartate by the one-pot method, along with easy operation, simple and convenient operation, low cost, short production period, and suitability for mass production.
Owner:HUBEI HOPE PHARMA
Fluorescent disperse dye ink for inkjet printing and preparation method of fluorescent disperse dye ink
The invention discloses fluorescent disperse dye ink for inkjet printing and a preparation method of the fluorescent disperse dye ink. The ink consists of a disperse dye, a fluorescent agent, a versatile dispersant, an organic solvent, a wetting agent and a surfactant. The preparation method comprises the following steps: stirring the disperse dye, the fluorescent agent, sodium lignin sulfonate, diethylene glycol and deionized water at a low speed by using a stirrer, and grinding in a grinding dispersion mill, thus obtaining disperse dye mill base with a solid content of 15 percent; stirring the dye mill base and the deionized water in the stirrer, adding an organic solvent formed by mixing isopropanol, thiodiglycol and biglycol ether and the wetting agent and the surfactant, and adding a preservative after the PH value is regulated to be 8-9; and filtering the mixed ink by using a millipore filter membrane, and further filtering the filtrate through the millipore filter membrane. The fluorescent disperse dye ink can be subjected to jet printing onto heat transfer paper from a piezoelectric inkjet printing machine, or is directly subjected to jet printing onto a chemical and blended fabric, and is suitable for large-scale industrial production.
Owner:绍兴旭泰纺织品有限公司 +1
Lansoprazole freeze-dried injection
ActiveCN101313895AGood resolubilityGood substance contentOrganic active ingredientsPowder deliveryForeign matterFiltration
The invention relates to a lansoprazole frozen powder injection agent and a preparation method thereof. The lansoprazole frozen powder injection agent prepared by the method can be taken as a medicine for treating gastric ulcer, duodenal ulcer, erosive gastroesophageal reflux disease, helicobacter pylori and Zollinger-Ellison Syndrome. The lansoprazole frozen powder injection agent prepared through taking a lansoprazole as an active ingredient, adopting crystallized filtration, freezing and drying. The preparation process comprises the following steps of: adding water for injection and alkali to the lansoprazole, stirring to dissolve and mix uniformly; performing the crystallized filtration; after measuring the content, adjusting pH value of filtrate; using the water for injection for fixing volume and a millipore filter with 0.22 mu m for filtration; filling, partially arranging corks, arranging in trays, freezing out, pressing the corks, taking out from boxes, rolling openings, checking quality and packaging. The prepared lansoprazole frozen powder injection agent has excellent re-dissolving performance, is more stable when used together with transfusion in clinical use, can not separate out, and has better inspection result for visible foreign matters and insoluble particles.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Device for evaluating dissolving/absorbing process of medical solid preparation
The invention provides a device for evaluating the dissolving / absorbing process of medical solid preparation, essentially consisting of a medicine dissolving room, a pH value adjusting room and a spreading pool, wherein the bottom of the medicine dissolving room is provided with a magnetic stir bar; the spreading pool consists of a feeding room, a receiving room and Caco-2 monolayer cell membraneor animal (such as rat) isolated intestine embedded between the feeding room and the receiving room; the medicine dissolving room, pH value adjusting room, the feeding room and the receiving room areconnected with each other by silicone tubes with inner diameter of 1.0mm-2.0mm; and the medicine dissolving room is provided with a medicine carrying device; a gap is remained between the medicine carrying device and the magnetic stir bar at the bottom of the medicine dissolving room; the top of the pH value adjusting room is provided with a stainless steel sieve mesh; and the silicone tube between the pH value adjusting room and the feeding room is internally additively provided with a Millipore filter. The device is applicable to wider solid preparation such as troche, soft capsule, hard capsule, and the like, solves the blocking problem caused by medical auxiliary materials, and truly simulate the affect of the pH change of gastrointestinal tract to the releasing rule of the medical solid preparation under the condition of human physiology.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Resuscitation fluids of VBNC salmonella and preparation method thereof and resuscitation method
InactiveCN101423808ASave raw materialsSimple and fast operationBacteriaMicroorganism based processesSterile waterCulture mediums
The invention discloses a recovery liquid for VBNC salmonella, a method for preparing the same and a reclaiming method. The recovery liquid consists of 1 portion of serum and 1 to 3 portions of sterile water which are mixed and stirred evenly and are filtered for sterilization through a millipore filter. The reclaiming method comprises the following steps: placing the salmonella in a VBNC into the recovery liquid and adopting an incremental temperature raising mode in pores of a thermal program control circulator, wherein the recovery temperature rise and an incubation time program of the thermal program control circulator are at a temperature of between 5 and 37 DEG C, and the incubation time is between 2 minutes and 1.5 hours; and then inoculating the salmonella in an SS liquid culture medium, and culturing the salmonella in a gas bath shaking table at a speed of between 200 and 220 revolutions per minute. The invention overcomes the defects that the prior art uses a plurality of materials, is inconvenient to get materials and the like; the invention only uses one material to recover the salmonella which is in the VBNC for nearly one year through procedural temperature rise; and few materials are used and the operation is simple and convenient, the concentration of the recovered salmonella can reach 10<8> CFU per milliliter in 12 hours, and each recovery index (time, bacterial population and the like) is greatly improved compared with that of the prior art.
