Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Etamsylate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
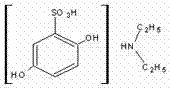
Etamsylate (sometimes spelled ethamsylate) is a antihemorrhagic agent which is believed to work by increasing resistance in the endothelium of capillaries and promoting platelet adhesion. It also inhibits biosynthesis and action of those prostaglandins which cause platelet disaggregation, vasodilation and increased capillary permeability.
Synergistic compositions and methods for enhancing potency and/or for prolonging the duration of action of anesthetics
InactiveUS7928141B2Improve effectivenessProlong the action timeBiocideOrganic chemistrySodium bicarbonateBerberine
Taught is a composition for enhancing potency and / or for prolonging the duration of action of an anesthetic comprising dexamethasone, compound vitamin B, metronidazole, berberine, etamsylate, gentamicin, chymotrypsin, methylene blue trihydrate, and 5% sodium bicarbonate aq. When administered with an anesthetic, the composition shortens the onset time of the anesthetic, and prolongs the duration of anesthesia.
Owner:LI FUCHAO
Synthetic method of etamsylate
ActiveCN102942509ANo water ensureHigh yieldOrganic compound preparationSulfonic acids salts preparationChlorosulfuric acidOrganic solvent
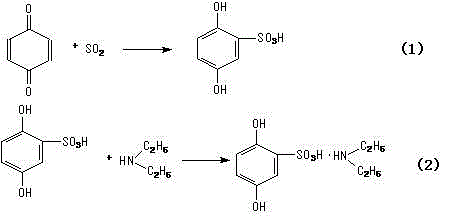
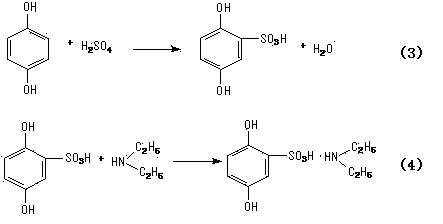
The invention relates to a synthetic method of etamsylate. The synthetic method comprises the following steps of (1) preparation of 2,5-dihydroxybenzenesulfonic acid; (2) preparation of a etamsylate crude product; and (3) purification. Compared with a conventional synthetic process of the etamsylate adopting concentrated sulfuric acid as a sulfonating agent, the synthetic method has the following advantages that the synthetic method employs chlorosulfonic acid as the sulfonating agent, and performs azeotropic dehydration on benzenediol and an organic solvent until anhydrous before sulfonation to ensure that no water is in the reaction system, thereby increasing product yield; hydrogen chloride gas produced during the sulfonation process can be absorbed by water to make hydrochloric acid, thereby decreasing discharge of three wastes (waste gas, waste water and waste residues); and usage amount of the sulfonating agent is decreased during the sulfonation process, so that production cost is reduced.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
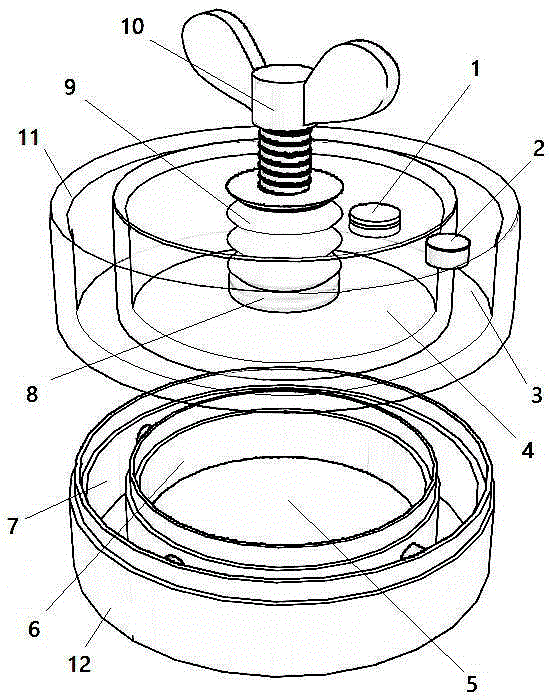
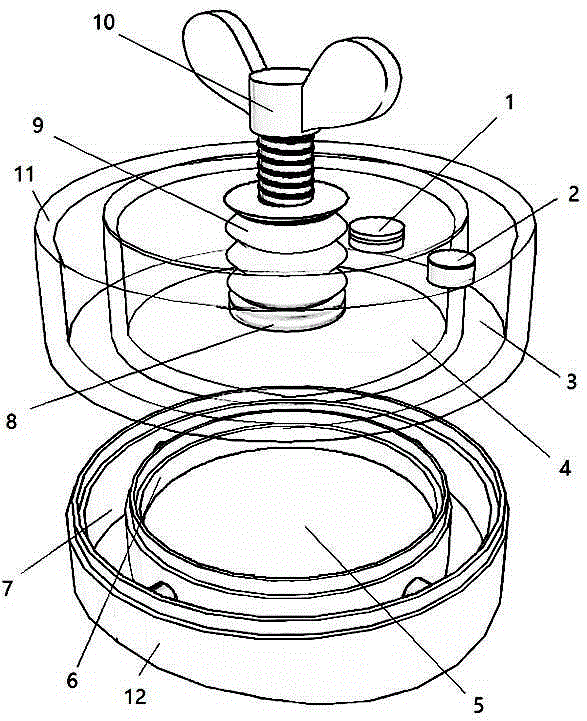
Air pressure type artery postoperation hemostat
The invention discloses an air pressure type artery postoperation hemostat and relates to the technical field of medical care instruments. The hemostat comprises an upper tank and a lower ring, wherein the upper tank is divided into an inner circular cavity A and an outer ring cavity A, and the inner circular cavity A and the outer ring cavity A open downwards; a one-way air inlet valve is mounted on one side of the top face of the inner circular cavity A, and a one-way air release valve is mounted on the top face of the outer ring cavity A; a puller bolt is arranged at the center of the top face of the inner circular cavity A in a threaded connection mode, and the outer side of the puller bolt is sleeved with a telescopic sealing sleeve; the lower end of the upper tank and the upper end of the lower ring are sealed in a loose joint mode; the lower ring is divided into an inner circular cavity B and an outer ring cavity B, the inner circular cavity B and the inner circular cavity A are identical in size and are sealed in a butt joint mode, and the outer ring cavity B and the outer ring cavity A are identical in size and are sealed in a butt joint mode; a cotton pad is arranged in the inner circular cavity B and soaked with etamsylate. The air pressure type artery postoperation hemostat has the advantages that negative pressure of the outer ring cavity A enables the device to be tightly adsorbed to the body of a patient without a bandage, positive pressure of the inner circular cavity A is used for balancing blood pressure to reduce bleeding, operation is easy, and time is saved.
