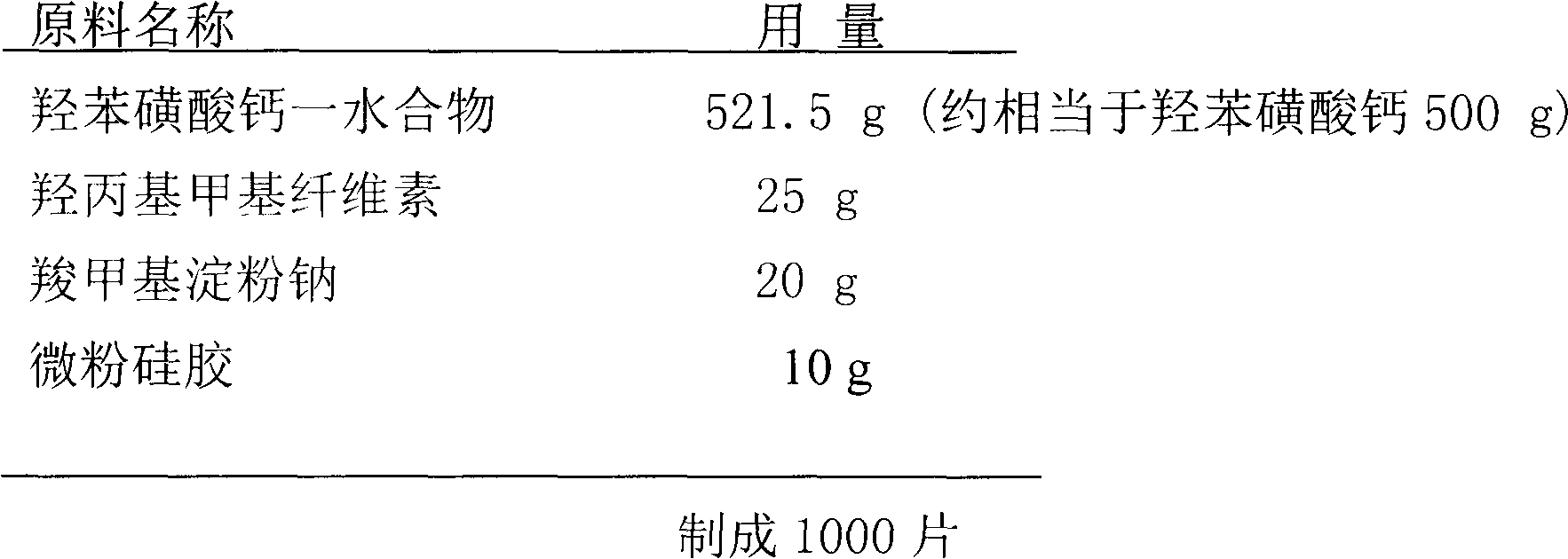
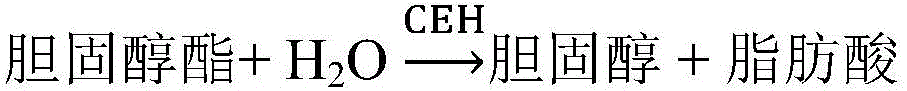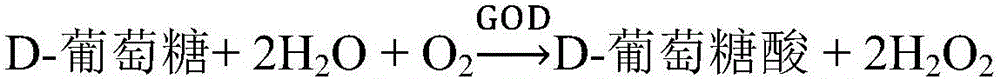Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
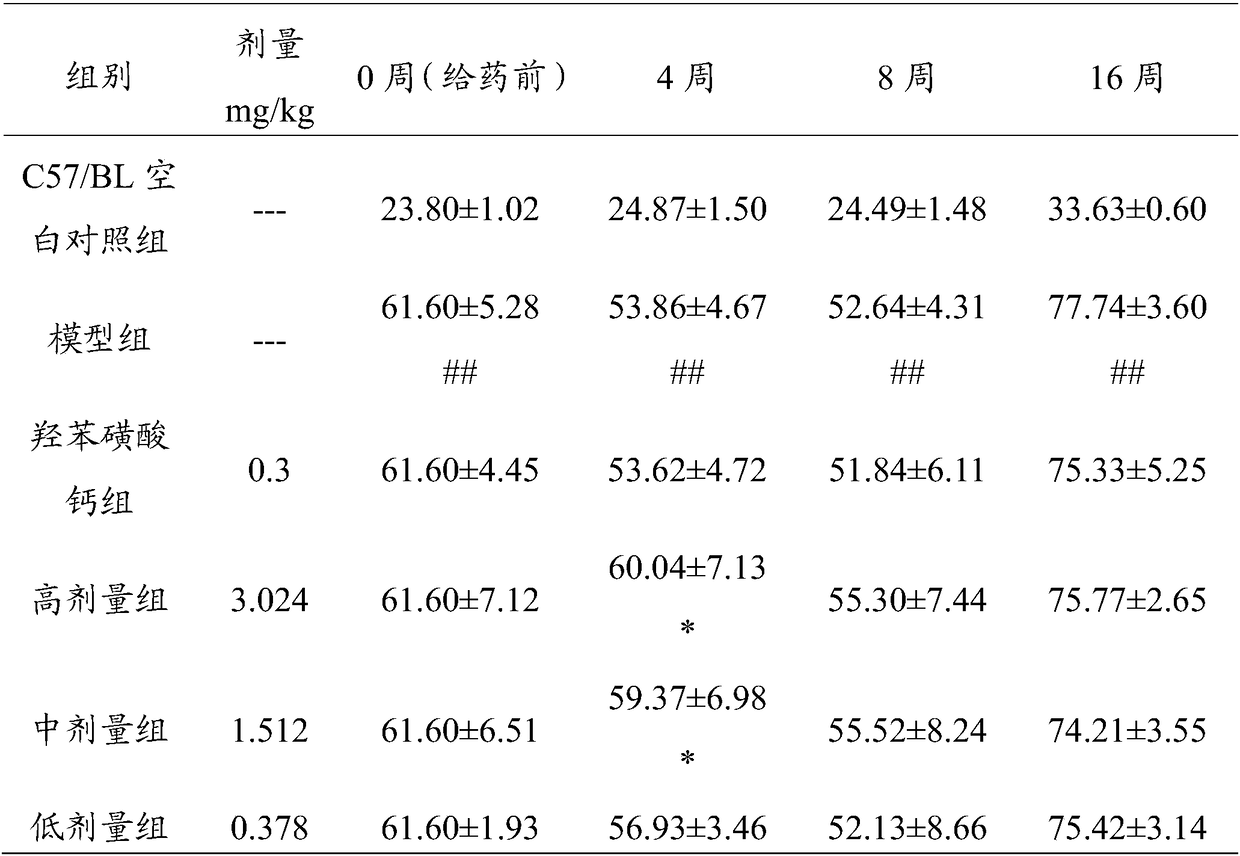
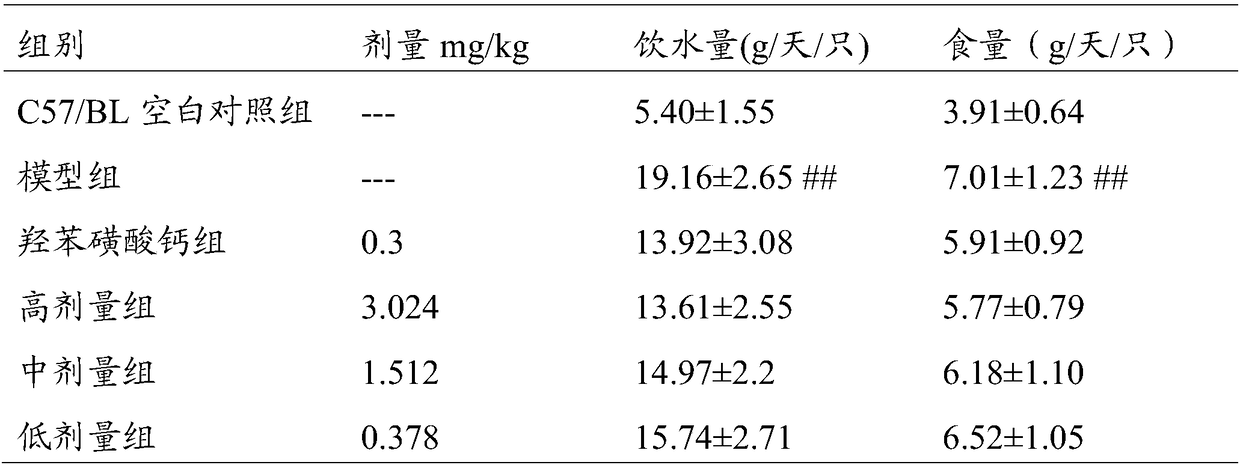
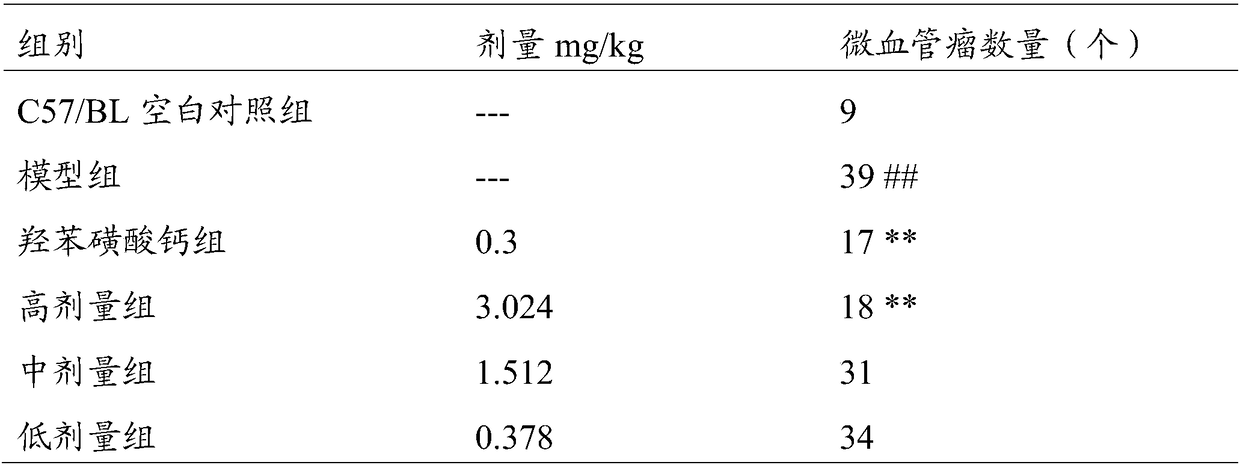
74 results about "Calcium dobesilate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
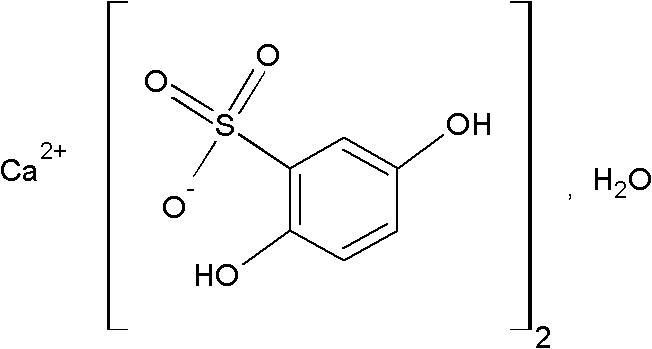
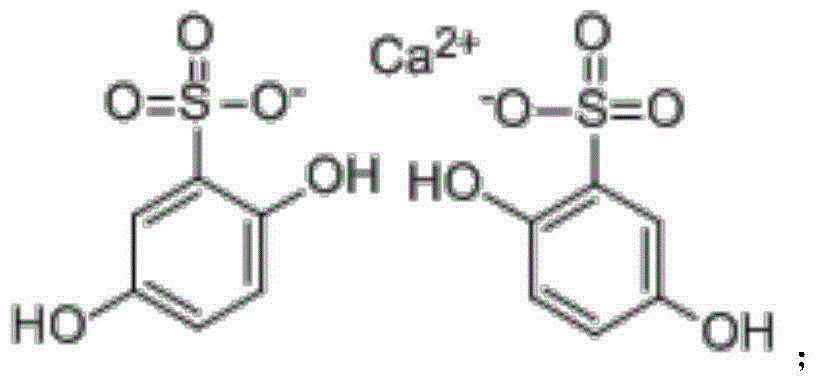
Calcium dobesilate is a vasoprotective. It is the calcium salt of dobesilic acid. It is a synthetic molecule with the ability to reduce capillary permeability in the body. In Italy the drug is sold by the pharmaceutical company OM Pharma under the trade name of Doxium in capsules containing 500 mg of active ingredient.
Cosmetic composition and uses thereof
The invention provides a cosmetic composition and uses thereof. The cosmetic composition comprises an active ingredient and a matrix, wherein the active ingredient is calcium dobesilate. The cosmetics can be made into solution, soap, cream, tincture, film, gel, shampoo and toothpaste type. The calcium dobesilate in the cosmetic composition has the effects of improving skin capillary circulation and reducing skin telangiectasia. The cosmetic composition can be used for preparing cosmetics for caring skin, preventing alopecia, promoting hair growth, protecting gums and strengthening teeth, removing facial blood streak and ecchymoses, and preventing and treating seborrheic dermatitis, rosacea and acne.
