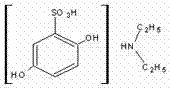
Medicinal preparation for preventing operative bleeding and preparation method thereof
A technology of pharmaceutical preparations and surgical bleeding, applied in the field of medicine, can solve problems such as unqualified foreign objects, plugging, and decreased yield of finished products, and achieve the effects of reducing product yield, improving safety, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
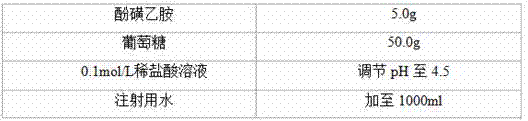
[0035] prescription
[0036]
[0037] Preparation Process
[0038] Step a, Ampoule treatment: Wash the ampoule through an ultrasonic bottle washing machine, sterilize and dry it in a tunnel-type sterilizing dryer at a temperature not lower than 290°C, and transfer it to the filling room for standby;
[0039] Step b, preparation: 1. Weigh sodium metabisulfite and sodium edetate calcium sodium in the prescribed amount and dissolve them in 20% water for injection, stir to dissolve;
[0040] ②. Weigh the prescription amount of methylparaben and etamsulfame into 50% water for injection, stir and dissolve for 15 minutes;
[0041] ③. Add the solution in ① to the solution in ②, stir and mix evenly, adjust the pH value with a pH regulator, after the pH adjustment is completed, use water for injection to make up the volume to the full amount, test the intermediate product solution, and prepare for potting after passing the test;
[0042] Step c, potting: the solution is filtered to...
Embodiment 2
[0051] prescription
[0052]
[0053] Preparation Process:
[0054] Step a, Ampoule treatment: Wash the ampoule through an ultrasonic bottle washing machine, sterilize and dry it in a tunnel-type sterilizing dryer at a temperature not lower than 290°C, and transfer it to the filling room for standby;
[0055] Step b, preparation: 1. Weigh the sodium metabisulfite and calcium sodium edetate in the prescribed amount and dissolve them in 15% water for injection, stir to dissolve;
[0056] ②. Weigh the prescribed amount of methylparaben and etamsulfame into 45% water for injection, stir and dissolve for 10 minutes;
[0057] ③. Add the solution in ① to the solution in ②, stir and mix evenly, adjust the pH value with a pH regulator, after the pH adjustment is completed, use water for injection to make up the volume to the full amount, test the intermediate product solution, and prepare for potting after passing the test;
[0058] Step c, potting: the solution is filtered to the...
Embodiment 3
[0067] prescription
[0068]
[0069] Preparation Process:
[0070] Step a, Ampoule treatment: Wash the ampoule through an ultrasonic bottle washing machine, sterilize and dry it in a tunnel-type sterilizing dryer at a temperature not lower than 290°C, and transfer it to the filling room for standby;
[0071] Step b, preparation: 1. Weigh the sodium metabisulfite and calcium sodium edetate in the prescribed amount and dissolve them in 25% water for injection, stir to dissolve;
[0072] ②. Weigh the prescribed amount of methylparaben and etamsulfame and dissolve it in 55% water for injection, stir and dissolve for 20 minutes;
[0073] ③. Add the solution in ① to the solution in ②, stir and mix evenly, adjust the pH value with a pH regulator, after the pH adjustment is completed, use water for injection to make up the volume to the full amount, test the intermediate product solution, and prepare for potting after passing the test;
[0074] Step c, potting: the solution is f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com