Owner:SHANDONG RES INST OF TUMOUR PREVENTION TREATMENT
Creatinine detection kit and use method thereof
PendingCN112029817AHigh accuracy of test resultsImprove accuracyMicrobiological testing/measurementBiological material analysisHigh creatinineCreatininase
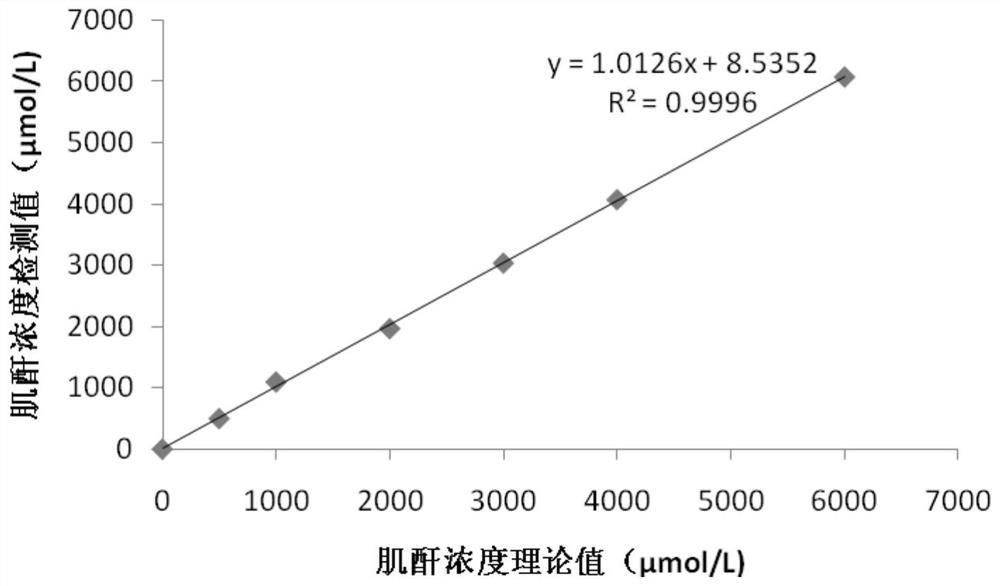
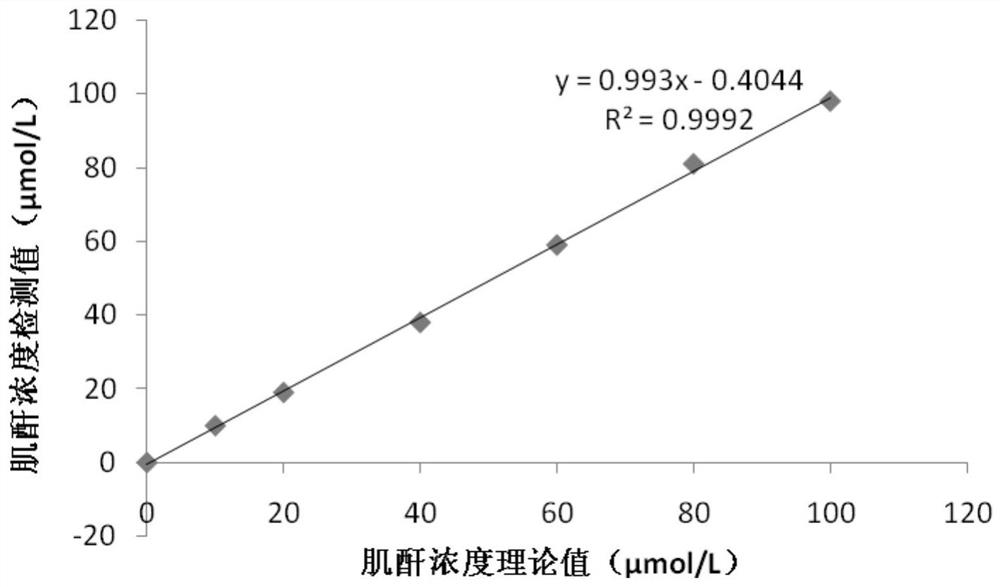
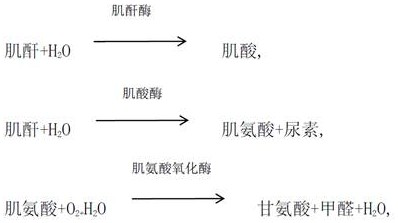
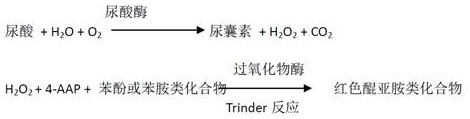
The invention provides a creatinine detection kit and a use method thereof. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises creatinase, sarcosine oxidase, peroxidase,ascorbic acid oxidase, 2, 4, 6-tribromo-3-hydroxybenzoic acid and 3, 5-dichloro-2-hydroxybenzenesulfonic acid; and the reagent R2 comprises creatinase, peroxidase, potassium ferrocyanide and 4-aminoantipyrine. The creatinine detection kit provided by the invention can resist interference of etamsylate with the concentration of 300mg / L (the highest blood concentration) or below in a to-be-detectedsample, has a wider linear range (0-6000 millimoles / L), is applicable to detection of samples with high creatinine content and can correctly evaluate renal functions of surgical patients.
Owner:LANZHOU BAIYUAN GENE TECH
Creatinine kit for eliminating calcium dobesilate and etamsylate and preparation method thereof
ActiveCN111733208AImprove stabilityEasy to operateMicrobiological testing/measurementBiological material analysisActive agentPeroxidase
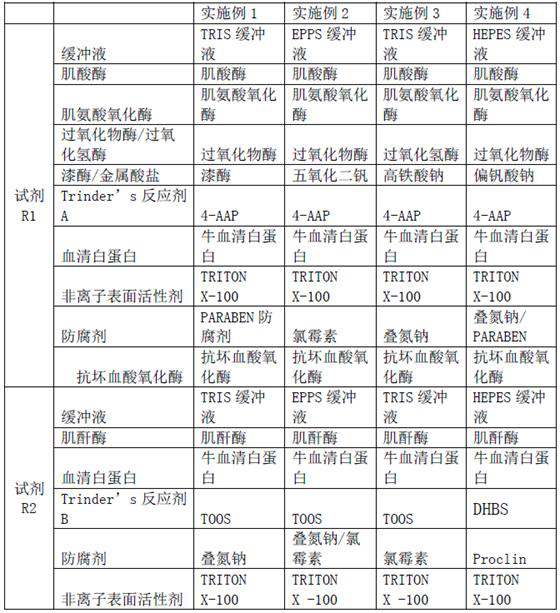
The invention relates to a creatinine kit capable of eliminating negative deviation interference in a creatinine determination caused by calcium dobesilate and etamsylate drugs in serum and a preparation method thereof. Key points of a technical scheme of the invention are that the creatinine kit includes a reagent R1 and a reagent R2, wherein the reagent R1 includes buffer, creatinase, sarcosineoxidase, ascorbic acid oxidase, peroxidase or catalase, serum albumin, nonionic surfactant, preservative, Trinder's reagent A, laccase or metalic acid salt; and the reagent R2 includes buffer, serum albumin, creatinine enzyme, preservative and Trinder's reagent B. The invention overcomes a defect of inaccurate creatinine determination due to the presence of the negative deviation caused by calciumdobesilate and etamsylat in serum samples when the clinical tests of creatinine determination are being carried out in various hospitals, and the invention is suitable for the detection of serum creatinine by an automatic biochemical analyzer.
