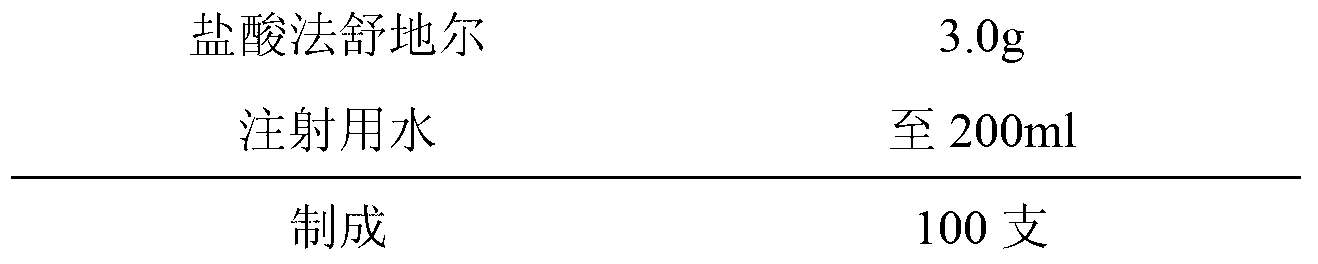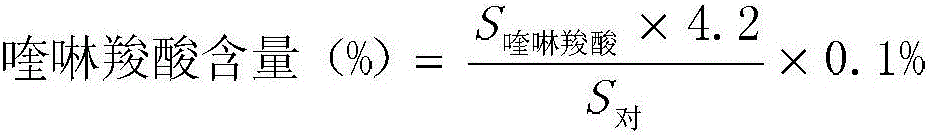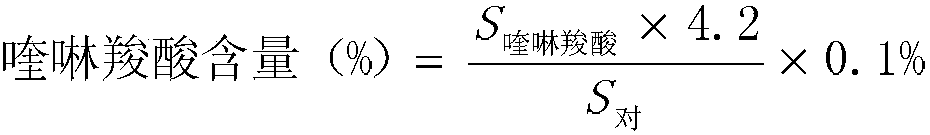Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
62 results about "Sodium calcium edetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
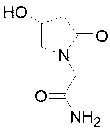
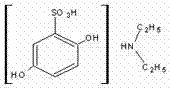
Sodium calcium edetate (sodium calcium EDTA), also known as edetate calcium disodium among other names, is a medication primarily used to treat lead poisoning. This includes short term and long term lead poisoning. For lead encephalopathy it is typically used together with dimercaprol. It does not appear to be useful for tetraethyllead toxicity. It is given by slow injection into a vein or into a muscle.
Efficient and environment-friendly remains preservative and application thereof
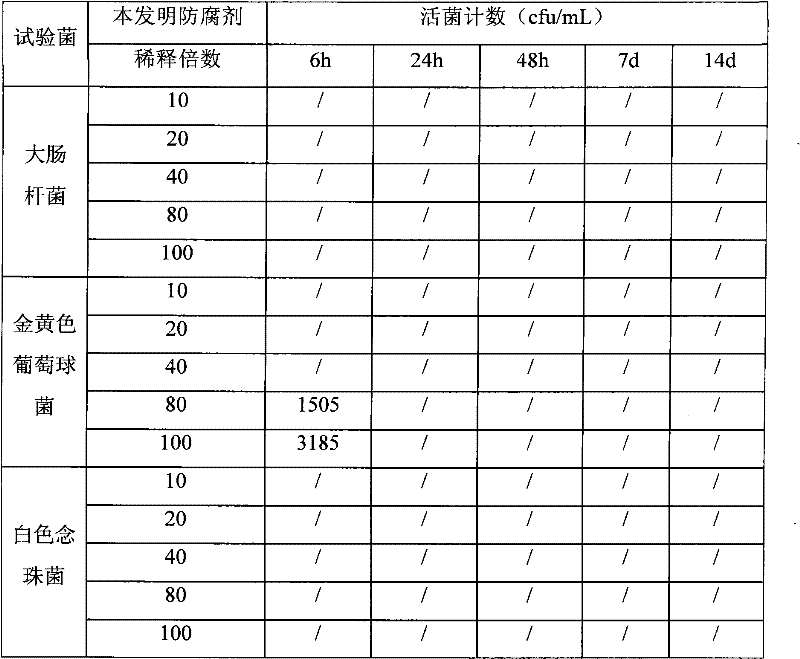
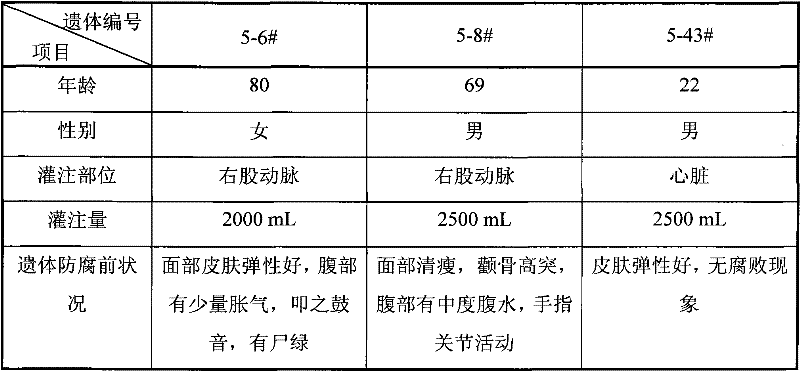
The invention relates to an efficient and environment-friendly remains preservative free of formaldehyde and application thereof. The remains preservative mainly comprises, by volume, 0.5-10% of phenoxyethanol, 0.2-1.5% of isothiazolinone, 3-15% of organic acid, 5-20% of propylene glycol, 5-30% of glycerol, 20-75% of ethanol and 5-35% of distilled water, and, by weight, 1-10% of hexamethylenetetramine and 0-2% of ethylenediamine tetraacetic acid disodium salt. The preservative can be poured through an aorta and an aortic arch and injected through the abdominal cavity, the thoracic cavity, the pharyngeal cavity and the cranial cavity so as to carry out antiseptic preservation, disinfection and smell removal to remains. Quantification sterilization experiments, animal experiments and funeral home field experiments prove that the remains preservative is efficient in antibacterial property, quick in action speed, low in toxicity and suitable for short-term or long-term remains antisepsis or specimen preservation of funeral homes and medical colleges at home and abroad.
Owner:民政部一零一研究所
Omeprazole sodium freeze-dried powder injection and preparation method thereof
ActiveCN101703483AReduce the risk of adverse reactionsComply with the requirements of human intravenous injectionPowder deliveryOrganic active ingredientsOmeprazole SodiumFiltration
The invention discloses an omeprazole sodium freeze-dried powder injection and a preparation method thereof. The omeprazole sodium freeze-dried powder injection contains an active ingredient, namely, omeprazole sodium monohydrate, and auxiliary materials, namely, calcium disodium edetate and sodium hydroxide. The preparation method of the omeprazole sodium freeze-dried powder injection is characterized by comprising the following steps: weighing the calcium disodium edetate of prescription amount and dissolving the calcium disodium edetate in water for injection, stirring, dissolving, and regulating pH value to 10.0-12.0 by using 10% of sodium hydroxide solution; weighing omeprazole sodium of the prescription amount and adding the omeprazole sodium in the mixture, stirring at room temperature for dissolution, supplementing and adding the water for injection to full amount; adding active carbon, stirring at room temperature for decoloration and endotoxin removal, conducting rough filtration to remove carbon firstly, and then conducting refining filtration by using a filter membrane of 0.22 Mum; taking refining filtrate to test intermediate, conducting encapsulation after meeting requirements; and freeze-drying and unboxing, thus obtaining the omeprazole sodium freeze-dried powder injection. The freeze-drying technology of the omeprazole sodium freeze-dried powder injection takes temperature below minus 40 DEG C as pre-freezing temperature; after pre-freezing for at least two hours, sublimation is started, wherein the sublimation temperature is 5-12 DEG C, the sublimation time is over 14 hours; and then drying is conducted for over 2 hours at the temperature of 20-35 DEG C. Unboxing is carried out after a stopper is added and a cover is put in place, thus obtaining the finished product of the omeprazole sodium freeze-dried powder injection.
Owner:HAINAN LEVTEC PHARMA
Levofloxacin hydrochloride medicinal composition for injection and preparation method thereof
InactiveCN102000083AMature technologyEasy to operateAntibacterial agentsOrganic active ingredientsSodium calcium edetatePharmaceutical Substances
The invention relates to a levofloxacin hydrochloride medicinal composition for injection and a preparation method thereof. The levofloxacin hydrochloride medicinal composition for injection comprises levofloxacin hydrochloride with curative dose and calcium disodium edetate and cysteine hydrochloride which can inhibit photolysis. The levofloxacin hydrochloride medicinal composition for injection adopts a special process and selects the calcium disodium edetate and the cysteine hydrochloride as a combinational antioxidant, so the stability of products is improved greatly. The invention also discloses the preparation method of the medicinal composition.