Owner:JILIN AGRICULTURAL UNIV
Test and evaluation method of density of fibers sucked from cigarettes or filter sticks
InactiveCN102788867AIntuitive calculation of aspirated fiber concentrationAspirated fiber concentration calculationMaterial analysisMicroscopic observationEngineering
A test and evaluation method of density of fibers sucked from cigarettes or filter sticks is characterized in that a millipore filter is covered on the surface of a filtering piece of a suction device, corresponding parameter is adopted on the suction device to conduct suction on the cigarettes or the filter sticks, a microscope is utilized to observe the number of silk fibers on the catching face of the filter after suction is finished, and ratio of the number of the fibers on the filter and the suction total capacity is utilized to calculate the density of the fibers sucked from the cigarettes or the filter sticks. The test and evaluation method has the advantages of being capable of intuitively calculating the density of the fibers sucked from the certain amount of cigarettes or filter sticks, being capable of obtaining size information of the fibers while quantitatively calculating the density of the fibers and being simple, convenient to operate and easy to popularize.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Leech aquarium and leech cultivation method
The invention discloses a leech aquarium. The leech aquarium comprises a closed aquarium body, the interior of the aquarium body is divided into a water pump chamber and a cultivation chamber left and right, the top of the water pump chamber and the top of the cultivation chamber are respectively provided with a cabin door, a water pump is arranged in the water pump chamber, a water outlet of the water pump is communicated with the cultivation chamber through a water outlet pipe, and an outlet of the water outlet pipe is provided with a Millipore filter screen which can prevent leeches from entering the water pump. A three-dimensional leech cultivation method based on the leech aquarium includes the steps that leech fries are thrown into the cultivation chamber of the leech aquarium, the stocking density is 1.5-2 times that of conventional cultivation, namely the density is 0.02-2.3 kg / m<2>, the fries are thrown in two batches, namely, the second batch is thrown when about one third of the leech fries are adsorbed on aquatic plants, feeding is performed regularly, the leech fries are fed according to a conventional method, the aquarium is placed indoors, the temperature is suitable for the leech fries to grow, particularly in winter, the leech fries in the cultivation chamber are located in a closed space and can not escape, and therefore economic benefits are improved.
Owner:SOUTHWEST UNIVERSITY
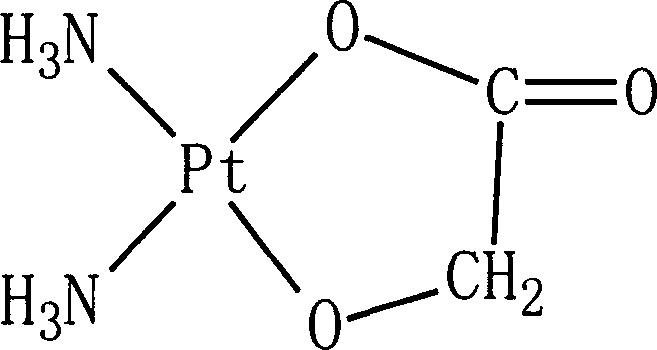
Preparation of nedaplatin freeze-dried powder injection
ActiveCN101015539AImprove stabilityLow content of related substancesPowder deliveryLyophilised deliveryFreeze-dryingBottle
This invention relates to a preparation method of freeze-dried injection of nedaplatin.nedaplatin freeze-dried injection prepared with the inventive method can be used as therapeutic drug of cancer. The invention improves the stability of nedaplatin during lyophilization and shortens the period of lyophilization through adding ethanol during preparation process. the preparation method comprises adding water for injection 80% of nedaplatin into nedaplatin, stirring for dissolving, adding dextran, stirring for dissolving, determing the content of intermediate, adding ethanol 1-10% of the cumulative volume of the solution, adding water for injection to full dose, filtrating with 0.22 mu m millipore filter under aseptic condition, encapsulating in sirin bottle, plugging, freeze drying, rolling the opening, testing, and packaging. The optimum amount of ethanol is 1-5% of cumulative volume of the solution.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Targeted hydrophobic antitumor drug nano preparation and preparation method thereof
ActiveCN105412024AUniform particle size distributionEnhanced inhibitory effectOrganic active ingredientsPowder deliveryDrugs solutionMedicine