Owner:ZHONGSHAN BGH BIOTECH CO LTD
Corallite herbal tooth powder
InactiveCN108451819ASmall friction hardnessNo grittinessCosmetic preparationsToilet preparationsOral diseasePollen
The invention relates to corallite herbal tooth powder. The corallite herbal tooth powder comprises active corallite powder, pollen typhae, folium artemisiae argyi, herba ecliptae, radix boehmeriae, radix glycyrrhizae, borneol, etamsylate, lauryl sodium sulfate and water. The active corallite powder obtained after corallite powder is activated and the rest of the raw materials are added into a ball grinder for wet grinding, milling is performed after drying, and the corallite herbal tooth powder is obtained. The corallite herbal tooth powder is good in stability, free of caking, and cool, refreshing, soft and smooth in taste, can effectively reduce the wear of enamel on the surfaces of teeth in the tooth brushing process and is suitable for removing ozostomia and preventing oral diseases.
Owner:SHAANXI SCI TECH UNIV
Etamsylate injection and preparation method thereof
InactiveCN107308105ARaise quality standardsSolve the problem of unstable pHOrganic active ingredientsInorganic non-active ingredientsSulfite saltSodium bisulfite
The present invention relates to a kind of ethylamine injection and preparation method thereof, the components of ethylamine injection are proportioned according to the following concentration: ethylamine is 120g / L to 130g / L; sodium bisulfite 1.65g / L to 1.85g / L; sodium sulfite 0.23g / L to 0.27g / L; solvent is water for injection.
Owner:BEIJING LISHIDA PHARMA
Citric acid and compound citric acid application in pharmacy
ActiveCN101530405AControl epidemicAvoid residueAntibacterial agentsSulfur/selenium/tellurium active ingredientsDiseaseBacterial disease
The invention relates to a new usage of citric acid and compound citric acid in the field of pharmacy. The product can prevent and control viral diseases and bacterial diseases of livestock and poultry; wherein, the compound citric acid comprises the following components according to weight portion: 93-98 portions of citric acid, 0.5-4 portions of gualfenesin and 1.5-3 portions of etamsylate. Proved by in vivo and vitro experiments, the compound citric acid can play a role in preventing and controlling fowl mycoplasma gallisepticum, avian influenza (H9 subtype), bursal virus disease and other diseases, and can effectively kill animal pathogenic bacterias such as cattle colon bacillus, mycoplasma gallisepticum, chicken colibacillosis, etc.
Owner:四川通达动物保健科技有限公司
Preparation method of etamsylate
InactiveCN104447428AImprove quality scoreSimple designAmino compound purification/separationOrganic compound preparationAlcoholOrganic solvent
The invention relates to a preparation method of etamsylate. The etamsylate is prepared by virtue of three steps, namely sulfonating, salifying and refining, and the entire preparation process is free from an organic solvent, so that cost and pollution are reduced; in addition, a crude product is refined by virtue of absolute ethyl alcohol which serves as a solvent; therefore, the entire process is not only lower in cost but also more environment-friendly; the etamsylate prepared by the method is relatively higher in mass percentage; in conclusion, the preparation method disclosed by the invention has the advantages of being simple in design, low in cost, environment-friendly and high in mass percentage.
Owner:HENAN LINGXIAN SCI & TECHN PHARMA
Uric acid test kit capable of eliminating drug interference of calcium dobesilate and etamsylate in serum and preparation method thereof
ActiveCN111733211AEliminate negative interferenceImprove stabilityMicrobiological testing/measurementBiological material analysisPeroxidaseActive agent
The invention discloses a uric acid test kit capable of eliminating drug interference of calcium dobesilate and etamsylate in serum and a preparation method thereof. According to the technical point,the test kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises a buffer solution, peroxidase, ascorbic acid oxidase, a preservative, a Trinder's reactant A, metallate or a nonionic surfactant; and the reagent R2 comprises a buffer solution, serum albumin, uricase, a preservative and a Trinder's reactant B or a nonionic surfactant. According to the uric acid test kit disclosed by the invention, one or more than one of high-redox-potential oxidized metallate, vanadium pentoxide and vanadium oxychloride is / are added into R1 to be mixed, and calcium dobesilate and etamsylate are oxidized by utilizing the metallate, H2O2 generated by uricase in decomposing uric acid after R2 is added is prevented from being consumed, and an accurate uric acid measured value is rapidly obtained.
Owner:ZHONGSHAN BGH BIOTECH CO LTD
Medicinal preparation for preventing operative bleeding and preparation method thereof
InactiveCN106963729AImprove securitySolve the phenomenon of bubblesOrganic active ingredientsInorganic non-active ingredientsSodium metabisulfiteSodium calcium edetate