Owner:TIANJIN HANKANG PHARMA BIOTECH
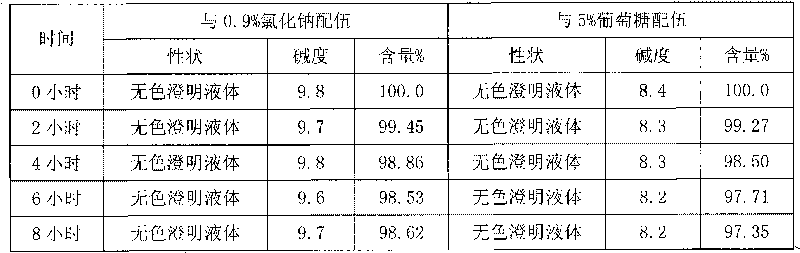
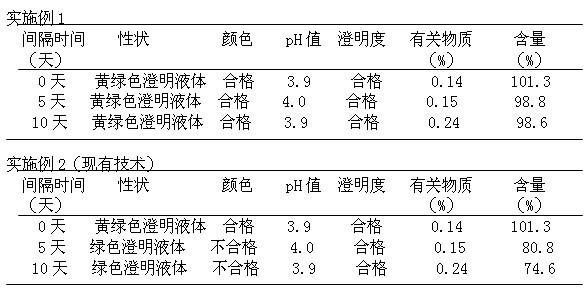
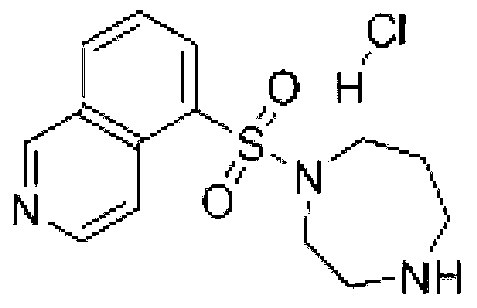
Fasudil hydrochloride injection composition and its preparation method
ActiveCN103222953AReduce contentSimple prescriptionOrganic active ingredientsPharmaceutical delivery mechanismSodium calcium edetateMedical prescription
The invention provides a novel Fasudil hydrochloride injection composition and its preparation method. A prescription and a preparation technology are simple. As a metal ion chelating agent, sodium calcium edetate in the prescription can be used to reduce degradation of the product under a high-light condition. In addition, the preparation technology provided by the invention is easy to operate. The quality of the prepared product is controllable, and the product has good stability and meets regulations of SFDA (State Food and Drug Administration).
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Exterior medicine composition of fluocinolone acetonide and ester of fluocinolone acetonide
The invention provides an exterior medicine composition of fluocinolone acetonide and ester of fluocinolone acetonide. The exterior medicine composition comprises the fluocinolone acetonide or the ester of the fluocinolone acetonide serving as the active component, and one or more auxiliary materials applied to skin; and the exterior medicine composition is characterized in that the auxiliary materials for skin include sodium calcium edetate or edetate disodium.
Owner:天津金耀药业有限公司
Stable parecoxib sodium pharmaceutical composition for injection
InactiveCN104434815AHigh yieldReduce market riskPowder deliveryAntipyreticParecoxib sodiumPharmaceutical drug
The invention relates to a stable parecoxib sodium pharmaceutical composition for injection. The pharmaceutical composition specifically includes parecoxib sodium and injection additives. Disodium hydrogen phosphate and sodium calcium edentate are adopted as the buffering agent, and sodium hydroxide is taken as the pH regulator. The production process is feasible, the quality is stable and controllable, the skin irritation is small, and at the same time the composition is convenient for transportation and storage. The invention provides the reasonable preparation prescription and preparation technology for clinical medication, and industrialization is easy to realize.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Modified silicon dioxide microspheres for absorbing heavy metal lead ion
InactiveCN106799209AGood dispersionGood emulsifying effectOther chemical processesWater contaminantsMicrosphereMordenite
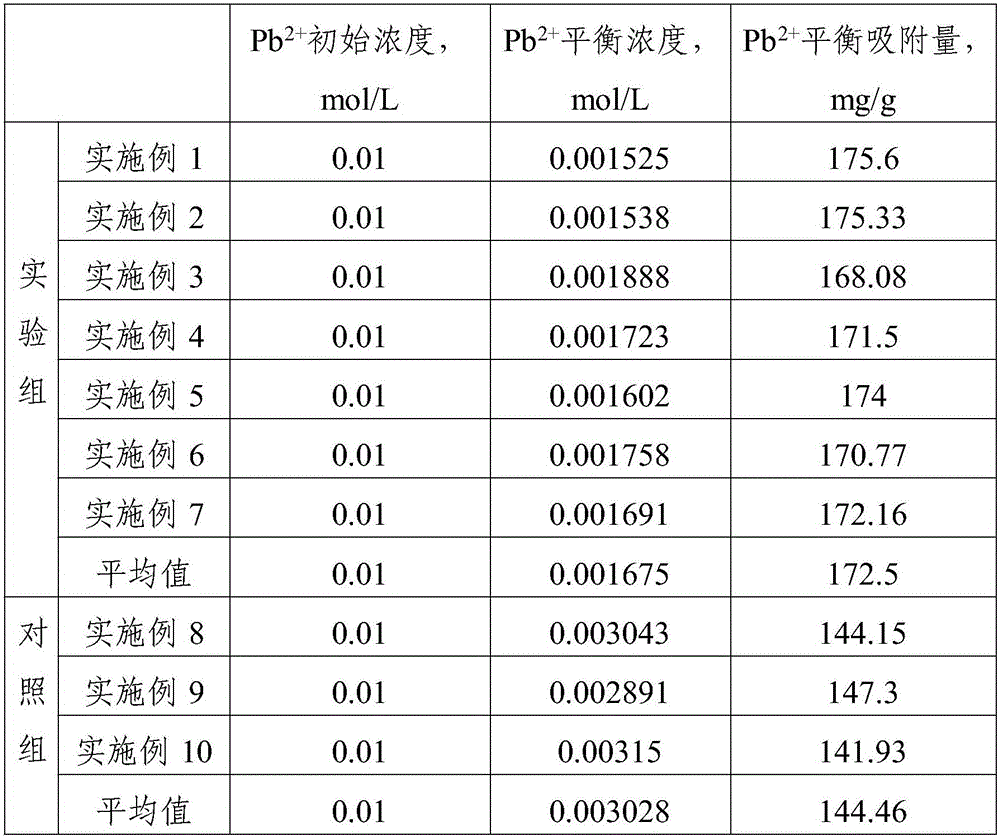
The invention provides a modified silicon dioxide microspheres for absorbing heavy metal lead ions. The modified silicon dioxide microspheres are prepared from the following raw materials in parts by weight: 8-10 parts of water glass, 12-15 parts of tetraethoxysilane, 3-6 parts of sodium calcium edetate, 4-6 parts of triethanolamine, 3-9 parts of ammonia water, 5-8 parts of humic acid, 3-7 parts of a magnetic nucleating agent, 4-10 parts of organobentonite, 15-25 parts of ethyl alcohol, 4-7 parts of aminopropyl trimethoxysilane, 3-8 parts of mordenite and 4-7 parts of fatty alcohol-polyoxyethylene ether. A magnetic nucleating agent is coated with water glass and tetraethoxysilane as silicon sources to prepare the silicon dioxide microspheres; the silicon dioxide microspheres are filled and modified by an adsorption material; and the adsorption rate of the prepared modified silicon dioxide microspheres reaches 172.5mg / g.