Owner:段亚东
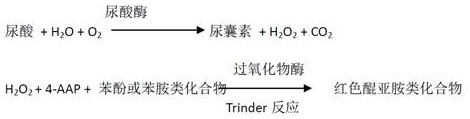
Kit and method for assaying creatinine
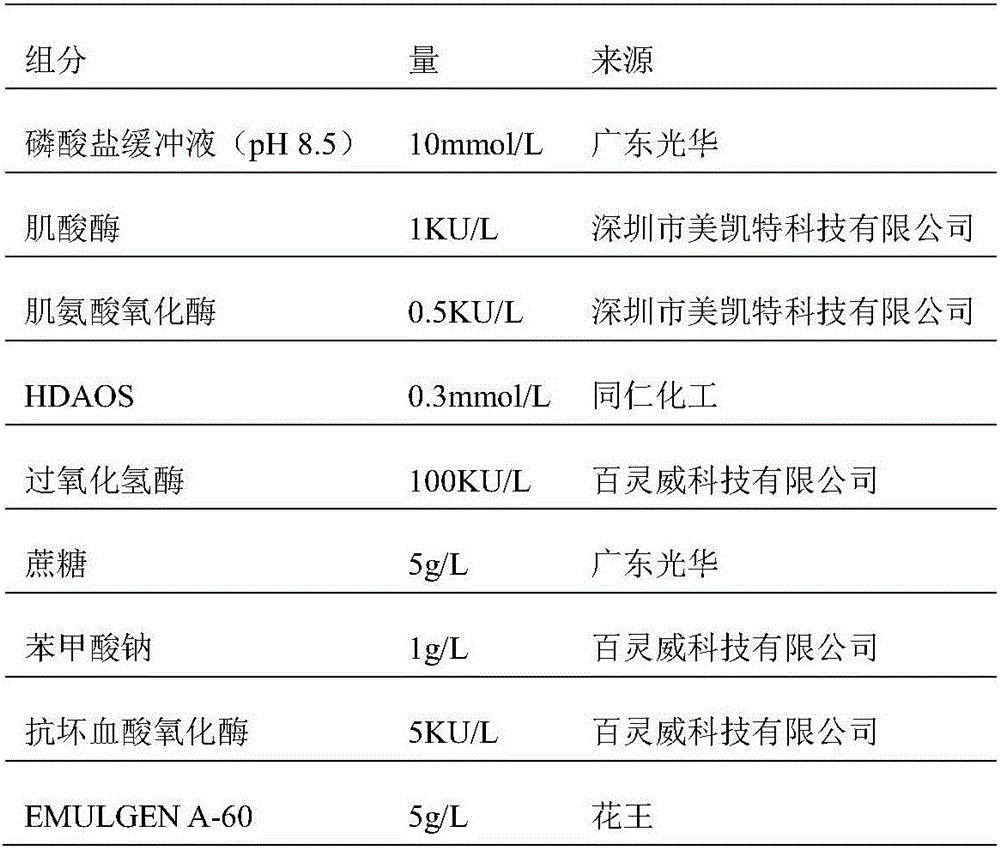
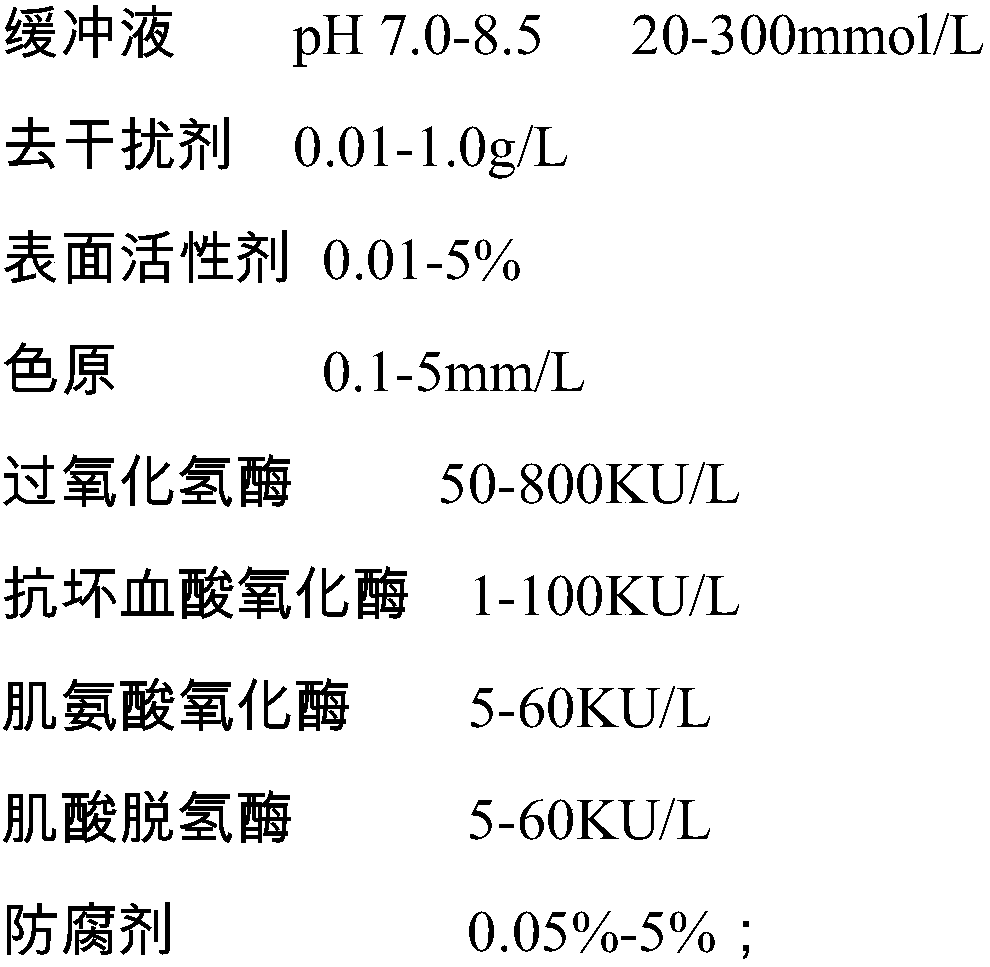
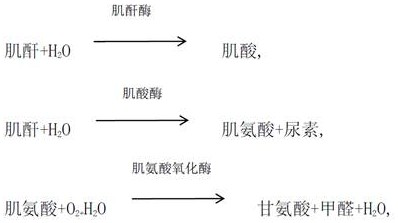
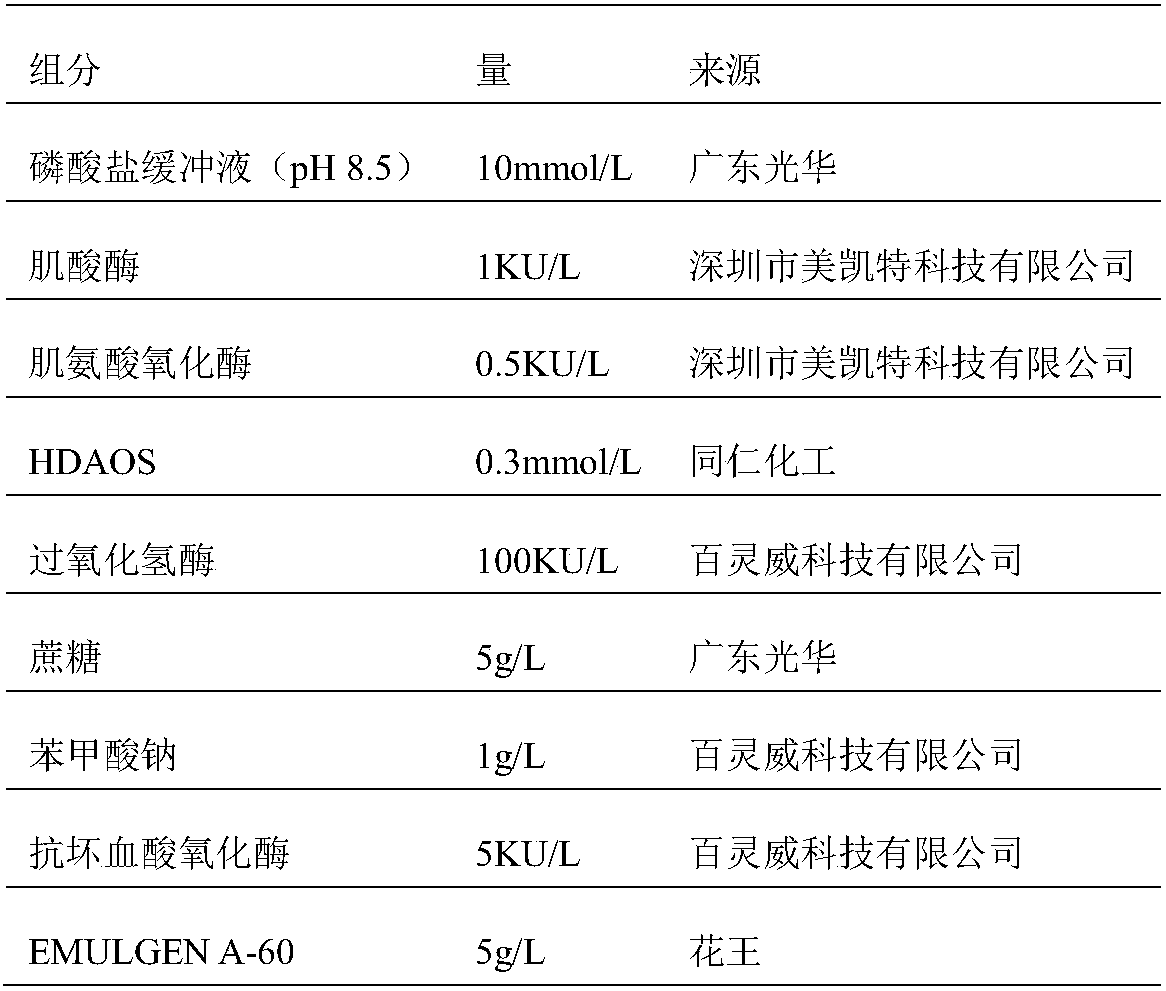
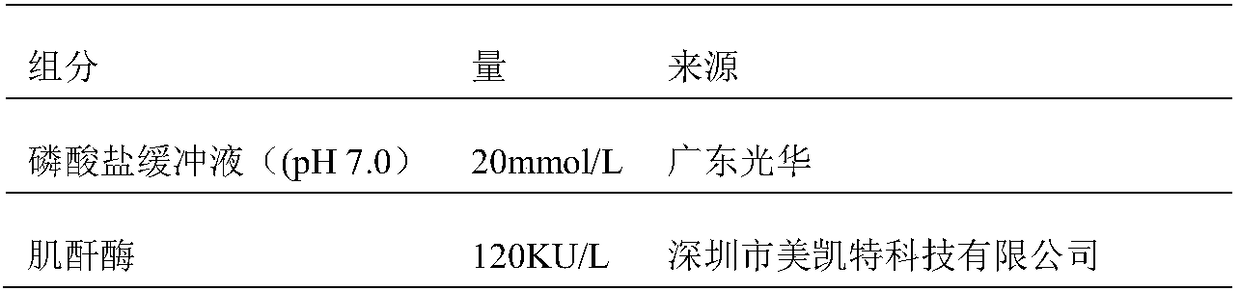
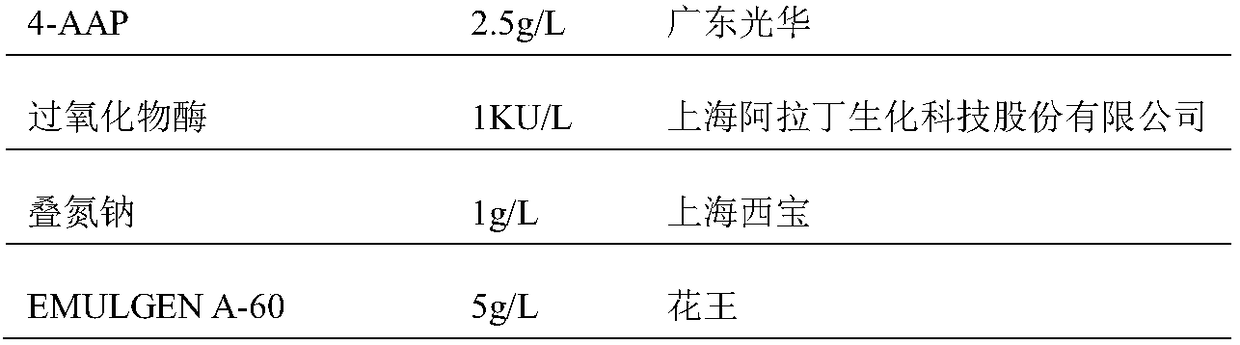
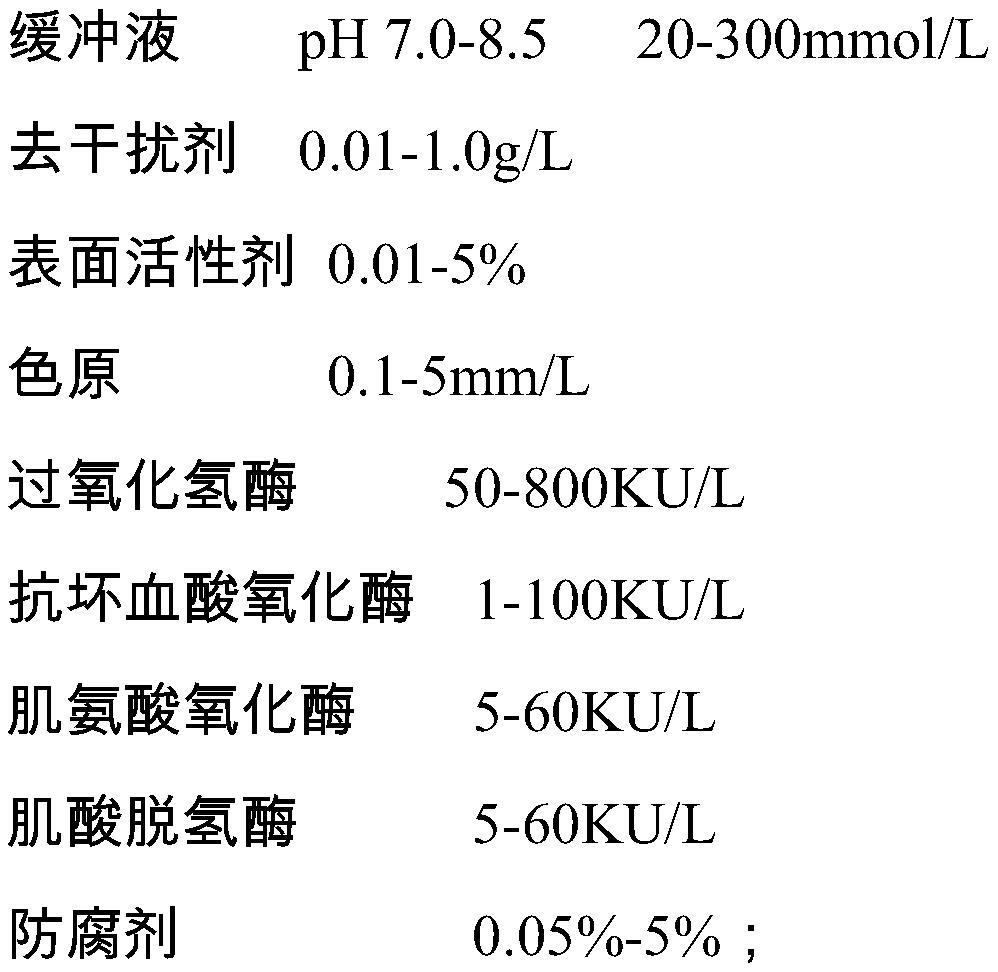
ActiveCN106198509AMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementSarcosine oxidaseCreatininase
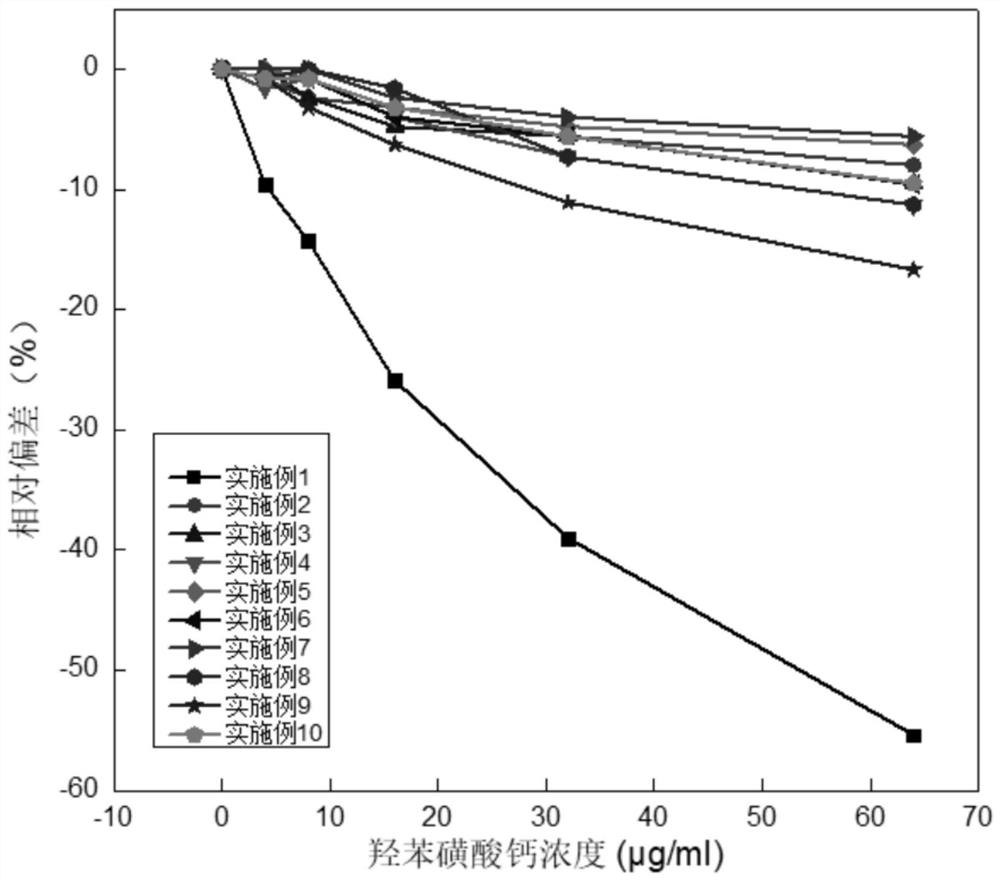
The invention relates to a kit and a method for assaying creatinine. The kit includes a first reagent group and a second reagent group. The first reagent group includes creatinase, sarcosine oxidase, chromogen and catalase; the second reagent group includes creatininase, 4-amino antipyrine