The invention belongs to the technical field of medicine and particularly relates to a medicinal preparation for preventing operative bleeding and a preparation method thereof. The medicinal preparation is prepared from active ingredients etamsylate, sodium metabisulfite, sodium calcium edetate, methylparaben, a pH regulator and water for injection. The phenomenon that bubbles are produced in the filling process of etamsylate injections is avoided, the problem of product yield reduction caused by bubbles is solved, the safety of the injection in clinical application is greatly improved, and the medicinal preparation is simple and easy to operate and facilitates large-scale production.
Owner:王爱美 +1
Biomarker having high-blood coagulation status and application of same
ActiveCN107290552AWide range of interventionsBiological testingSignalling pathwaysBiomarker (petroleum)
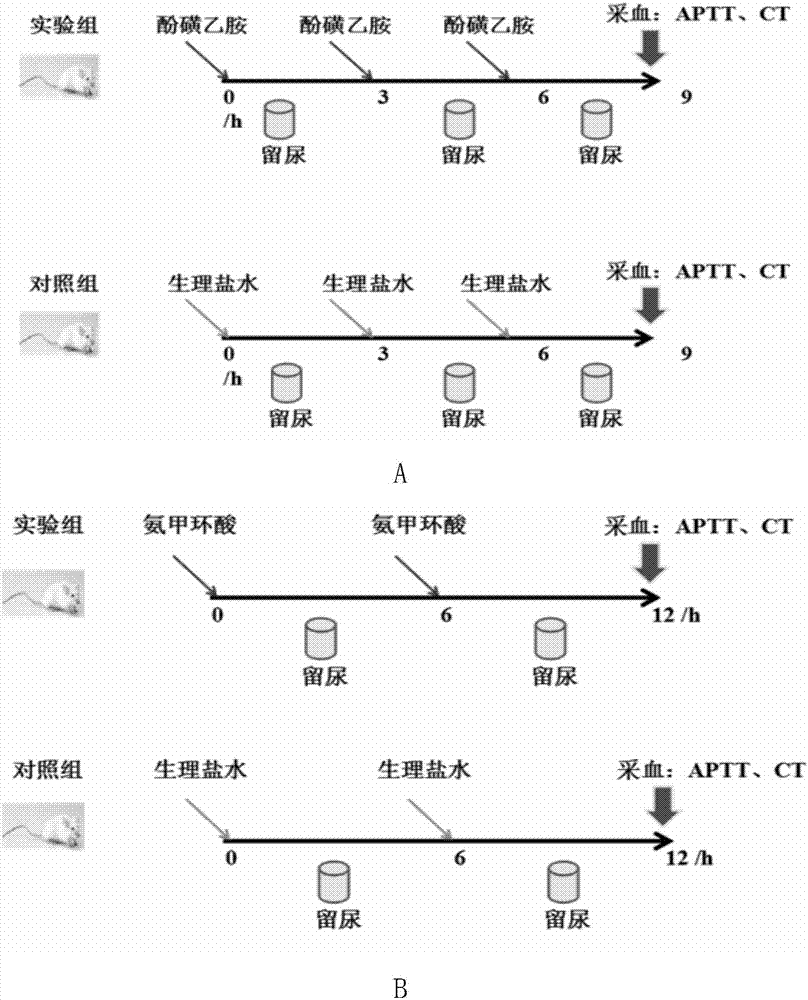
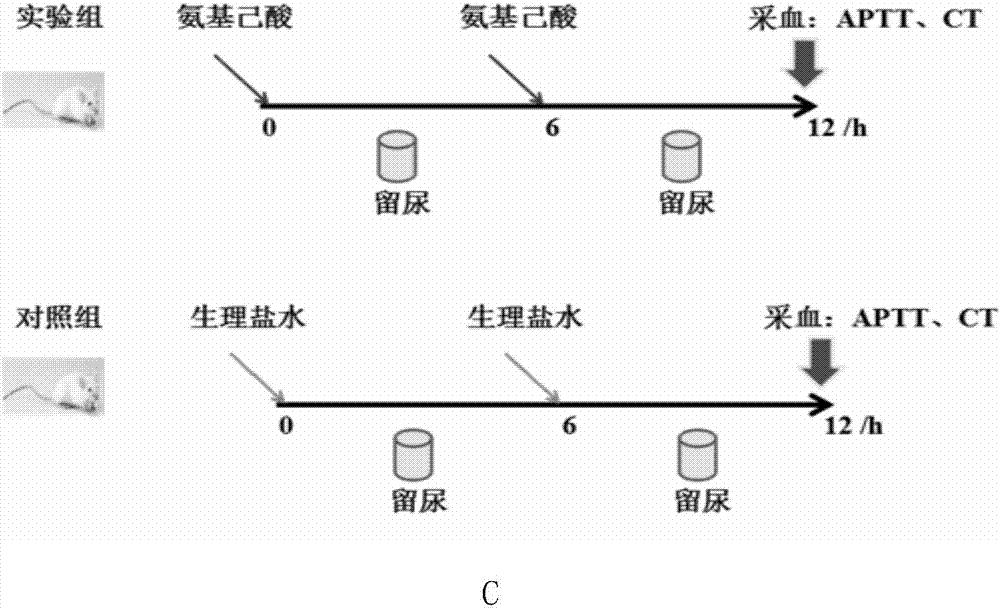
The invention discloses a biomarker having a high-blood coagulation status and an application of same. In the invention, three different blood coagulation promoting drugs (etamsylate, tranexamic acid and aminocaproic acid) are employed to respectively intervene in blood coagulation status of adult rats, thus successfully obtaining an adult rat acute high-blood coagulation status model; and then change on urine proteomes of the adult rats in intervention groups with different blood coagulation promoting drugs is analyzed; for the first time, the change feature of the urine proteomes under the high-blood coagulation status is systematically researched, and a biomarker, which is related to the high-blood coagulation status, is found in the urine. The invention initially discloses body intervention range and change factors of the high-blood coagulation status, which not only prompt related physiological and pathological mechanisms and the change feature of a signal pathway, but also are applied as an important biomarker in detection and / or diagnosis for related pathological change of the high-blood coagulation status or supply assistant information to the aspect.
Owner:BEIJING NORMAL UNIVERSITY
Method for producing high-purity etamsylate
ActiveCN109467522AImprove liquidityImprove conversion rateOrganic compound preparationAmino compound preparationSolventGenotoxicity
Owner:云南元顺医药有限公司
Hemostatic non-latex self-adhesive elastic bandage and preparation method thereof
InactiveCN109731122AGuaranteed stickinessGood hemostatic effectAbsorbent padsBandagesPolyphenolAntibacterial agent
The invention provides a hemostatic non-latex self-adhesive elastic bandage and a preparation method thereof, and relates to the technical field of elastic bandage processing. The elastic bandage comprises an adhesive and an antibacterial elastic fabric, wherein the adhesive is prepared from, by weight, 25-30 parts of polyurethane, 15-20 parts of acrylate, 1-2 parts of polyacrylate, 4-5 parts of tea polyphenol, 5-7 parts of aloe gel, 3-5 parts of chitosan, 2-3 parts of isolated soybean protein, 0.5-0.8 part of etamsylate, 1-2 parts of a thickener and 0.2-0.4 part of a dispersant. The antibacterial elastic fabric is composed of an antibacterial agent and an elastic fabric. The hemostatic non-latex self-adhesive elastic bandage and the preparation method thereof have the advantages that thedisadvantages in the prior art are overcome, various ingredients stopping bleeding and promoting wound healing are added into the adhesive to enhance the hemostatic effect of the product while effectively ensuring the viscosity of the adhesive, the antibacterial agent is adopted to treat the elastic fabric to effectively prevent wound infection and enhance the practicability of the product.
Owner:安徽省康富医疗用品有限公司
Local anesthesia gel preparation and preparation method thereof
InactiveCN105616478APrevent corruptionExtended shelf lifeAnthropod material medical ingredientsHydroxy compound active ingredientsCelastrus orbiculatusGel preparation
The invention discloses local anesthesia gel preparation and a preparation method thereof. The local anesthesia gel preparation is prepared from, by weight, 8-20 parts of Celastrus orbiculatus, 8-20 parts of benzoin, 2-2.8 parts of bee venom, 0.1-2.0 parts of etamsylate, 0.3-1.0 part of benzoate, 3-5 parts of menthol, 2-3.6 parts of diclofenac, 1.0-1.5 parts of sodium fusidate, 1.5-3.5 parts of local anesthetic, 2.3-4.0 parts of viscous polymer, 0.05-0.3 part of preservative, 0.05-0.1 part of antioxidant, 0.2-0.6 part of surfactant and 10-16 parts of gel matrix. The local anesthesia gel preparation can be used for local administration of trauma or during small external surgery, can play a good role in controlling pain, resisting bacteria, diminishing inflammation and preventing wound infection, can reduce local damage, has high affinity with skin and is convenient to use and quick in action; anesthetic action generated by the local anesthesia gel preparation can quickly seep into collenchyme and can last for a long time, and the local anesthesia gel preparation is safe and nontoxic, simple in preparation process and low in cost.