Owner:吴中区穹窿山天仲高分子材料技术研究所
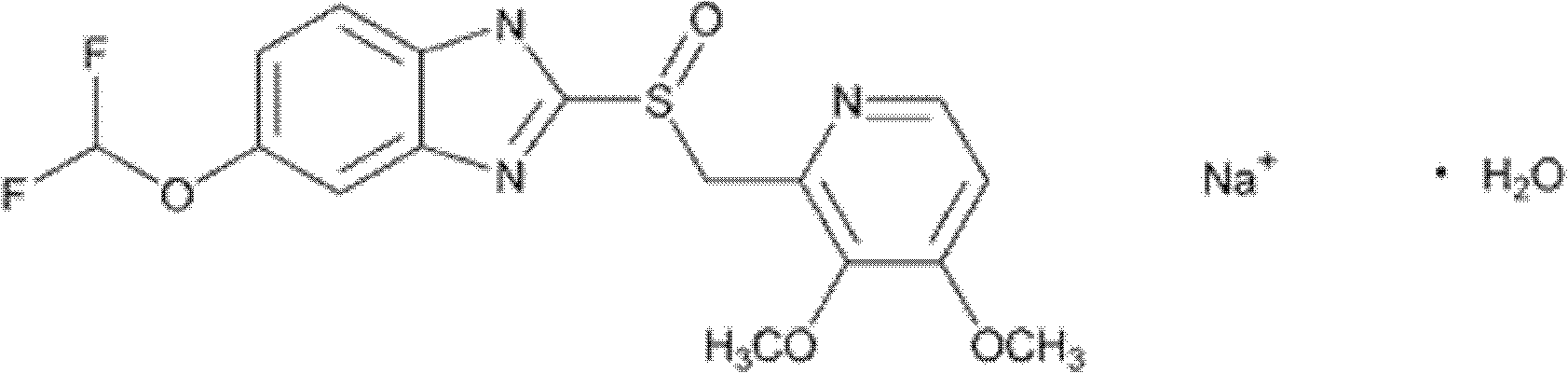
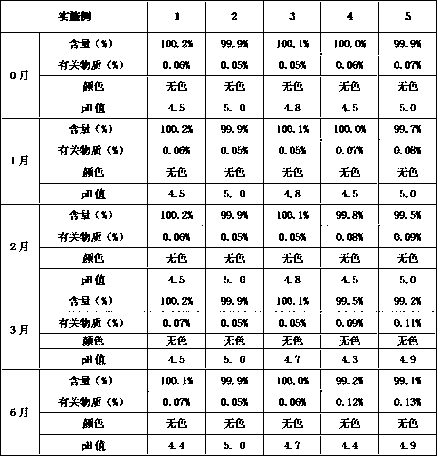
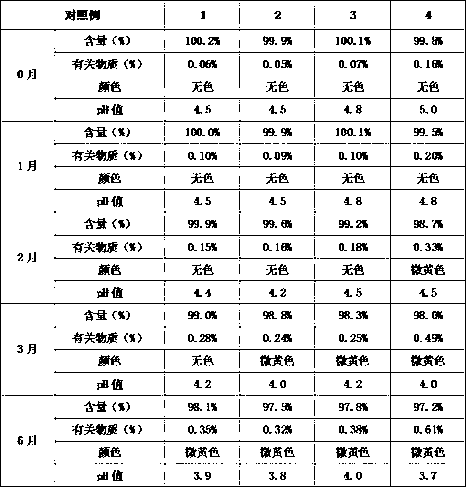
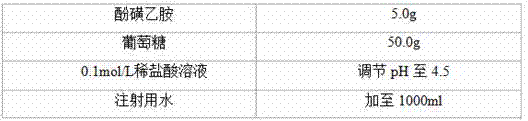
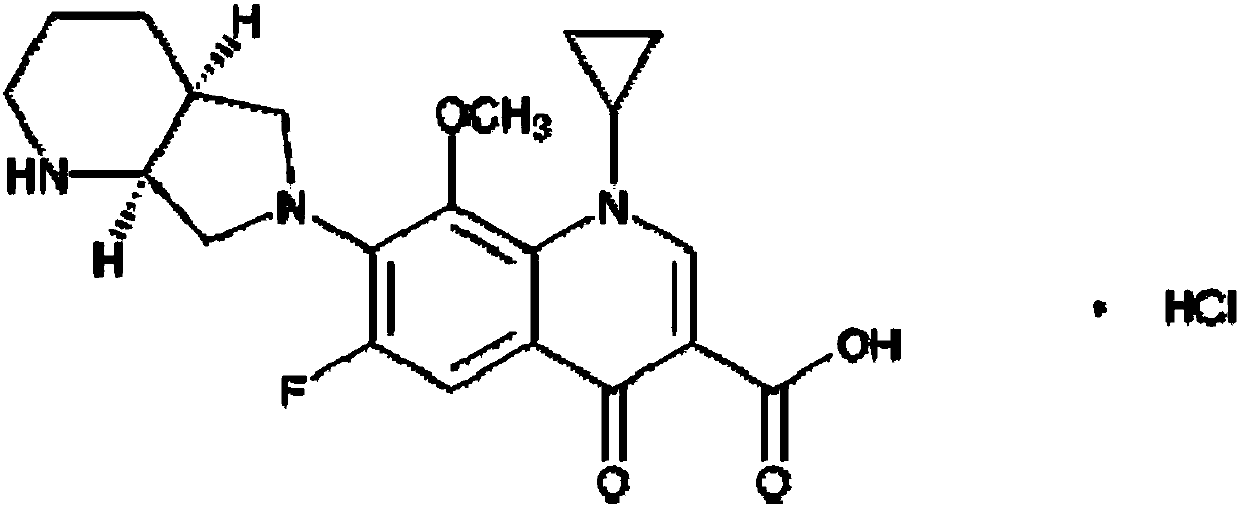
Detection method and content determining method of sodium calcium edetate in pantoprazole sodium for injecting
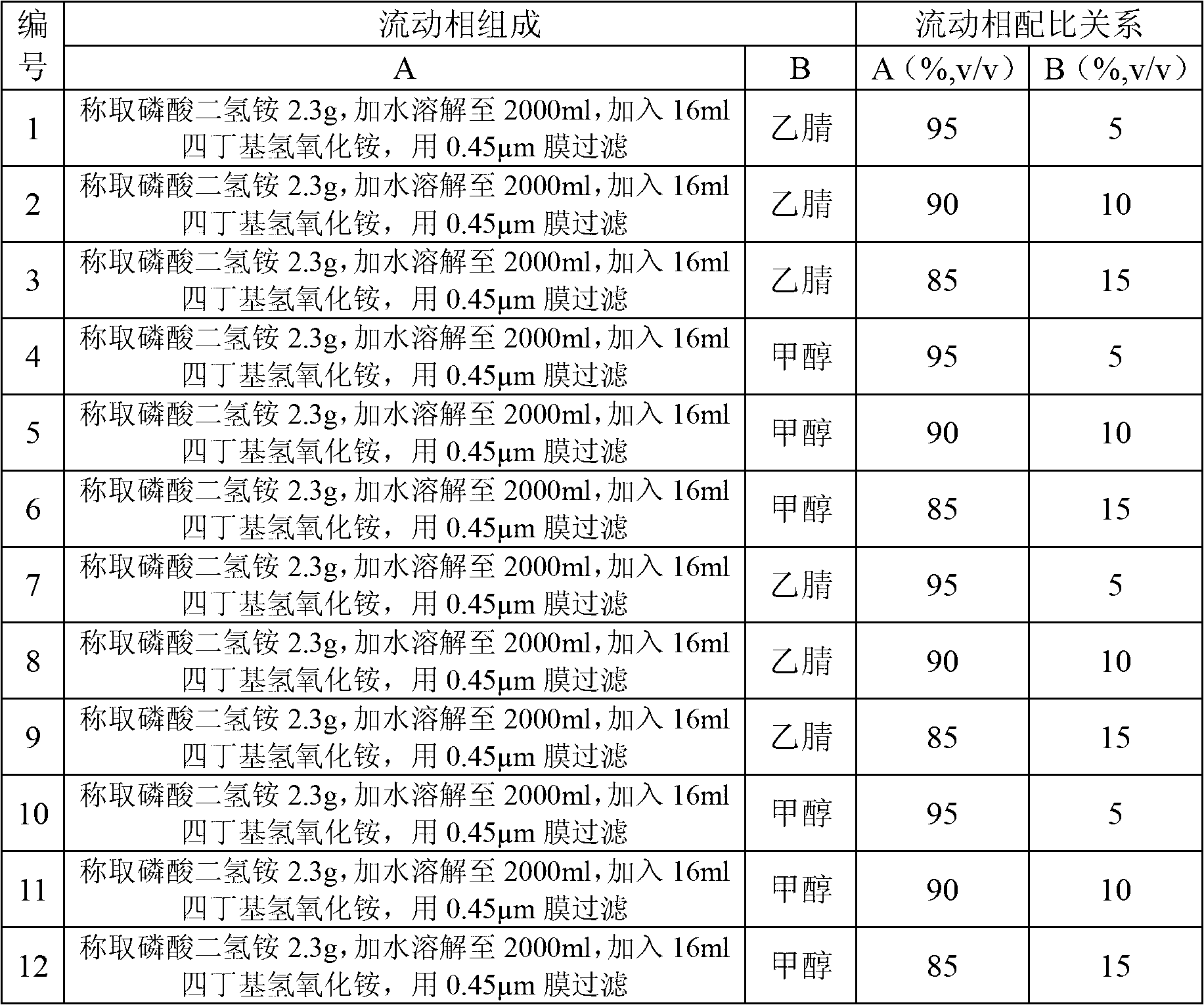
The invention belongs to the field of medicament analysis and particularly relates to a detection method and a content determining method for sodium calcium edetate as an accessory in pantoprazole sodium for injecting. The invention aims at solving the technical problem of providing a method with the advantages of simpleness in and convenience for operation, quickness and accuracy for detecting the sodium calcium edetate as the accessory in the pantoprazole sodium for injecting. HPLC (High Performance Liquid Chromatography) detection conditions are as follows: a stationary phase: octadecylsilane bonded silica gel is used as a filling agent; a mobile phase: in terms of volume, an ion pair buffer solution is 85-95 percent, acetonitrile is 5-15 percent and the pH value is adjusted to 2.2-2.6 with phosphoric acid; the flowing speed is 0.8-1.2 ml / min; the column temperature is 30-40DEG C; the detection wavelength is 250-260 nm; and the theoretical plate number is required not to be lower than 2000 calculated in terms of a sodium calcium edetate peak. The detection method has the advantages of simple and convenient operation, accurate and reliable determination result, stronger specificity and shorter detection time; and the retention time of a main peak is about 6 minutes.