The invention relates to a targeted hydrophobic antitumor drug nano preparation. The nano preparation comprises a hydrophobic antitumor drug and a carrier in a mass ratio being 1: (4-32.5), wherein the carrier comprises 37.5wt%-95.3wt% of albumin and 4.7wt%-62.5wt% of a hyaluronic acid-albumin conjugate, and the hyaluronic acid-albumin conjugate is prepared from albumin and hyaluronic acid in a mole ratio being 1: (1-20). The preparation method of the nano preparation comprises steps as follows: preparing the hyaluronic acid-albumin conjugate; preparing a carrier solution; preparing a drug solution; preparing the nano preparation. The nano preparation has more uniform particle size distribution and good dispersability, is stable and avoids agglomeration, the particle size is nearly unchanged after freeze-drying and redissolving, and the yield is high after the nano preparation is filtered by a millipore filter (0.22 mu m), and the pharmacological function is good.
Owner:AC PHARMA CO LTD
Preparing method for ambroxol hydrochloride injection
ActiveCN103126978ASimple production processImprove product qualityOrganic active ingredientsPharmaceutical delivery mechanismNitrogen gasMedical prescription
The invention discloses a preparing method for ambroxol hydrochloride injection. The preparing method for the ambroxol hydrochloride injection includes the following steps that each 2ml of the injection comprises, by weight, 15 mg of ambroxol hydrochloride, 4.0 mg of disodium hydrogen phosphate, 1.16 mg of citric acid and 14 mg-16 mg of sodium chloride; water for injection is cooled to be below 30 DEG C; the disodium hydrogen phosphate and the citric acid are added into the water for injection to be dissolved; a prescribed dosage of ambroxol hydrochloride raw medicine is added to be dissolved; the sodium chloride is added to adjust solution to be isotonic; the solution is filtered through a 0.22-micrometer millipore filter; and the solution is filled and sterilized after high purity nitrogen gas is let in. The preparing method for the ambroxol hydrochloride injection is simple in process and stable in product quality.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Method for analyzing content of adenosine and cordycepin in cordyceps militaris by virtue of high performance liquid chromatography (HPLC)
InactiveCN102928526AOptimize extraction conditionsEasy to handleComponent separationBiotechnologyAdenosine
The invention discloses a method for analyzing the content of adenosine and cordycepin in the sporocarp of cordyceps militaris through artificial culture by virtue of high performance liquid chromatography (HPLC). The method comprises the following specific steps: 1, weighing the dried sporocarp powder of cordyceps militaris in a 50ml centrifuge tube with a plug, adding water, ultrasonically extracting the centrifuge tube in water bath, then centrifuging and taking a supernate as a test solution; 2, weighing a certain amount of standard substances of adenosine and cordycepin, adding water and dissolving to prepare a mixed solution as a mixed standard solution; 3, performing content measuring, namely taking the mixed standard solution and test solution respectively, filtering the solutions through a 0.45um aqueous phase millipore filter and injecting the filtered solutions into a small bottle, and measuring the chromatographic peak of the cordyceps militaris sporocarp test solution and the adenosine cordycepin standard solution with the same chromatographic retention time. The invention provides a new, simple and convenient method for evaluating the quality of the cordyceps militaris sporocarp.
Owner:SHANGHAI JIAOTONG UNIV
Rotational flow microporous filter
ActiveCN1958114AReduce pollutionAvoid depositionFiltration circuitsNon-miscible liquid separationCycloneSewage
A millipore filter with cyclone is composed of 4 modules, a millipore tube with small conic tube, a cyclone tube, middle bulkhead, inlet pad of cyclone cavity, top cover plate of cyclone cavity, and the screw bolts for connecting said units together to form a raw water cavity and a filtered water cavity. It has high filter precision and no pollution to membrane.
Owner:DAQING OILFIELD CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com