and peroxidase. The chromogen is selected from N-(2-hydroxyl-3-sulfopropyl)-3,5-dimethoxyaniline or a sodium salt thereof, and N-ethyl-N-(2-hydroxyl-3-sulfopropyl)-3,5-dimethoxyaniline. The kit and the method can reduce interference during the detection of the creatinine due to the calcium dobesilate and / or etamsylate that exist in a sample.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
Composition for eliminating interference of calcium dobesilate medicine on creatininase-method detection
ActiveCN108627654ASolve the problem of serious negative interferenceDisease diagnosisBiological testingHigh concentrationCreatininase
The invention relates to a composition for eliminating the interference of a calcium dobesilate medicine on creatininase-method detection. The composition comprises an R1 reagent, an R2 reagent and ahigh molecular oxidant, wherein the high molecular oxidant is one or more of high molecular peroxy acid, high molecular selenium oxide, high molecular sulfur chloride ether, N-substituted imide, water-soluble tetrazole and a Dess-Martin oxidant. The high molecular oxidant in the composition disclosed by the invention can react with the calcium dobesilate in a sample to be detected, the calcium dobesilate loses the effect of interfering enzyme-method creatinine detection and the accuracy of sarcosine-oxidase-method detection is improved, so that the problem of serious negative interference, caused by high-concentration calcium dobesilate in blood of a patient, on an enzyme-method creatinine project detection result when the patient orally takes the calcium dobesilate medicine is solved.