Owner:CHENGDU BAIYU JINGELAI PHARMA CO LTD
Pantoprazole sodium freeze-dried powder injection and preparation method thereof
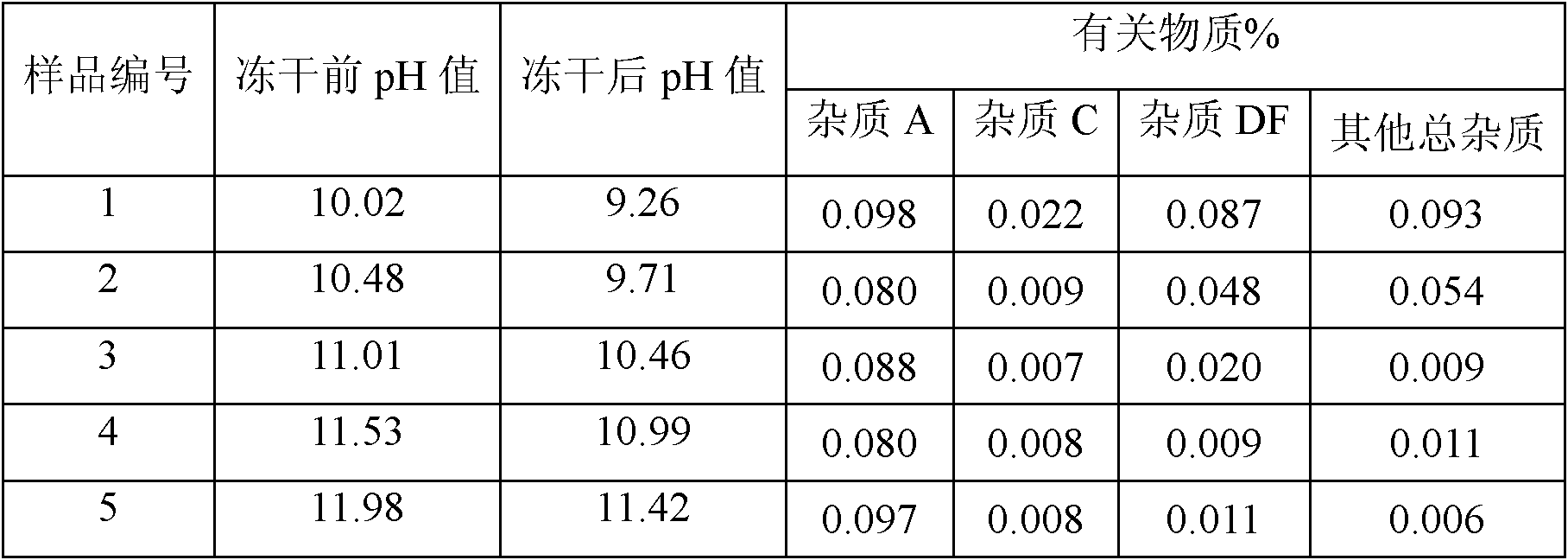
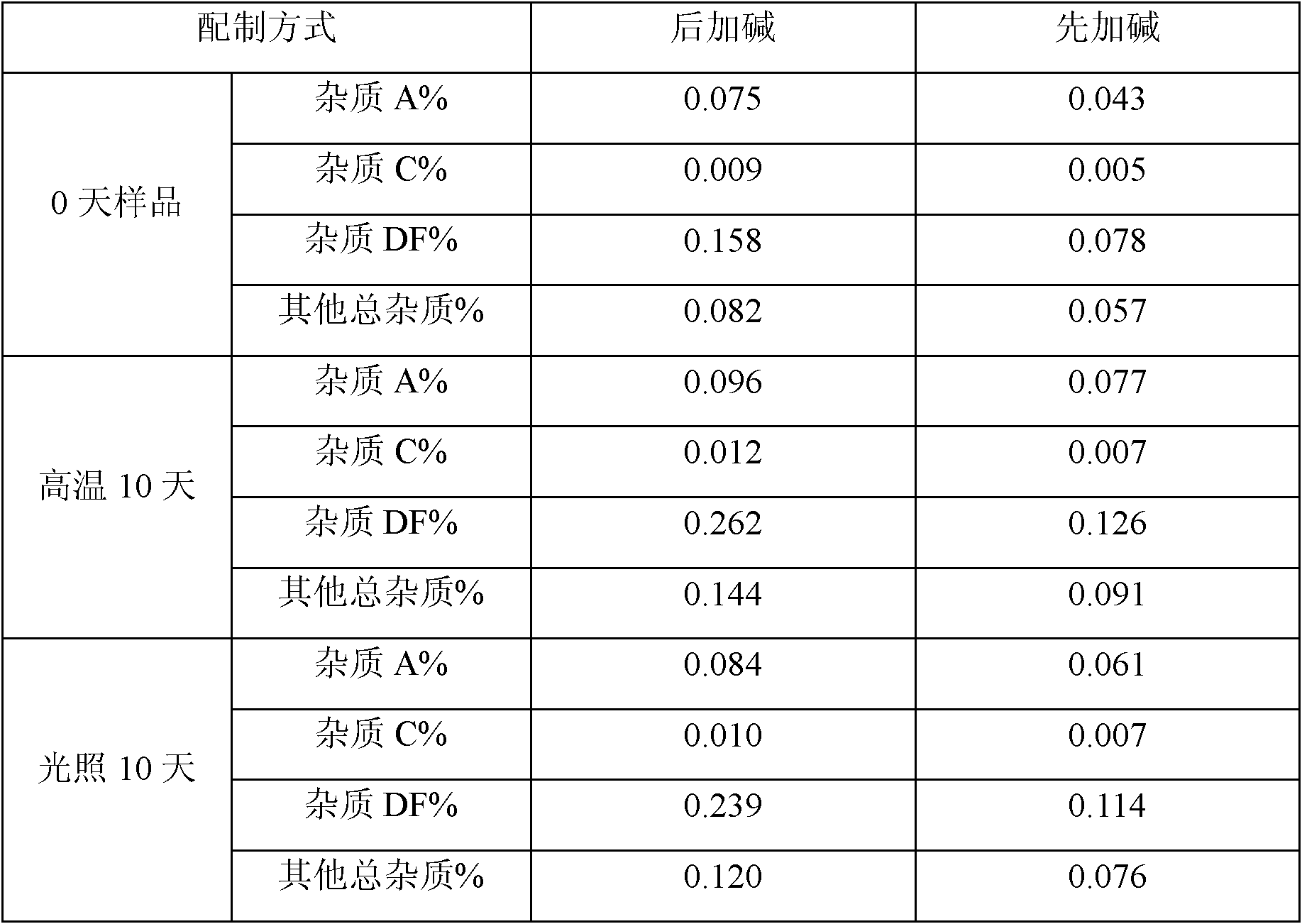
InactiveCN102552186AAvoid low calciumRaise the pHPowder deliveryOrganic active ingredientsFreeze-dryingPantoprazole
The invention belongs to the field of pharmacy and in particular relates to a pantoprazole sodium freeze-dried powder injection and a preparation method thereof. The invention achieves the purposes of increasing the pH value of the solution before freeze-drying, reducing the production of foreign substances before freeze-drying of the product, and improving stability. The freeze-dried powder injection contains the following components in parts by weight: 1 part of pantoprazole sodium, 0 to 3 parts of cyoprotectants, 0.025 to 0.1 part of sodium calcium edetate and an appropriate amount of pH regulators. The pH value of the solution before freeze-drying is increased to aid the control of foreign substances before freeze-drying of the product. Besides, sodium calcium edetate is used as a metal ion complexing agent for the preparation of pantoprazole sodium freeze-dried powder injection, which protects the product from metal complexation and prevents the injection from chelating with calcium ions during intravenous infusion to avoid low calcium levels.
Owner:成都金典药物科技开发有限公司
Adapalene gel
ActiveCN103099775AOrganic active ingredientsPharmaceutical delivery mechanismSodium calcium edetateALLYL SUCROSE
The invention provides an adapalene gel. The adapalene gel comprises adapalene serving as an active ingredient, water, humectant, surface active agent, carbopol and neutralizer, wherein the water, humectant, surface active agent, polyacrylic acid and neutralizer are adopted as the auxiliary materials; and the adapalene gel is characterized by comprising sodium calcium edetate.