Owner:卢连伟
Etamsylate injection and preparation method thereof
PendingCN111437253AImprove stabilityChanging medicinal valueOrganic active ingredientsInorganic non-active ingredientsForeign matterDisodium Edetate
The invention belongs to the technical field of medicine preparation, and particularly relates to an etamsylate injection and a preparation method thereof. The preparation method comprises the following steps: (1) preparing a saturated solution of etamsylate, edetate disodium, sodium pyrosulfite and sodium chloride for standby use; and dissolving mannitol, potassium chloride and calcium chloride in 10ml of water for injection to obtain a trace element solution for later use; (2) uniformly mixing the saturated solution of etamsylate, edetate disodium, sodium pyrosulfite and sodium chloride withthe trace element solution to obtain a concentrated raw material solution; (3) putting the concentrated raw material solution into a concentrated mixing tank, adsorbing, and filtering until the concentrated solution is clear; (4) putting the concentrated solution into a diluting tank, adding water for injection, adsorbing, and filtering to obtain a diluted solution; (5) carrying out three-stage filtration treatment on the diluted solution to obtain a finished stock solution; (6) supplementing water for injection into the finished stock solution until the weight of the final solution is reached; (7) sealing; (8) sterilizing for 10 minutes; (9) detecting whether visible suspending foreign matters and particles exist in sterilized ampoule bottles or not; and (10) storing.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Compound dimetridazole premix for preventing and treating poultry enterovirus syndrome
ActiveCN103690940BEfficient therapeutic effectReasonable formulaOrganic active ingredientsPeptide/protein ingredientsEnterovirusTherapeutic effect
The invention discloses a compound dimetridazole premix for preventing and treating the poultry enterovirus syndrome. The premix is prepared by uniformly mixing the following components in percentage by weight: 10 to 30 percent of dimetridazole, 4 to 10 percent of neomycin sulfate, 2 to 8 percent of decoquinate econazole nitrate, 10 to 30 percent of lysozyme, 0.5 to 5 percent of etamsylate, 3 to 10 percent of lactic acid TMP and the balance of pharmaceutically applicable auxiliary material. According to the invention, the novel high-efficiency anticoccidial drug decoquinate econazole nitrate, the dimetridazole and the neomycin sulfate which have a special curative effect on intestinal infection, the lysozyme with effects of resisting to bacteria, resisting to viruses, diminishing inflammation, relieving pain and repairing intestinal mucosa, the high-efficiency hemostatics etamsylate and the drug synergist are compounded into a compound preparation; and the compound dimetridazole premix has a high-efficiency treatment effect on the poultry enterovirus syndrome. The compound dimetridazole premix has a reasonable and scientific formula, is simple to prepare, has wide application and can be used as a priority drug for preventing and treating the poultry enterovirus syndrome.
Owner:NANYANG XINXIANFENG PHARMA
Citric acid and compound citric acid application in pharmacy
ActiveCN101530405BControl epidemicAvoid residueAntibacterial agentsSulfur/selenium/tellurium active ingredientsFowlViral disease
The invention relates to a new usage of citric acid and compound citric acid in the field of pharmacy. The product can prevent and control viral diseases and bacterial diseases of livestock and poultry; wherein, the compound citric acid comprises the following components according to weight portion: 93-98 portions of citric acid, 0.5-4 portions of gualfenesin and 1.5-3 portions of etamsylate. Proved by in vivo and vitro experiments, the compound citric acid can play a role in preventing and controlling fowl mycoplasma gallisepticum, avian influenza (H9 subtype), bursal virus disease and otherdiseases, and can effectively kill animal pathogenic bacterias such as cattle colon bacillus, mycoplasma gallisepticum, chicken colibacillosis, etc.
Owner:四川通达动物保健科技有限公司
Etamsylate tablet and preparation method thereof
InactiveCN107823169AIncrease surface areaLarge porosityOrganic active ingredientsPharmaceutical non-active ingredientsSucroseAcrylic resin
The present invention provides a kind of ethylamine tablet and preparation method thereof, relate to the field of medical technology, consist of a tablet core and a coating layer, comprising the following steps: (1) combining ethylamine, low-substituted hydroxypropyl cellulose, Sorbitol and dextrin are mixed together, add 5 times the volume of distilled water, and ultrasonically treat for 2-3min to obtain a mixture. Add pectin and animal glue to the mixture, ultrasonically treat for 5-10min, and vacuum-dry to obtain solid particles. The granules are subjected to tabletting treatment to obtain a core pre-finished product; (2) magnesium stearate is evenly coated on the outside of the chip pre-finished product, and compressed again to obtain a tablet core; (3) microcrystalline cellulose, sucrose, citric acid Add triethyl ester and acrylic resin to 10 times the volume of distilled water, and ultrasonically treat for 2-3min to obtain a coating solution; (4) put the tablet core into the coating solution for coating to obtain phensulfame tablets. The phensulfame tablet of the present invention is beneficial to maintaining the stability of the drug effect, and has higher bioavailability after use by users.
Owner:HUAYI PHARMA ANHUI CO LTD
Medicine for treating rheumatism
InactiveCN106667990ARelieve joint swellingRelief the painHydrocarbon active ingredientsSkeletal disorderSide effectSterol
The invention discloses a medicine for treating rheumatism. The medicine for treating the rheumatism contains the following main raw materials in parts by weight: 8-13 parts of tea polyphenol, 10-16 parts of etamsylate, 4-11 parts of diazepam, 20-30 parts of lidocaine, 5-10 parts of ofloxacin, 8-12 parts of corn sterol, 4-10 parts of lycopene and 6-9 parts of lucidum polysaccharide. The medicine is capable of effectively relieving swelling and pains of limbs and joints caused by rheumatism and rheumatoid diseases and effectively improving the life quality of patients with rheumatism and rheumatoid diseases, the symptoms relief cycle is short after the medicine is taken, and obvious toxic and side effects are not found at present.