Owner:WUHAN HANHAI NEW ENZYMES BIOLOGICAL TECH CO LTD
Spawning-induced addition agent for artificial propagation of sea water fish
InactiveCN101480396AImprove survival rateEasy to expandSexual disorderAnhydride/acid/halide active ingredientsAdditive ingredientMortality rate
A parturition hastening accessory ingredient used for artificially propagating briny fishes relates to medicine used for artificially propagating briny fishes. The invention aims to provide accessory medicine for artificially propagating briny fishes, which enables gonadotropic hormones to more quickly act on a target organ, shortens the effect time of ripe fishes, enhances the effect on promoting the sexual maturity and accelerating maturity, improves the rate for hastening parturition and simultaneously reduces the death rate in the post-partum period of the ripe fishes. Adopting hexanone theobromine and calcium hydroxybenzosulfonate which are medical raw materials in the technical scheme, the invention is characterized in that the hexanone theobromine and the calcium dobesilate which are the medical raw materials are finely ground and uniformly mixed to form the parturition hastening accessory ingredient and is used for artificially propagating briny fishes.
Owner:EAST CHINA SEA FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Human serum creatinine content detecting reagent and method for resisting calcium dobesilate interference
PendingCN111394424AEliminate distractionsGuaranteed accuracyMicrobiological testing/measurementBiological material analysisElevated creatinineCalcium dobesilate
The embodiment of the invention discloses a human serum creatinine content detecting reagent for resisting calcium dobesilate interference. The detection reagent comprises a first reagent, a second reagent and a third reagent, wherein the first reagent comprises sodium carbonate. The detection reagent is used for determining human serum creatinine based on an enzyme method, and besides, interference of calcium dobesilate drugs in serum can be avoided. The defect for detecting creatinine by a conventional enzyme method is overcome. The embodiment of the invention further provides a human serumcreatinine content detecting method for resisting calcium dobesilate interference.
Owner:海丰生物科技(北京)有限公司
Kit and method for determining triglyceride
ActiveCN106399460AMicrobiological testing/measurementBiological material analysisPeroxidaseMagnesium salt