Owner:TIANJIN JINYAO GRP
Flurbiprofen cataplasm
InactiveCN107157962AFast transdermal absorptionLow drug releaseOrganic active ingredientsAntipyreticCarboxymethyl celluloseGlycine
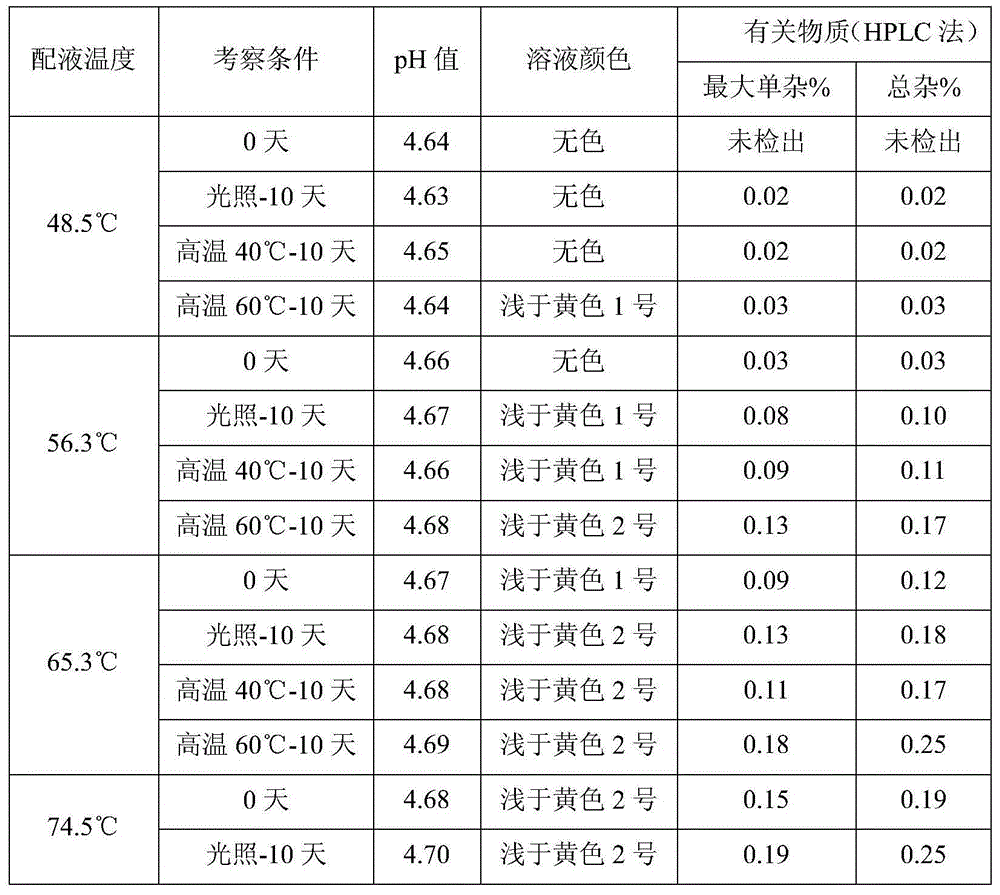
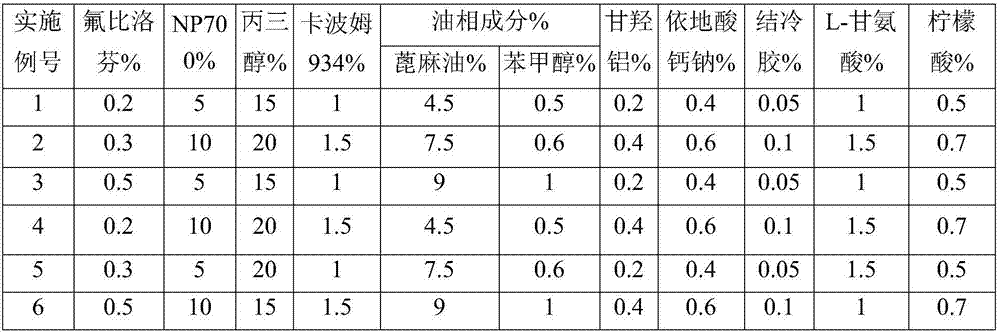
The invention provides flurbiprofen cataplasm. The cataplasm is composed of a backing layer, a drug reservoir and a protective layer. The cataplasm is characterized in that the drug reservoir is prepared from the following components in percentage by weight: 0.2%-0.5% of flurbiprofen as an active component, 5%-10% of an oil phase component, 5%-10% of partially neutralized sodium polyacrylate as an aqueous phase component, 15%-20% of glycerol, 0.2%-0.4% of aluminum glycinate, 0.1%-0.3% of sodium calcium edetate, 1%-1.5% of carbomer 934, 1.5%-3% of sodium carboxymethyl cellulose (CMC-Na), 0.05%- 0.1% of a pH adjusting agent and gellan gum, 1%-1.5% of L-glycine, 1%-3% of a filler and the balance of water, wherein the oil phase component is prepared from castor oil and benzyl alcohol, the ratio of castor oil to benzyl alcohol is 1 to (0.08 to 0.12), the aqueous phase component and water form hydrogel, the filler is arranged in the hydrogel dispersedly, and the oil phase component is emulsified and disperses in the hydrogel to form the drug reservoir.
Owner:北京茗泽中和药物研究有限公司
Perhexiline injection liquid and preparation method thereof
InactiveCN104415091AQuality improvementImprove clinical drug safetyInorganic non-active ingredientsPharmaceutical delivery mechanismEthanol precipitationBiology
The invention discloses a perhexiline injection liquid and a preparation method thereof, wherein the perhexiline injection liquid is prepared from radix salviae miltiorrhizae, ligusticum chuanxiong, sodium metabisulfite, sodium calcium edetate, HS-15 and water for injection. The preparation method of the perhexiline injection liquid comprises the steps: adding water to radix salviae miltiorrhizae and ligusticum chuanxiong, decocting, filtering the decoction, concentrating, carrying out precipitation treatment for 2 times with ethanol, filtering, concentrating the filtrate, diluting with water for injection, regulating the pH value with dilute hydrochloric acid, refrigerating, filtering, regulating the pH value with a sodium hydroxide solution, concentrating the filtrate, refrigerating, and filtering to obtain a solution 1; taking 100 ml of water for injection, adding sodium calcium edetate and sodium metabisulfite, stirring until being dissolved, and filtering with activated carbon to obtain a solution 2; adding the HS-15 into 100 ml of water for injection, evenly stirring to obtain a solution 3; and merging the three solutions, filtering, regulating the pH value, adding water for injection to the full amount, filling and sealing, and thus obtaining the product. With adopting of the new prescription and process, the perhexiline injection liquid has an outstanding advantage of greatly enhancing the medication security of the perhexiline injection liquid.
Owner:CHENGDU LIST PHARMA
Compound amino acid injection and preparation method thereof
InactiveCN102940628AGood metal ion chelating performanceInhibition of catalysisOrganic active ingredientsMetabolism disorderAntioxidantArginine
A compound amino acid injection provided by the invention comprises the following solvents in every 1000 mL: 11.6-14.2g of leucine, 6.8-8.3g of threonine, 8.2-10.0g of isoleucine, 6.3-7.7g of glycine, 12.6-15.4g of valine, 6.3-7.7g of phenylalanine, 1.2-1.4g of tryptophan, 4.0-4.8g of methionine, 0.4-0.6g of glutamic acid, 6.4-7.8g of alanine, 1.5-1.9g of serine, 4.5-5.5g of proline, 0.35-0.45g of tyrosine, 9.0-11.0g of lysine acetate, 8.1-9.9g of arginine, 4.5-5.5g of histidine, 0.9-1.1g of aspartate, 0.3-0.4g of cysteine, and 0.04-0.06g of calcium disodium edetate. The injection does not contain sulfite antioxidant, and employs cysteine and sodium calcium edetate as antioxidants and metal chelators; and nitrogen is used for protection in a production process, so as to reduce oxygen content in solution as much as possible and produce safer product for clinical usage.
Owner:湖北长联杜勒制药有限公司
Ozagrel sodium medicinal composition for injection
InactiveCN102429903AFix stability issuesHigh yieldOrganic active ingredientsPharmaceutical delivery mechanismMedicineSodium calcium edetate
The invention discloses an ozagrel sodium medicinal composition for injection. Ozagrel sodium injection consists of ozagrel sodium, sodium citrate and calcium disodium edetate, wherein each piece of injection contains 40-100 mg of ozagrel sodium, 4-10 mg of sodium citrate and 0.02-0.05 mg of calcium disodium edentate. A preparation method of the composition comprises the following steps of: adding a prescription dose of sodium citrate and calcium disodium edentate into a prescription dose of 90 percent injection water at the temperature of 55-65 DEG C and stirring to dissolve; adding a prescription dose of ozagrel sodium and stirring until the ozagrel sodium is fully dissolved; measuring an initial pH value, and adjusting the pH value to be within a range of 7.5-9.5 by using 4 percent sodium hydroxide solution and 10 percent sodium citrate solution according to the initial pH value; adding medicinal carbon and stirring; performing extraction filtration, replenishing injection water to full amount and uniformly mixing; performing fine filtration; canning; sterilizing; performing light inspection; and warehousing to obtain the ozagrel sodium injection. The ozagrel sodium medicinal composition has high light stability, clarity and stability and does not produce crystals, and has the more obvious advantages of improving the product yield, reducing the cost, realizing industrialization and guaranteeing better application to clinical use.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Oxiracetam injection composition and preparation method thereof
The invention relates to an oxiracetam injection composition and a preparation method thereof. The oxiracetam injection provided by the invention comprises 0.1-0.3 g of oxiracetam, 0.2-0.4 mg of sodium dihydrogen phosphate, 0.02-0.04 mg of sodium calcium edetate, 0.02-0.04 mg of glycine, 0.02-0.04 mg of vitamin B6, a pH value regulator (for regulating the pH value to the range from 4.5 to 5.0) and water for injection per 1 ml. The oxiracetam injection provided by the invention has the advantages of good quality and high stability.