Owner:ZHENGZHOU ZHENGXIAN PHARMA CO LTD
P-hydroxybenzene sulfonate compound as well as preparation method and application thereof
PendingCN114380723AOrganic compound preparationSulfonic acids salts preparationPhenolBenzenesulfinic acid
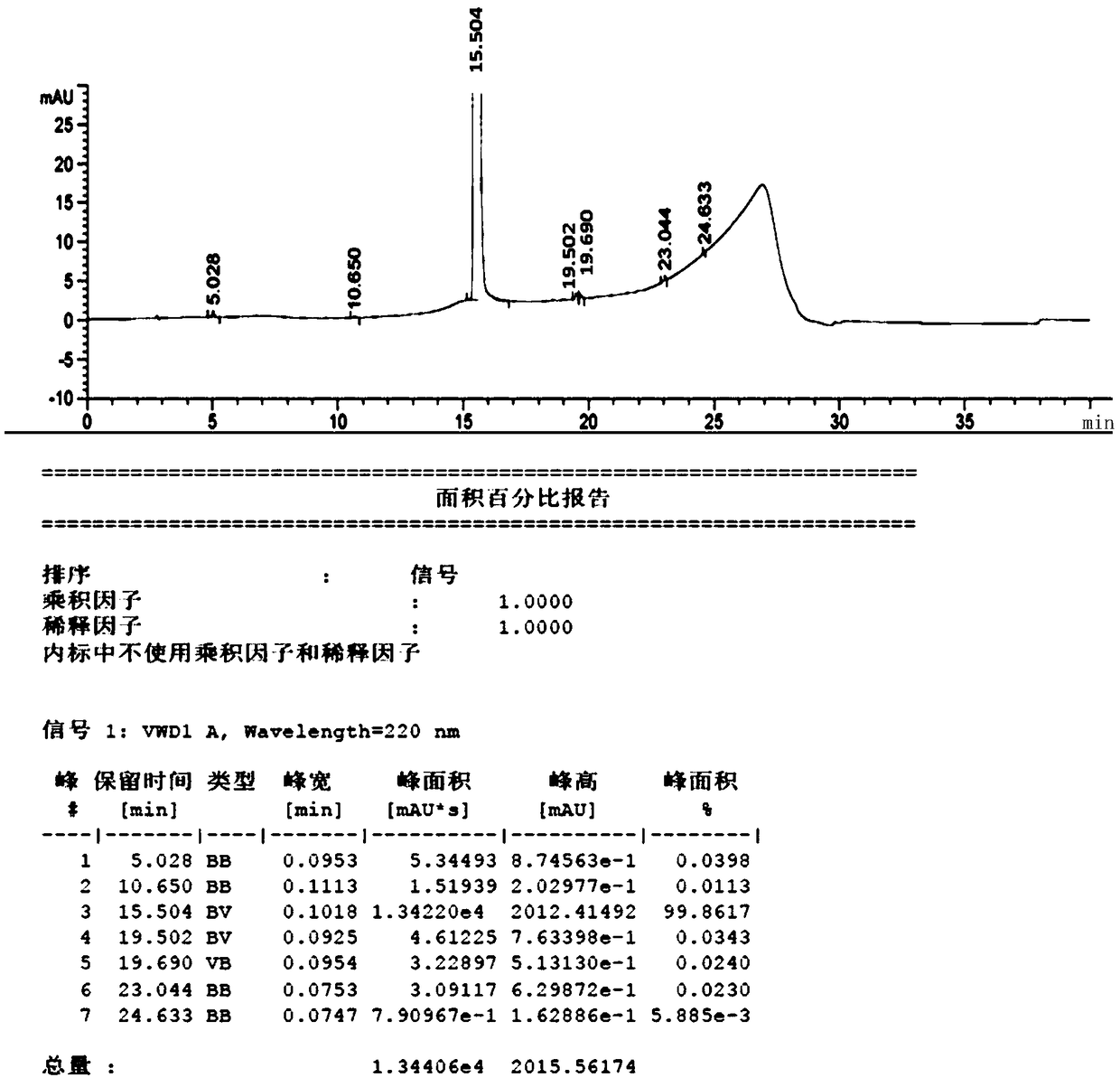
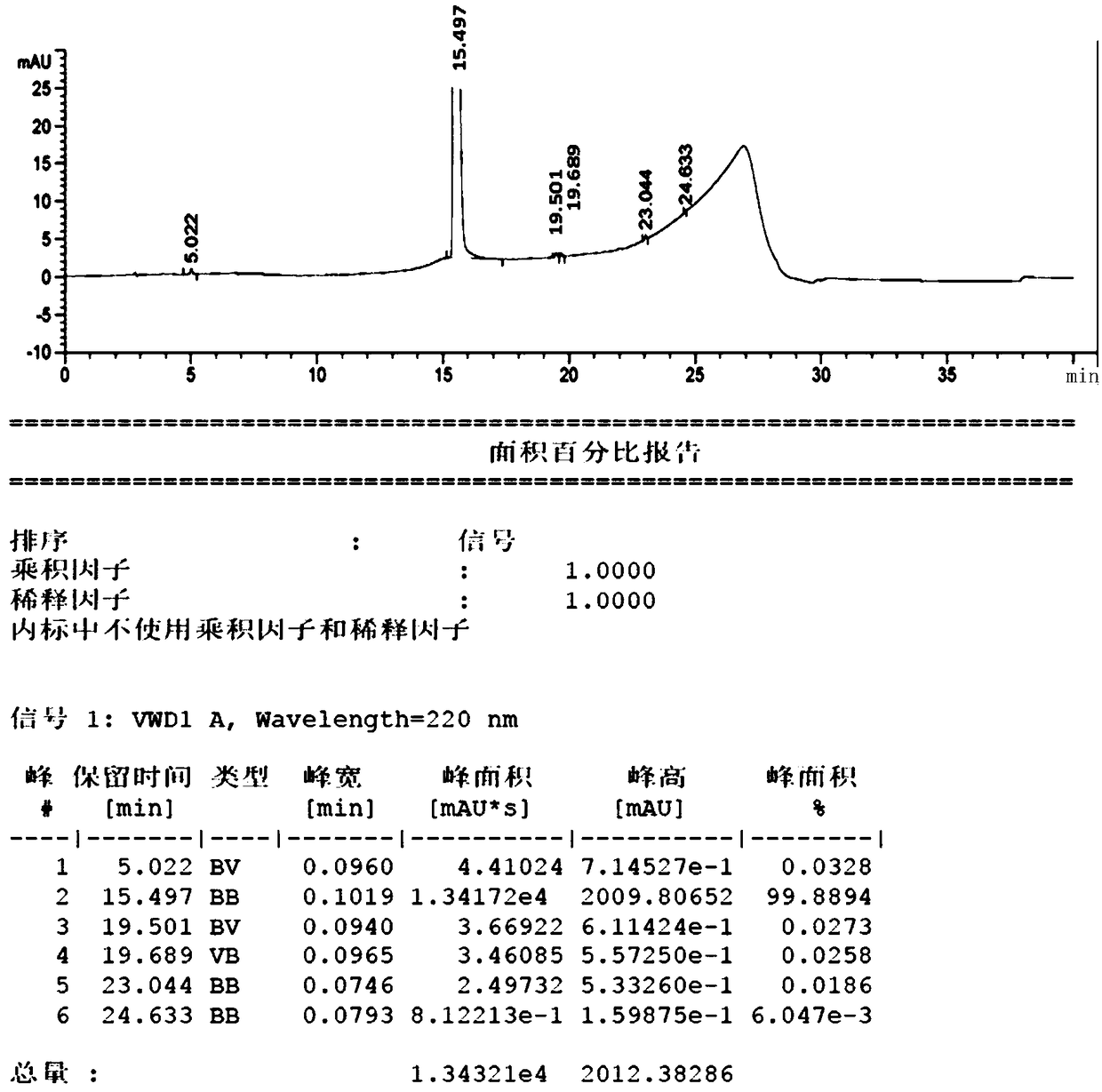
The invention relates to a p-hydroxybenzene sulfonate compound and application thereof, the p-hydroxybenzene sulfonate compound is specifically calcium dobesilate and etamsylate impurities, and the p-hydroxybenzene sulfonate compound is an impurity phenol brought into a starting material when hydroquinone is used as the starting material for synthesis, a process impurity generated in a sulfonation process, a process impurity generated in a sulfonation process and a process impurity generated in a sulfonation process. The compound can be used as an impurity reference substance of etamsylate bulk drugs and calcium dobesilate bulk drugs. The method disclosed by the invention has certain guiding significance on quality research of etamsylate and calcium dobesilate raw material medicines.
Owner:成都益安成贸易有限公司
A pharmaceutical preparation for preventing surgical bleeding and its preparation method
InactiveCN106963729BImprove securitySolve the phenomenon of bubblesOrganic active ingredientsInorganic non-active ingredientsSodium metabisulfiteSodium calcium edetate
The invention belongs to the technical field of medicine and particularly relates to a medicinal preparation for preventing operative bleeding and a preparation method thereof. The medicinal preparation is prepared from active ingredients etamsylate, sodium metabisulfite, sodium calcium edetate, methylparaben, a pH regulator and water for injection. The phenomenon that bubbles are produced in the filling process of etamsylate injections is avoided, the problem of product yield reduction caused by bubbles is solved, the safety of the injection in clinical application is greatly improved, and the medicinal preparation is simple and easy to operate and facilitates large-scale production.