The invention relates to a kit and a method for determining triglyceride. The kit comprises a reagent group 1 and a reagent group 2, wherein the reagent group 1 comprises magnesium salts, triphosadenine, glycerokinase, glycerol lipase oxidase, peroxidase and chromogen; the reagent group 2 comprises lipoprotein lipases and 4-amino-antipyrine, wherein the chromogen is N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3,5-dimethoxyaniline (DAOS) or comprises DAOS. The kit and the method provided by the invention have the advantage that the interference caused by calcium dobesilate and / or etamsylate existing in the sample during the triglyceride detection can be reduced.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
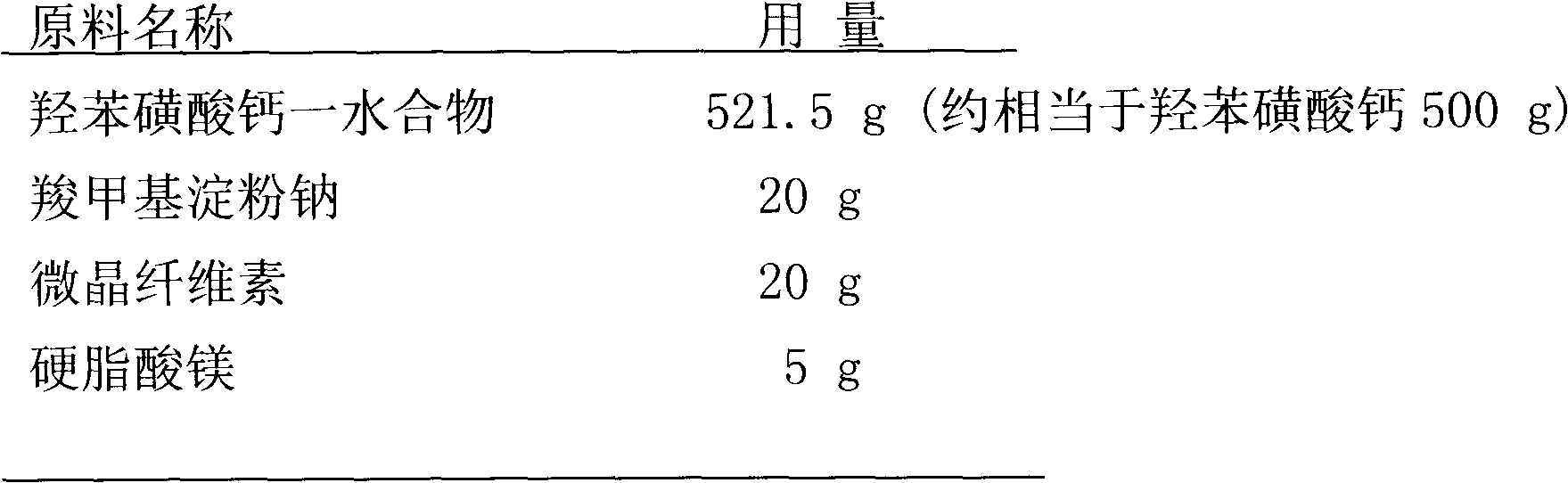
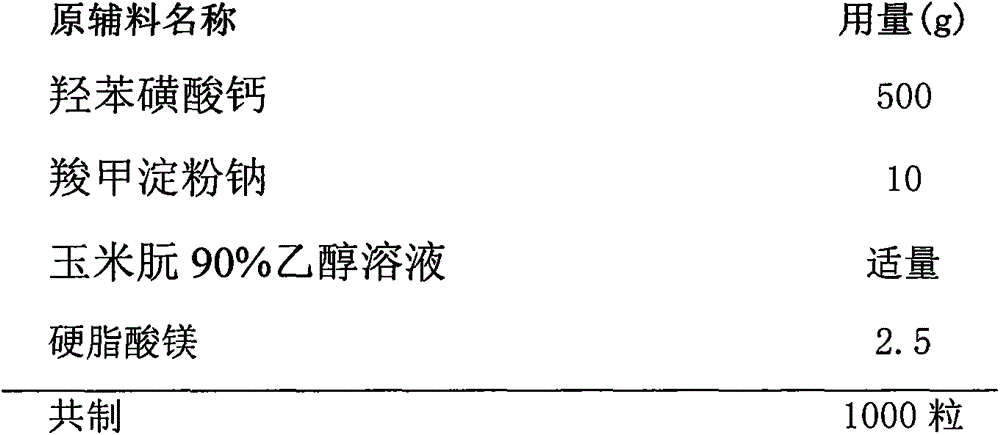
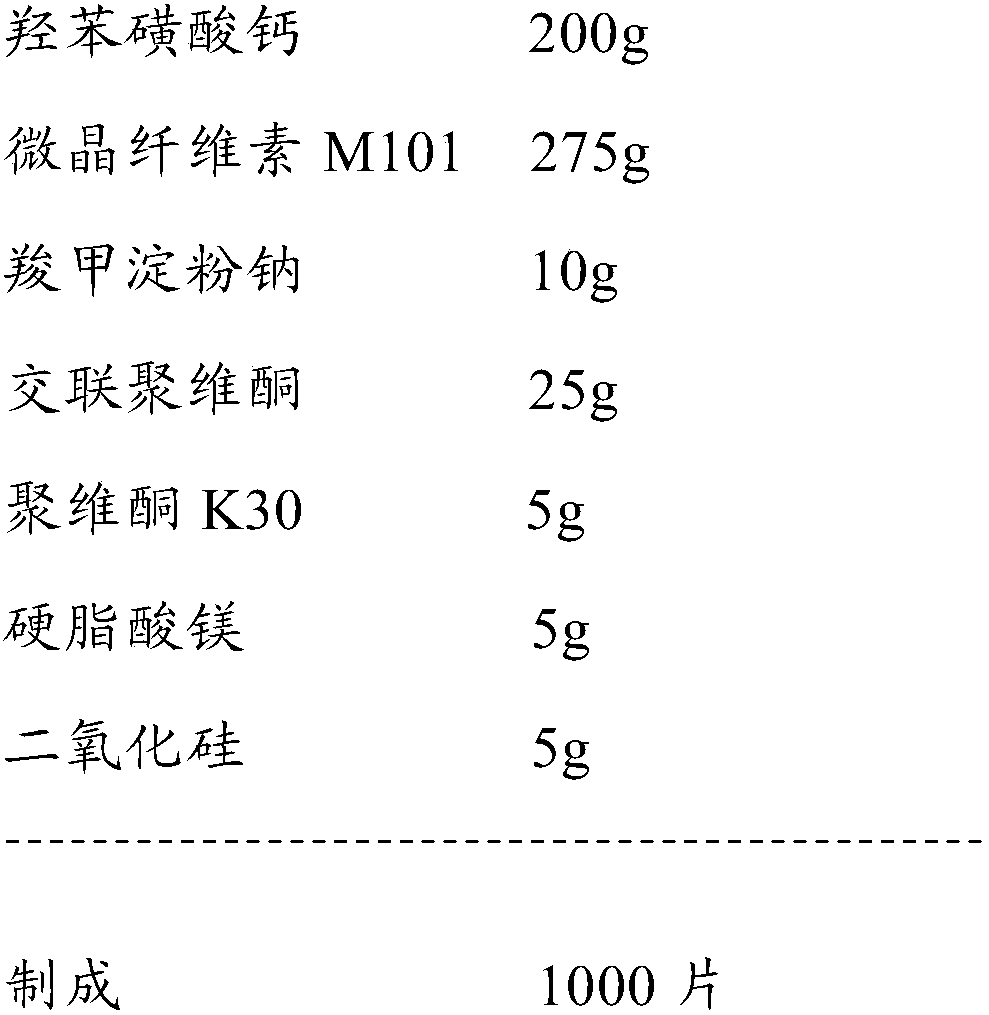
Calcium dobesilate capsule and preparation method thereof
InactiveCN102091055AThe effect is fully verifiedAvoid loading differencesSenses disorderMetabolism disorderDissolutionCroscarmellose sodium
The invention discloses a calcium dobesilate capsule and a preparation method thereof. The calcium dobesilate capsule is prepared from calcium dobesilate, croscarmellose sodium, magnesium stearate and polyvinyl pyrrolidone, wherein every 1000 calcium dobesilate capsules contain 500g of calcium dobesilate, 20-50g of croscarmellose sodium and 2-6g of magnesium stearate. In addition, a traditional preparation method is improved in the invention, and the improved method comprises the following steps: on the basis of taking the croscarmellose sodium as a disintegrating agent and taking the magnesium stearate as a lubricating agent, adding a proper amount of the polyvinyl pyrrolidone to prepare a bonding agent; preparing the bonding agent and the calcium dobesilate as well as the croscarmellosesodium into granules by a multi-step granulation method; and spraying the ethanol solution of the magnesium stearate onto the surfaces of the granules to obtain calcium dobesilate capsules with stable quality, high dissolution rate and small content uniformity.
Owner:HAINAN JINRUI PHARMA CO LTD
Method for preparing medicinal high-purity calcium dobesilate
The invention relates to a method for preparing medicinal high-purity calcium dobesilate. The method comprises the following steps of: 1, treating a coarse product of calcium dobesilate by using an organic solvent; and 2, re-crystallizing by using an aqueous solution of ethanol, wherein the organic solvent is acetone, ethyl acetate or a mixed solvent of acetone and ethyl acetate, and a volume ratio (V / V) of the acetone to the ethyl acetate is 1:5-5:1.