Owner:HEBEI RENHE YIKANG PHARMA
Levofloxacin hydrochloride injection and preparation method thereof
ActiveCN108685846AReduce adsorptionIncrease contentAntibacterial agentsOrganic active ingredientsActivated carbonForeign matter
The invention relates to a preparation method of a levofloxacin hydrochloride injection. The method comprises the following steps: adding glycerin, lipoic acid and / or sodium calcium edetate, and sodium sulfite into water for injection, adding pre-treated activated carbon used for needles in two batches, and decarbonizing to obtain a solution A; adding levofloxacin hydrochloride into the water forinjection, dissolving, then adding the pre-treated activated carbon used for needles, and decarbonizing to obtain a solution B; adding the solution B into the solution A, supplementing the water for injection to a full dose, adjusting the pH to 4.0-4.6, filtering, carrying out ultrafiltration, sampling and inspecting ultrafiltrate, filling after the product is qualified, filling nitrogen, carryingout fused sealing, sterilizing, carrying out lamp inspection after cooling, packaging, and testing to obtain the finished product. The product prepared by the method is high in content of principal agents and good in stability, no visible foreign matter appears in a storage process, and the color of the product does not change.
Owner:JIANGXI GUOYAO PHARMA LLC
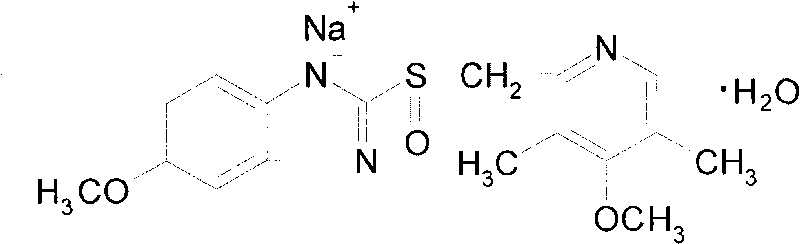
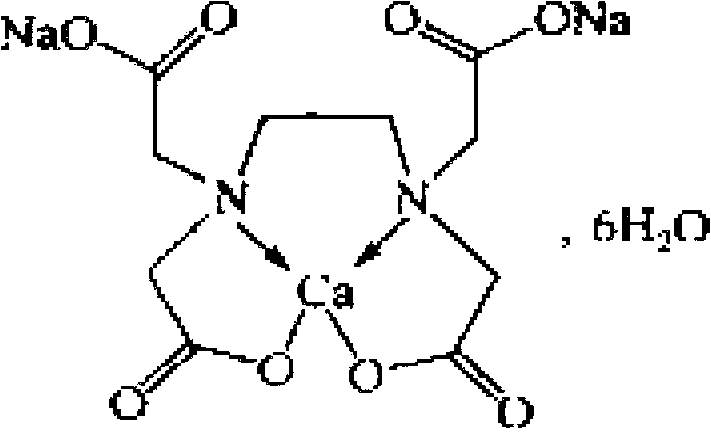
Preparation method of ethylenediaminetetraacetic acid calcium disodium salt
ActiveCN103172532ALow priceMild responseOrganic compound preparationAmino-carboxyl compound preparationRoom temperatureSodium calcium edetate
The invention discloses a preparation method of ethylenediaminetetraacetic acid calcium disodium salt, comprising the following steps: step 1 of firstly preparing a solvent and a calcium-containing compound, adding 1-2g of the calcium-containing compound into 160-180g of the solvent at a first stirring-up state to form a mixed solution, heating the mixed solution to 35-45 DEG C, and stirring the solution for the second time; step 2 of adding 7-9 g of ethylenediaminetetraacetic acid disodium salt into the above mixture solution and then stirring the mixture solution for the third time, simultaneously heating and refluxing the mixture solution for 1.5 h, then concentrating the heated mixture solution under reduced pressure while hot to 1 / 3 of the original volume, subsequently cooling the concentrated solution to the room temperature, and filtering the cooled solution; step 3 of slowly dropping 350-450ml of a crystallization solution into the filtered solution and simultaneously stirring the solution, with a large number of crystal appearing, and after the dropping process, stirring the solution for the fourth time, standing and filtering the solution, washing filter cakes with a washing solution, and finally drying the filter cakes to obtain the ethylenediaminetetraacetic acid calcium disodium salt with yield of 95%.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP
Netilmicin sulfate injection and preparing method
The invention relates to a netilmicin sulfate injection and a preparing method. Particularly, the netilmicin sulfate injection is prepared from netilmicin sulfate, sodium sulfite or sodium hydrogen sulfite, cysteine, edetate disodium or sodium calcium edentate and water for injection. The invention further relates to the preparing method for the netilmicin sulfate injection. The prepared netilmicin sulfate injection has one or more excellent characteristics described in the description.
Owner:成都天台山制药股份有限公司
Medicinal preparation for preventing operative bleeding and preparation method thereof
InactiveCN106963729AImprove securitySolve the phenomenon of bubblesOrganic active ingredientsInorganic non-active ingredientsSodium metabisulfiteSodium calcium edetate