Owner:王爱美 +1
Western medicine for curing cold of bamboo rat and preparation method of western medicine
InactiveCN106581028ADoes not affect eatingEasy to solveRespiratory disorderHeterocyclic compound active ingredientsRoxithromycinEphedrine
The invention discloses a western medicine for curing a cold of a bamboo rat and a preparation method of the western medicine. The western medicine for curing the cold of the bamboo rat comprises the following components by weight: 5-8 parts of chlortrimeton, 20-25 parts of calcium gluconate, 4-8 parts of cetirizine, 10-15 parts of ephedrine, 2-8 parts of acetylspiramycin, 4-10 parts of roxithromycin, 15-20 parts of promethazine, 8-15 parts of omeprazole, 10-15 parts of etamsylate, 8-10 parts of cedilanid, 2-6 parts of bromhexine, and 2-8 parts of aminophylline. Compared with the prior art, the western medicine is mixed into a feed to feed the bamboo rat and has good effects of preventing and treating the cold of the bamboo rat, the marked effective rates of the prevention and the treatment are up to 99% and 95%, and in addition, the western medicine has no strong flavor of the western medicine, is mixed into the feed to feed the bamboo rat, does not affect the feeding of the bamboo rat, and is convenient to use.
Owner:南宁市浩特竹鼠养殖场(微型企业)
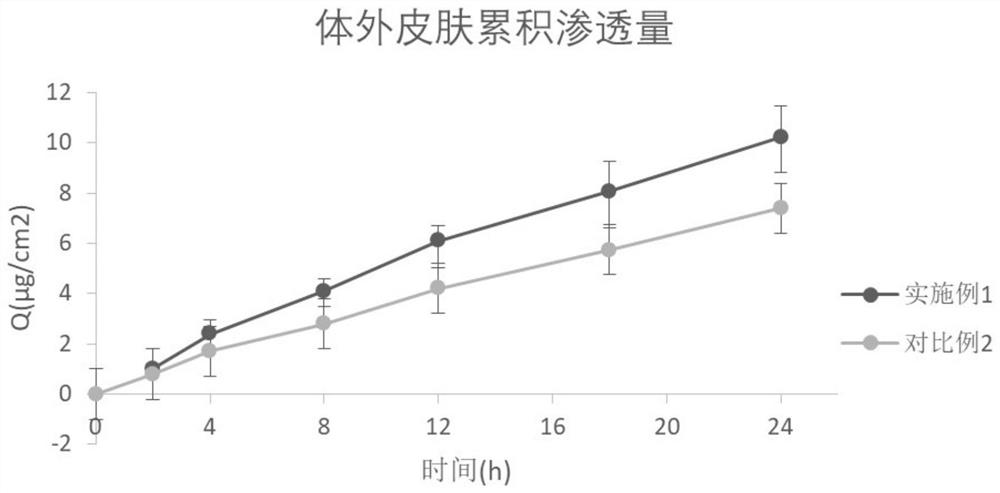
Etamsylate gel patch and preparation method thereof
PendingCN111773201ALittle side effectsNot easy to get dirtyOrganic active ingredientsPharmaceutical non-active ingredientsSkin penetrationPharmaceutical drug
The invention relates to the technical field of medicine, and discloses an etamsylate gel patch and a preparation method thereof. The etamsylate gel patch is composed of a backing layer, an adhesive layer, a gel layer and an anti-adhesive layer in sequence, wherein the gel layer comprises a gel substrate and a medicine active ingredient etamsylate. Adhesion tests, in-vitro dissolution tests and in-vitro skin penetration tests prove that the etamsylate gel patch has good adhesion performance, can be used repeatedly, can effectively release medicines through the skin, and can be stopped administrating at any time as needed to reduce systemic adverse reactions, and is safe and reliable.
Owner:YANGZHOU ZHONGBAO PHARMA
Biomarkers of Hypercoagulation State and Its Application
The invention discloses a biomarker having a high-blood coagulation status and an application of same. In the invention, three different blood coagulation promoting drugs (etamsylate, tranexamic acid and aminocaproic acid) are employed to respectively intervene in blood coagulation status of adult rats, thus successfully obtaining an adult rat acute high-blood coagulation status model; and then change on urine proteomes of the adult rats in intervention groups with different blood coagulation promoting drugs is analyzed; for the first time, the change feature of the urine proteomes under the high-blood coagulation status is systematically researched, and a biomarker, which is related to the high-blood coagulation status, is found in the urine. The invention initially discloses body intervention range and change factors of the high-blood coagulation status, which not only prompt related physiological and pathological mechanisms and the change feature of a signal pathway, but also are applied as an important biomarker in detection and / or diagnosis for related pathological change of the high-blood coagulation status or supply assistant information to the aspect.
Owner:BEIJING NORMAL UNIVERSITY
Compound tablet for treating functional uterine bleeding and preparation method thereof
InactiveCN105497870APromote circulationPhysical conditioningOrganic active ingredientsHeavy metal active ingredientsBenzoic acidLiver and kidney
The invention discloses a compound tablet for treating functional uterine bleeding and a preparation method thereof. The compound table comprises the following main ingredients: etamsylate, hoof nail polypeptide, lecithin, soyabean protein, estradiol benzoate, aminomethyl benzoic acid, norethindrone and ferrous fumarate, and further comprises polysaccharide-iron complex, pavlova viridis se-polysaccharide, seabuckthorn flavone, galactooligosaccharide, progesterone and iron-protein succinylate. By combining with various medical ingredients, the compound tablet has the functions of astringing proctoptosia, nourishing yin and supplementing blood, tonifying liver and kidney, performing antisepsis and anti-inflammation, removing blood stasis and stopping bleeding, and invigorating spleen and supplementing qi; besides, the compound tablet can improve the blood circulation, shortens the wound healing time, nurses the health of patients while stopping the bleeding, and enhances the immunity of the patients; furthermore, the compound tablet is convenient to take, definite in curative effect and low in costs, can relieve the surgery pain of the patients and reduce the economical burden of the patients, and has a wide application prospect.
Owner:许筱妹
A kind of method for producing high-purity phenolsulfonethylamide
ActiveCN109467522BImprove liquidityImprove conversion rateOrganic compound preparationAmino compound preparationOrganosolvSolvent
The invention discloses a method for producing high-purity etamsylate. The method comprises the following steps: carrying out a sulfonation reaction on hydroquinone serving as an initial material, a sulfonating agent, a dispersing agent and an organic solvent to obtain 2,5-dihydroxybenzenesulfonic acid; cooling the reaction solution to 45-70 DEG C, adding a mixed solution of diethylamine and waterto form a salt, cooling and crystallizing, so as to obtain the etamsylate. According to the reaction system used in the method, the fluidity of the system is improved, and the three-transfer-one-reaction efficiency is improved, so that the conversion rate of the materials is improved by 5-10%; when the system is cooled to 45-70 DEG C after the reaction is ended, the mixed solution of diethylamineand water is directly added into the system, the operation is simplified, and the after-treatment time is shortened; concentrated water is avoided, the energy consumption is reduced, and the yield ofthe product after salt formation reaches 80-85%; the product does not need to be re-crystallized or subjected to activated carbon discoloration, the purity directly reaches 99.5% or higher, and the content of all single impurities is lower than 0.05%; in addition, the use of a type of solvents and reagents containing genotoxicity warning structures is avoided, and totally safe and low-toxicity class-2 and class-3 solvents friendly to the humans and environment are used.
