Synthetic method of etamsylate
A synthesis method and a technology of sulfoethylamine, applied in the field of pharmaceutical synthesis, can solve the problems of poor product quality, many by-products, low yield and the like, and achieve the effects of reducing production costs, reducing three waste discharges and improving product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
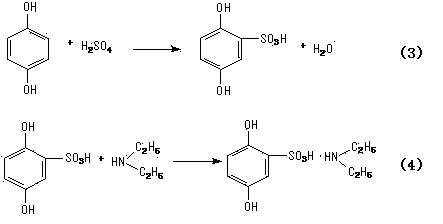
[0038] (1) Preparation of 2,5-dihydroxybenzenesulfonic acid
[0039] In a 500L reaction tank equipped with stirring, reflux condenser, and thermometer, add 50kg of hydroquinone and 150g of dichloroethane, start stirring, heat distillation and azeotropic dehydration until anhydrous, then cool down to 30°C, add chlorine dropwise 50kg of sulfonic acid was dropped within 3 hours, and the reaction was cooled with ice water, and the temperature was slowly raised to 90°C for 1 hour, then the temperature was lowered, dichloroethane was separated, and 36L of water was added to the residue to make 2,5-dihydroxy Benzenesulfonic acid is dissolved to obtain 2,5-dihydroxybenzenesulfonic acid solution;
[0040] (2) Preparation of the crude product of phensulfame
[0041] In a 500L reaction tank equipped with stirring, high-level tank, and thermometer, add the 2,5-dihydroxybenzenesulfonic acid solution obtained in step (1), add diethylamine into the high-level tank, and 2,5-dihydroxybenzenes...
Embodiment 2
[0046] (1) Preparation of 2,5-dihydroxybenzenesulfonic acid
[0047] In a 500L reaction tank equipped with stirring, reflux condenser, and thermometer, add 50kg of hydroquinone and 250g of dichloromethane, start stirring, heat distillation and azeotropic dehydration to anhydrous, then lower the temperature to 50°C, and add chlorosulfur dropwise Add 55kg of acid, drop it within 3 hours, and cool the reaction with ice water, react at 30°C for 3 hours, then lower the temperature, separate dichloroethane, add 40L of water to the residue, and dissolve 2,5-dihydroxybenzenesulfonic acid , to obtain 2,5-dihydroxybenzenesulfonic acid solution;
[0048] (2) Preparation of the crude product of phensulfame
[0049] In a 500L reaction tank equipped with stirring, high-level tank, and thermometer, add the 2,5-dihydroxybenzenesulfonic acid solution obtained in step (1), add diethylamine into the high-level tank, and 2,5-dihydroxybenzenesulfonic acid The mass ratio to diethylamine is 1:1, a...
Embodiment 3
[0054] (1) Preparation of 2,5-dihydroxybenzenesulfonic acid
[0055]In a 500L reaction tank equipped with stirring, reflux condenser, and thermometer, add 50kg of hydroquinone and 180g of chloroform, start stirring, heat distillation and azeotropic dehydration to anhydrous, then cool down to 40°C, and dropwise add 52kg of chlorosulfonic acid , dropped within 3 hours, and cooled the reaction with ice water, slowly raised the temperature to 50°C for 2 hours, then lowered the temperature, separated dichloroethane, and added 38L of water to the residue to make 2,5-dihydroxybenzenesulfonic acid Dissolving to obtain 2,5-dihydroxybenzenesulfonic acid solution;
[0056] (2) Preparation of the crude product of phensulfame
[0057] In a 500L reaction tank equipped with stirring, high-level tank, and thermometer, add the 2,5-dihydroxybenzenesulfonic acid solution obtained in step (1), add diethylamine into the high-level tank, and 2,5-dihydroxybenzenesulfonic acid The mass ratio to die...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com