P-hydroxybenzene sulfonate compound as well as preparation method and application thereof
A technology of hydroxybenzenesulfonate compound and hydroxybenzenesulfonate, which is applied in the field of synthesis of hydroxybenzenesulfonate compound, and can solve problems such as lagging research work on phensulfonate and calcium dobesilate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
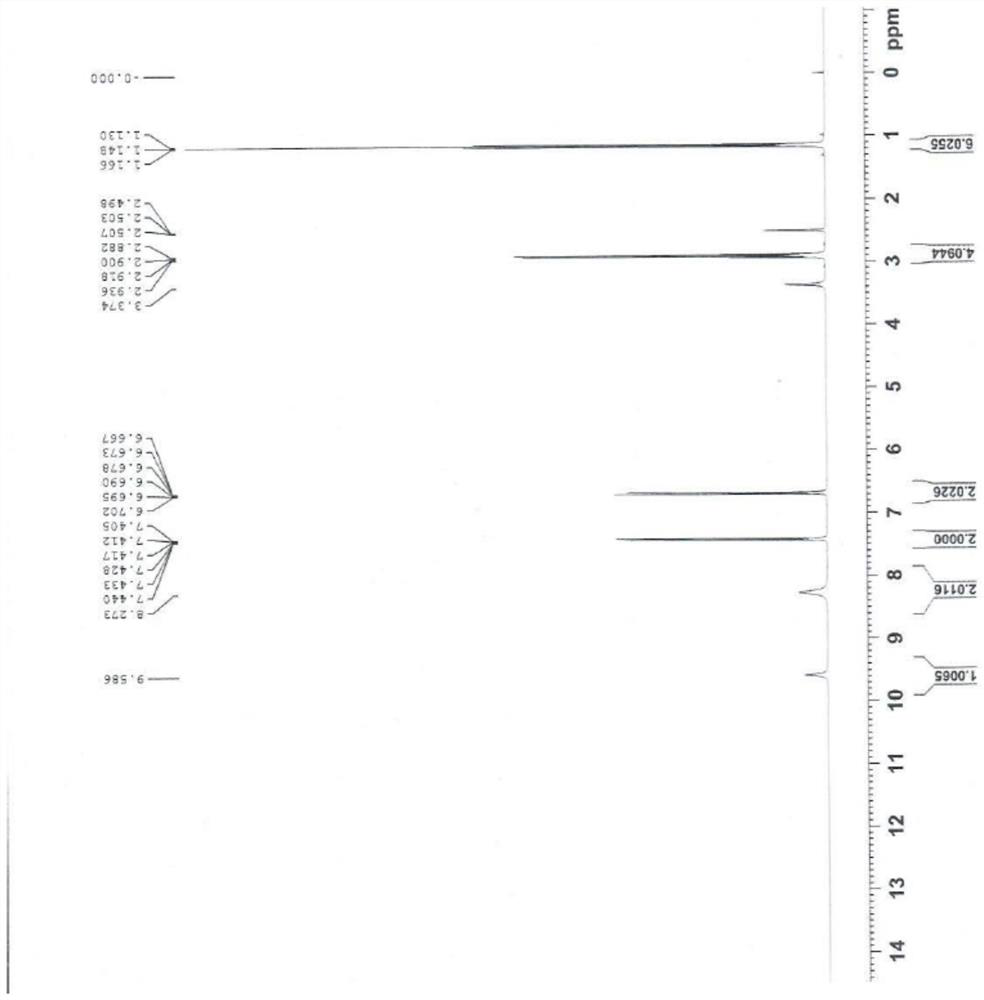
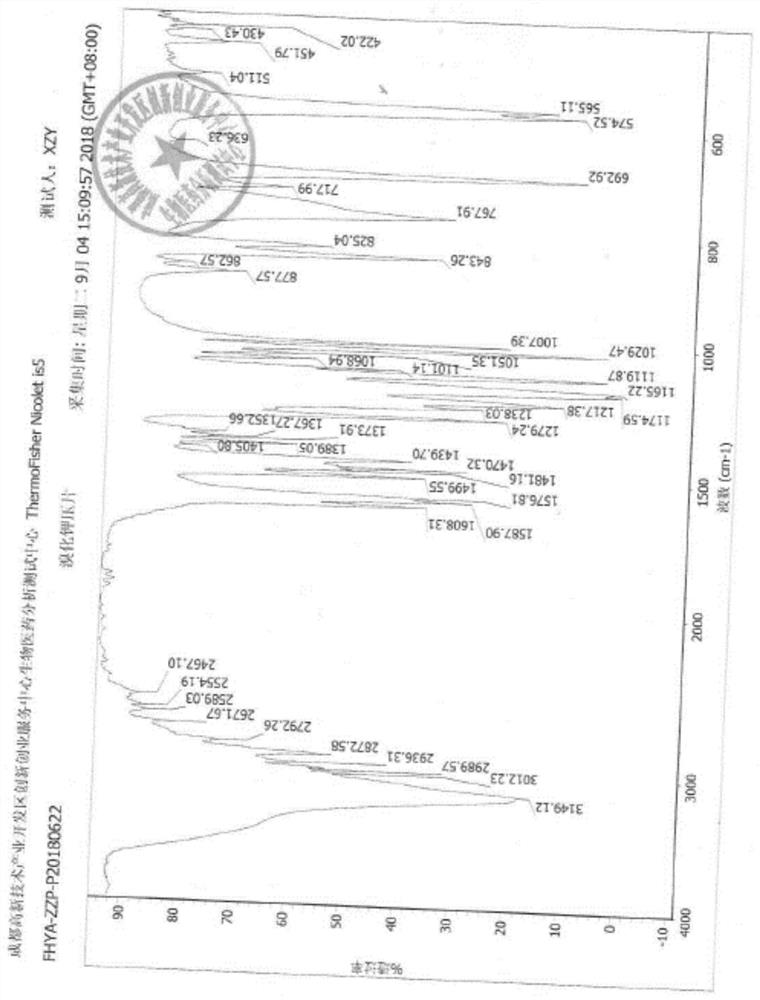
Image
Examples
Embodiment 1
[0047] Embodiment 1 p-Hydroxybenzenesulfonic acid diethylamine salt is synthesized
[0048] Take 94.10g of phenol and add 282ml of n-heptane (3 times the volume of phenol feeding mass) and 142.10g of sulfuric acid (the amount is 1.45 equivalents of phenol), heat and stir until reflux, stir and keep warm for 4h. After the reaction is complete, remove the solvent, cool down to room temperature, add 56ml of water (0.6 times the mass volume of phenol) to disperse, then control 50-60°C and add 87.68g of diethylamine (1.2 equivalents of phenol) to form a salt. After salt formation, lower the temperature to 0-5°C, stir and heat-preserve for crystallization for 6 hours, filter with suction, wash the filter cake with 150ml of isopropanol, and dry the filter cake under vacuum at 60°C for 4 hours to obtain 209.02g of off-white powdery crude product, molar yield The rate is 84.52%.
[0049] Remove 200.04g of the crude product, add 160ml of isopropanol aqueous solution for recrystallizati...
Embodiment 2
[0051] Embodiment 2 p-Hydroxybenzenesulfonic acid calcium salt is synthesized
[0052] Take 94.23g of phenol, add 424ml of n-hexane (the amount is 4.5 times the mass volume of the phenol feed) and 147.51g of sulfuric acid (the amount is 1.5 equivalent of the phenol feed), heat and stir until reflux, stir and keep warm for 8h. Complete reaction, after being cooled to room temperature, pour off solvent, add 122.5ml into water (consumption is 1.3 times of mass volume of phenol charging capacity) after dispersing, add 114.13g calcium carbonate in batches (addition is 1.14 equivalents of phenol charging capacity) adjustment pH to 5 into a salt. After salt formation, insoluble matter was filtered off, and the filtrate was concentrated under reduced pressure at 50°C until the system was slightly turbid, and the concentration was stopped. The concentrated raffinate was cooled to 0-5°C, stirred and crystallized for 8 hours, filtered with suction, and the filter cake was rinsed with 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com