The invention belongs to the technical field of medicine and particularly relates to a medicinal preparation for preventing operative bleeding and a preparation method thereof. The medicinal preparation is prepared from active ingredients etamsylate, sodium metabisulfite, sodium calcium edetate, methylparaben, a pH regulator and water for injection. The phenomenon that bubbles are produced in the filling process of etamsylate injections is avoided, the problem of product yield reduction caused by bubbles is solved, the safety of the injection in clinical application is greatly improved, and the medicinal preparation is simple and easy to operate and facilitates large-scale production.
Owner:王爱美 +1
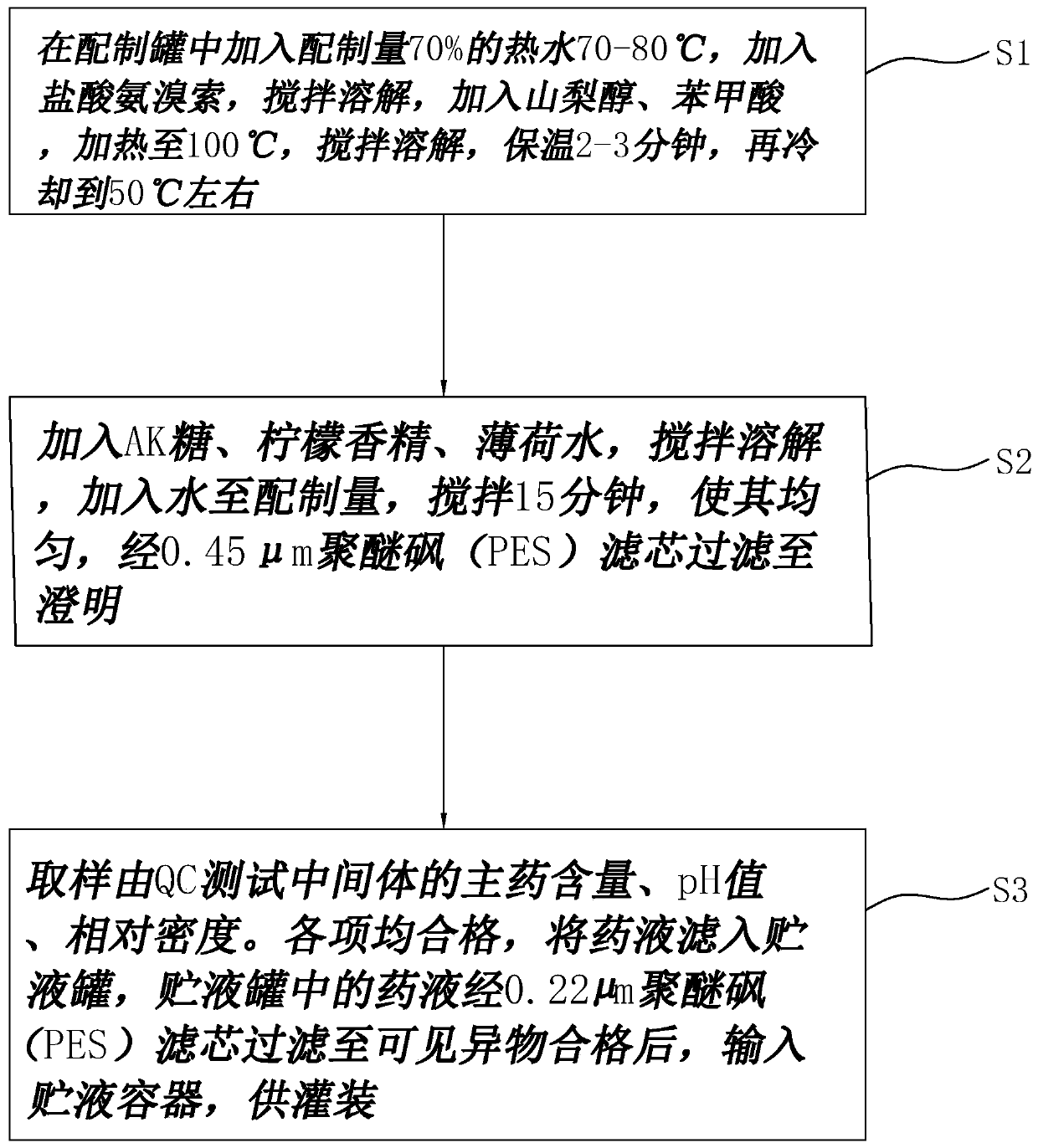
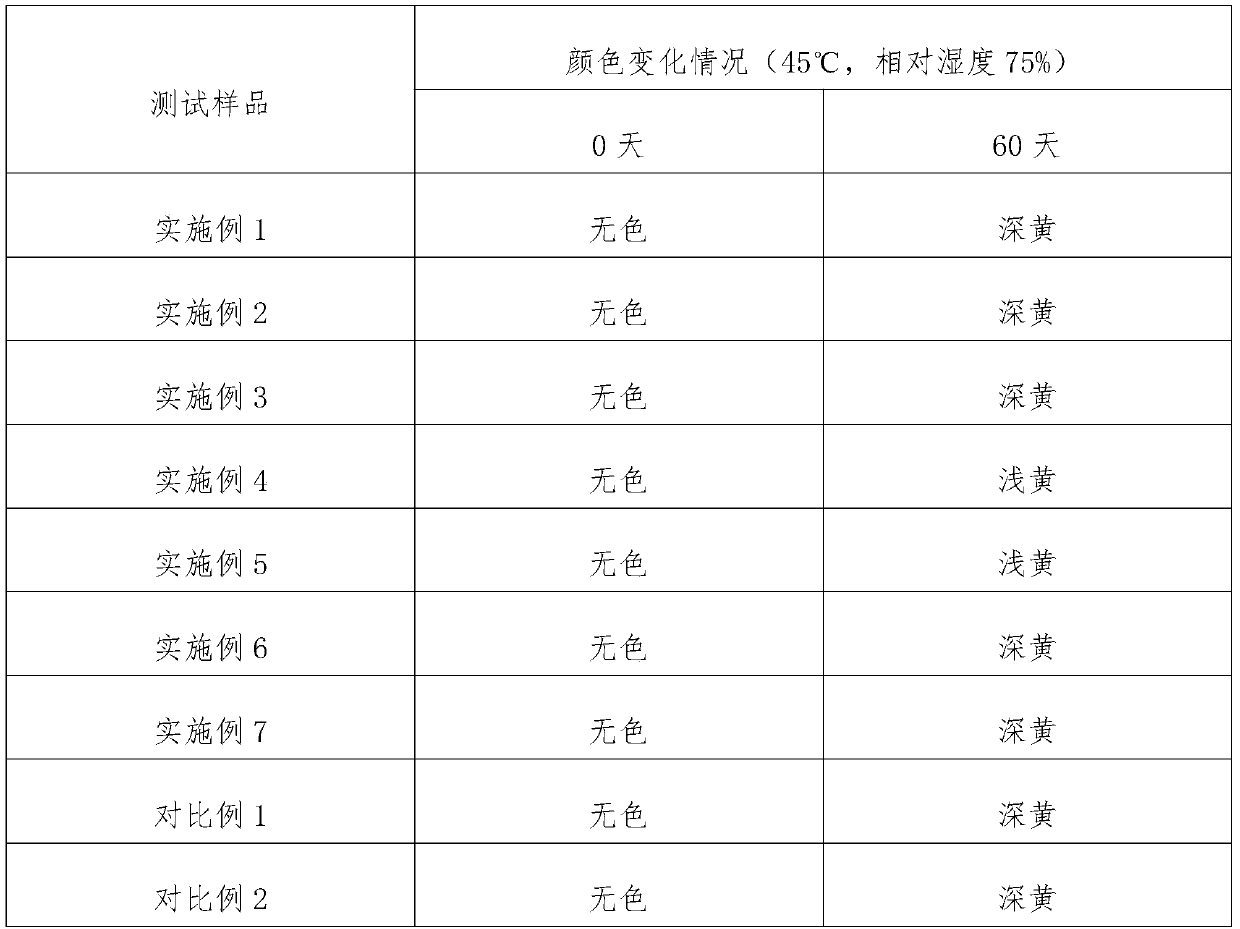
Ambroxol hydrochloride oral solution and preparation method thereof
ActiveCN111494314AImprove securityImprove stabilityOrganic active ingredientsDispersion deliveryBenzoic acidMedicine
The invention discloses an ambroxol hydrochloride oral solution and a preparation method thereof, and relates to the technical field of phlegm eliminating medicines. The ambroxol hydrochloride oral solution is characterized by comprising the following raw material components in parts by weight: 2-5 parts of ambroxol hydrochloride; 150 to 250 parts by weight of sorbitol; 0.5 to 1 part by weight ofbenzoic acid; 0.5 to 1.5 parts by weight of AK sugar; 0.05 to 1 part by weight of sodium calcium edetate; 0.1 to 0.5 part by weight of lemon essence; 0.05 to 0.2 part by weight of peppermint water; and purified water added to 1000 parts by volume; and when the unit of the weight part is g, the unit of the volume part is mL. The ambroxol hydrochloride oral solution prepared by the formula has the advantages of high safety and good stability.
Owner:SHANGHAI XINYI JINZHU PHARMA
Moxifloxacin hydrochloride injection pharmaceutical composition and preparation method and quality control method thereof
ActiveCN106821972AExcellent methodological featuresAntibacterial agentsOrganic active ingredientsQuality controlSodium calcium edetate
The invention relates to a moxifloxacin hydrochloride injection pharmaceutical composition and a preparation method and a quality control method thereof. The moxifloxacin hydrochloride injection pharmaceutical composition comprises moxifloxacin hydrochloride, aspartic acid, sodium calcium edetate, an optional acidifying or alkalizing agent and water for injection. The quality control method is a method for determining content of RR isomers in the moxifloxacin hydrochloride injection pharmaceutical composition. The moxifloxacin hydrochloride injection pharmaceutical composition and the preparation method and the quality control method thereof have the superior advantages specified in the description.
Owner:成都天台山制药股份有限公司
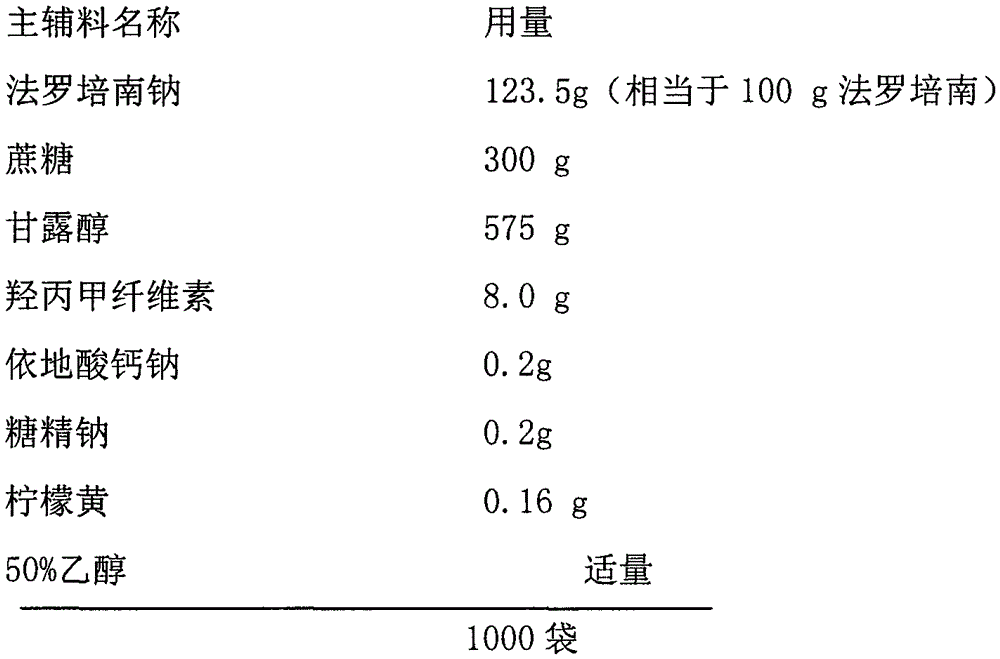
Faropenem sodium granules
InactiveCN106667917AHigh hardnessGood water solubilityAntibacterial agentsPharmaceutical non-active ingredientsSolubilitySide effect
The invention provides faropenem sodium granules. The faropenem sodium granules are characterized by being prepared from the following components in parts by weight: 100 parts of faropenem sodium calculated on the basis of faropenem, 300 to 400 parts of saccharose, 400 to 575 parts of mannitol, 8.0 to 80 parts of hydroxypropyl methylcellulose, 0.1 to 0.2 part of sodium calcium edentate, 0 to 50 parts of a taste correcting agent and 0 to 0.2 part of a color correcting agent. The prepared faropenem sodium granules have good granule hardness, high water solubility and moderate taste and color, solve the defect of low stability of the conventional granules, and are stable in release, durable in action, stable in curative effect, small in toxic and side effect and particularly favorable for children and people suffering from dysphagia to take.