Owner:云南元顺医药有限公司
Synthetic method of etamsylate
ActiveCN102942509BNo water ensureHigh yieldOrganic compound preparationSulfonic acids salts preparationChlorosulfuric acidOrganic solvent
The invention relates to a synthetic method of etamsylate. The synthetic method comprises the following steps of (1) preparation of 2,5-dihydroxybenzenesulfonic acid; (2) preparation of a etamsylate crude product; and (3) purification. Compared with a conventional synthetic process of the etamsylate adopting concentrated sulfuric acid as a sulfonating agent, the synthetic method has the following advantages that the synthetic method employs chlorosulfonic acid as the sulfonating agent, and performs azeotropic dehydration on benzenediol and an organic solvent until anhydrous before sulfonation to ensure that no water is in the reaction system, thereby increasing product yield; hydrogen chloride gas produced during the sulfonation process can be absorbed by water to make hydrochloric acid, thereby decreasing discharge of three wastes (waste gas, waste water and waste residues); and usage amount of the sulfonating agent is decreased during the sulfonation process, so that production cost is reduced.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
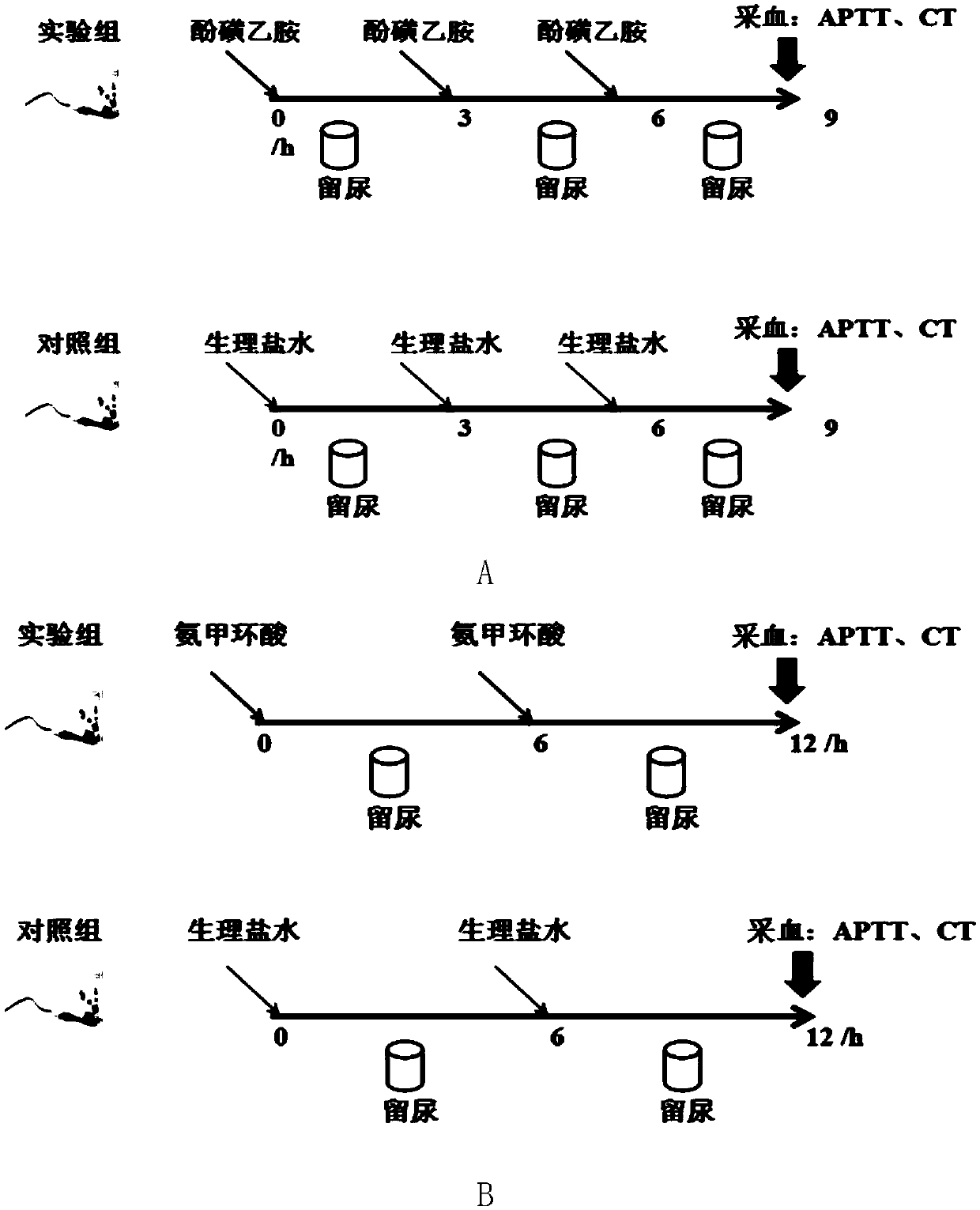
Etamsylate induced rat hypercoagulable state animal model and establishment method thereof
ActiveCN107308151AIncrease success rateEasy to operateOrganic active ingredientsBlood disorderHypercoagulable statesIntraperitoneal route
The invention discloses an etamsylate induced rat hypercoagulable state animal model and an establishment method thereof. According to the model, etamsylate is adopted for interference, three times of intraperitoneal injection are implemented, then a hypercoagulable state is achieved within 1.5-9 hours, the operation is simple, the model success rate is high, and the hypercoagulable state can last for a relatively long time. The rat hypercoagulable state animal model based on an etamsylate medicine is successfully established, a base for related rat hypercoagulable state research is made, and the establishment method is a novel rat hypercoagulable state animal model method.
Owner:BEIJING NORMAL UNIVERSITY
Uric acid kit for rapidly and simply eliminating drug interference of calcium dobesilate and etamsylate in serum
ActiveCN112522364AEliminate negative interferenceImprove stabilityMicrobiological testing/measurementBiological material analysisCreatininasePeroxidase
The invention relates to a uric acid kit for rapidly and simply eliminating drug interference of calcium dobesilate and etamsylate in serum. The uric acid kit is characterized by comprising a reagentR1 and a reagent R2, wherein the reagent R1 comprises a buffer solution, creatinase, creatine oxidase, ascorbic acid oxidase, peroxidase or catalase, serum albumin, a nonionic surfactant, a preservative, a Trinder's reactant A and laccase or metallate; and the reagent R2 comprises a buffer solution, serum albumin, creatininase, a preservative and a Trinder's reactant B. According to the invention,the defect that creatinine determination is inaccurate due to negative deviation caused by the calcium dobesilate and the etamsylate contained in serum samples when creatinine is determined by clinical examination in various hospitals at present is overcome, and the uric acid kit is suitable for detection of serum creatinine by a full-automatic biochemical analyzer.
Owner:ZHONGSHAN BGH BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com