Owner:HAINAN HONZ PHARMA
Glycerin fructose sodium chloride injection and preparation method thereof
InactiveCN106727680AGuarantee product quality5-HMF content controlHydroxy compound active ingredientsPharmaceutical delivery mechanismActivated carbonGlycerol
The invention relates to a glycerin fructose sodium chloride injection and a preparation method thereof. The glycerin fructose sodium chloride injection is prepared from glycerin, fructose, sodium chloride, activated carbon, sodium calcium edetate and citric acid, wherein the glycerin content accounts for 10% of the total content, the fructose content 5%, the sodium chloride content 0.9%, the activated carbon content 0.06% and the sodium calcium edetate content 0.03%. According to the formula and preparation method of the injection, the content of 5-hydroxymethylfurfural can be effectively controlled, the stability is good, and the product quality is guaranteed.
Owner:SHANDONG QIDU PHARMA
Magnetic nano adsorbent for metallurgical wastewater and preparation method of magnetic nano adsorbent
InactiveCN110302744AHigh surface activityLarge specific surface areaOther chemical processesWater contaminantsNickel saltSorbent
The invention discloses a magnetic nano adsorbent for metallurgical wastewater and a preparation method of the magnetic nano adsorbent. The magnetic nano adsorbent is composed of magnetic ferroferricoxide / nickel oxide modified sepiolite, p-tert-butylphenylhydroxamic acid, sodium calcium edetate, ethylene diamine tetramethylene phosphonic aci, cation exchange resin and polymeric ferric silicate sulfate; the magnetic ferroferric oxide / nickel oxide modified sepiolite is prepared by ferric ion, divalent nickel salt, and activated sepiolite which are taken as raw materials in a hydrothermal method; and the preparation method includes the steps of activating sepiolite with nitric acid, preparing magnetic ferroferric oxide / nickel oxide precursor, preparing magnetic ferroferric oxide / nickel oxide modified sepiolite in a hydrothermal method, and preparing the magnetic nano adsorbent. According to the magnetic nano adsorbent, surface activity is high, the specific surface area is large, and non-ionic magnetic conducting components such as iron filings, oxidized ores and rare earth ores in wastewater can be selectively adsorbed while heavy metal ions are removed to avoid resource waste andsecondary pollution.
Owner:淮北市菲美得环保科技有限公司
Meptazinol hydrochloride injection medicine composition and preparation method thereof
The invention provides a meptazinol hydrochloride injection medicine composition and a preparation method thereof. Only a small amount of acetic acid-sodium acetate is used to serve as a buffer system in the prescription, the pH value of liquid medicine can be controlled within the reasonable range, meanwhile, meptazinol hydrochloride injection is prevented from being degraded in the placement process, and product quality is remarkably improved; meanwhile, use of antioxidant is omitted, adverse reaction caused by sodium calcium edetate is avoided, and safety is guaranteed; a preparation process of introducing nitrogen twice is adopted, and product stability is greatly improved.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Moxifloxacin hydrochloride injection pharmaceutical composition and its preparation and quality control method
ActiveCN106821972BExcellent methodological featuresAntibacterial agentsOrganic active ingredientsSodium calcium edetateMoxifloxacin Injection
The invention relates to a moxifloxacin hydrochloride injection pharmaceutical composition and a preparation method and a quality control method thereof. The moxifloxacin hydrochloride injection pharmaceutical composition comprises moxifloxacin hydrochloride, aspartic acid, sodium calcium edetate, an optional acidifying or alkalizing agent and water for injection. The quality control method is a method for determining content of RR isomers in the moxifloxacin hydrochloride injection pharmaceutical composition. The moxifloxacin hydrochloride injection pharmaceutical composition and the preparation method and the quality control method thereof have the superior advantages specified in the description.
Owner:CHENGDU TIANTAISHAN PHARMA
Application of calcium disodium edetate in preparation of medicine for inhibiting connective tissue growth factor and monoamine oxidase of human beings and mammal
InactiveCN101780065AGood treatment effectAchieve targeted enrichmentOrganic active ingredientsPharmaceutical delivery mechanismDiseaseMicrosphere
The invention relates to the application of calcium disodium edetate in the preparation of medicine for inhibiting connective tissue growth factor and monoamine oxidase of human beings and mammal; the calcium disodium edetate is injection when being applied to prepare the medicine of the diseases, and the injection contains 5-30wt % of calcium disodium edetate and auxiliary material used for the injection; the preferable lung target injection contains 0.1-5wt% of calcium disodium edetate and one or more auxiliary material(s) which is / are applicable to lung target preparation; the lung target injection is microsphere preparation, microcapsule preparation or liposome injection; preferably, the particle sizes of microspheres, microcapsules and liposome are 7-30 mu m; and the lung target preparation is preferably liposome injection liquid.
Owner:TIANJIN JINYAO GRP
Flurbiprofen cataplasm
InactiveCN107951864AEnhance drug release propertiesFast transdermal absorptionOrganic active ingredientsAntipyreticGlycerolOil phase
The invention provides Flurbiprofen cataplasm. The Flurbiprofen cataplasm comprises a back lining layer, a drug storage cavern and a protective layer, and is characterized in that the drug storage cavern is prepared from components in percentage by weight as follows: 0.2%-0.5% of Flurbiprofen as an active component, 5%-10% of oil-phase composition and aqueous phase composition, wherein the oil-phase composition is prepared from castor oil and benzyl alcohol in a ratio of 1:(0.08-0.12), and Flurbiprofen is dissolved in benzyl alcohol firstly and then dispersed in the castor oil; the aqueous phase composition is prepared from 5%-10% of partially neutralized sodium polyacrylate, 15%-20% of glycerol, 0.2%-0.4% of aluminium glycinate, 0.1%-0.3% of sodium calcium edetate, 1%-1.5% of carbomer 934, 1.5%-3% of HPMC (hydroxypropyl methylcellulose), a pH regulator, 0.05%-0.1% of gellan gum, 1%-1.5% of L- glycine, 1%-3% of filler and the balance of water; hydrogel is formed from the aqueous phasecomposition and water, the filler is dispersed in the hydrogel in a filling manner, the oil-phase composition is emulsified and dispersed in the hydrogel, and the drug storage cavern is formed.
Owner:北京茗泽中和药物研究有限公司
Composite plant acid copper zincium bactericidal agent
InactiveCN101322498AInhibition and treatment of diseasesThe formula is scientific and reasonableBiocideArthropodicidesSide effectDissolution
The invention relates to a composite plant acid copper and zinc bactericide which can effectively solve the problems of high toxicity, impact on the quality of agricultural products and serious threat to human health of the existing biological bactericidal products, the technical proposal for the solution is as follows, the composite plant acid copper and zinc bactericide is counted by the weight percentage: 47 to 83 percent of plant acid, 5 to 28 percent of copper carbonate, 5 to 19 percent of zinc carbonate and 4 to 19 percent of sodium ethylene diamine tetraacetate, the total amount is 100 percent, the plant acid is firstly poured into a container, the copper carbonate is slowly added for carrying out the reaction for 1 to 3 hours at the temperature of 18 to 30 DEG C, the zinc carbonate is further added after the complete dissolution for carrying out the reaction for 1 to 3 hours, the sodium ethylene diamine tetraacetate is added to be stirred for 2 to 3 hours after the complete dissolution, the even mixing is carried out, and then the bactericide finished product with the pH value of 3 to 5.5 and the specific weight of 1.0 to 1.2 is prepared. The bactericide has scientific and reasonable formula, easy production, no pollution and no toxicity or side effects, the bactericide can effectively inhibit the diseases of crops caused by fungi, the application is wide and the bactericide is another creation in agricultural biological bactericides.
Owner:李海涛
Oxaliplatin pharmaceutical composition for injection
InactiveCN104434816ANo stimulationAvoid harmPowder deliveryPharmaceutical non-active ingredientsMedicineSodium calcium edetate
The invention aims to provide an oxaliplatin pharmaceutical composition for injection and a preparation method thereof. A certain amount of sodium calcium edetate, meglumine and sodium hydroxide are used, the pH value of a solution is regulated firstly, and then oxaliplatin is dissolved, so that the stability of the preparation can be effectively improved, the preparation is unlikely to precipitate and relevant substances are free of changes. The provided new oxaliplatin pharmaceutical composition can be used for completely realizing stability of a preparation solution of oxaliplatin in clinical application.
Owner:TIANJIN SONGRUI MEDICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com