Owner:BEIJING ZHENDONG GUANGMING PHARMA RES INST +1
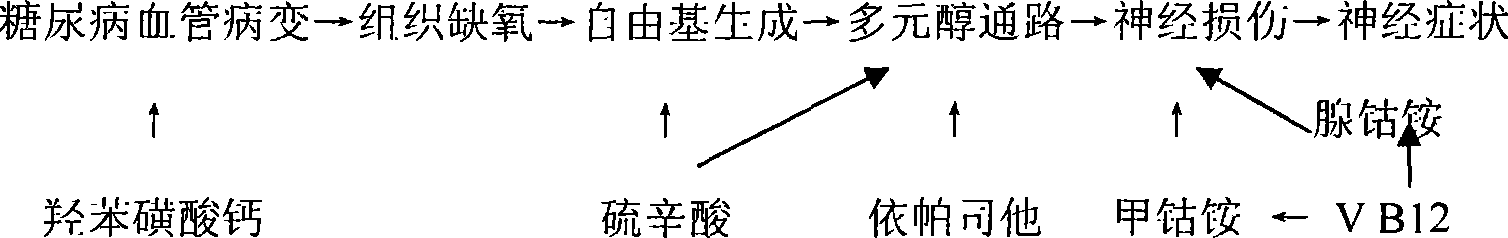
Medicinal composition for treating diabetes chronic complication and preparation method thereof
InactiveCN101214257AReduced mechanical pain thresholdImprove neurological symptomsNervous disorderMetabolism disorderAdditive ingredientVitamin B12
The present invention relates to a series of medicine combinations for remedying diabetic chronic complication and a preparation method thereof, which are compound oral preparations (comprising troche, dispersible tablet, effervescent tablet, capsule preparation, soft capsule and granule preparation) which are mainly made from at least two active components of mecobalamin, cobamamide, vitamin B12, epalrestat, lipoic acid and calcium dobesilate and pharmic acceptable auxiliary materials. The preferential combinations of the present invention are the mecobalamin plus the lipoic acid, the mecobalamin and the lipoic acid plus the calcium dobesilate, the mecobalamin and the epalrestat plus the calcium dobesilate, the mecobalamin, the lipoic acid and the epalrestat plus the calcium dobesilate, and the mecobalamin and the vitamin B12 plus cobamamide. The present invention is mainly used for remedying the nervous lesion of the diabetic chronic complication.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
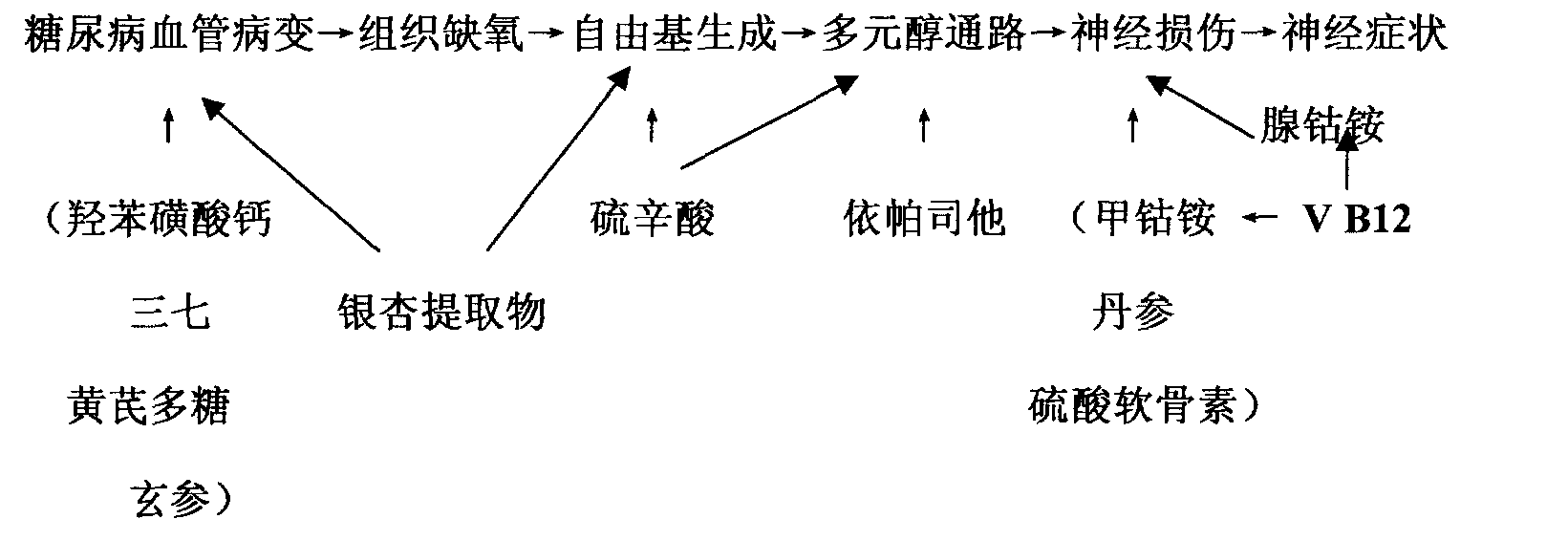
Chemical medicine and natural medicine composition for treating diabetes chronic complication and preparation method thereof
The invention relates to a series of combinations of chemical drug and natural drug used for treating diabetic chronic complications and the preparation method thereof, which are the combinations formed by one or a plurality of chemical drugs and one or a plurality of natural drugs, wherein, the chemical drug is any of mecobalamin, cobamamide, vitamin B12, epalrestat, lipoic acid and calcium dobesilate, and the natural drug is any of chondroitin sulfate, ginkgo biloba extract, astragalus polysaccharide, salvia miltiorrhiza, panax notoginseng and scrophularia. The preferred combination is: mecobalamin + lipoic acid + ginkgo biloba extract, mecobalamin + lipoic acid + ginkgo biloba extract + astragalus polysaccharide + salvia miltiorrhiza + panax notoginseng + scrophularia, mecobalamin + lipoic acid + calcium dobesilate + ginkgo biloba extract, mecobalamin + lipoic acid + chondroitin sulfate+ epalrestat + calcium dobesilate + ginkgo biloba extract, mecobalamin + lipoic acid + chondroitin sulfate + epalrestat + calcium dobesilate + ginkgo biloba extract + astragalus polysaccharide + salvia miltiorrhiza + panax notoginseng + scrophularia. The combinations are used for treating the microvessel and nerve lesions of diabetic chronic complications.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Calcium dobesilate injection
InactiveCN101062011AImprove stabilityLong validity periodPowder deliveryPharmaceutical non-active ingredientsVitamin CAdditive ingredient
The invention discloses an injection and powder needle injection for eyeball rear injection of benzyl sulfonic acid calcium, which is characterized by the following: allocating 0. 2-20 / mL benzyl sulfonic acid calcium and 0-50% medicinal findings; setting the medicinal findings as bone holding agent and antioxidant; choosing the bone holding agent from one of manna sugar, vitamin C, carbamide, antacidin, dextran, polyvinylpyrrolidone and aminoacetic acid or their mixture; choosing the antioxidant from one of vitamin C, vitamin E, sodium sulfite, sodium acid sulfite, sodium metabisulfite, natrium hyposulfidum, methilanin, thiourea, editic acid disodium and citric acid or their mixture. This powder needle injection is one of spraying or hot air dry injection, powder needle injection and freezing dry powder needle injection.
Owner:SHENYANG XINGQI PHARM CO LTD
Externally-applied skin gel as well as preparation method and application thereof
InactiveCN102641267AFunction increaseReduce in quantityAerosol deliveryOintment deliveryTinidazoleAdditive ingredient
The invention discloses externally-applied skin gel as well as a preparation method and application thereof, belongs to the technical field of medicines and relates to preparation of the externally-applied skin gel from calcium dobesilate and any one active pharmaceutical ingredient of metronidazole, tinidazole and ornidazole for the first time. In weight, per 100.0g of externally-applied skin gel contains 1.0g to 8.0g of calcium dobesilate and 0.1g to 3.0g of metronidazole, or tinidazole or ornidazole. The externally-applied skin gel can be used for treating acne rosacea and acne; the calcium dobesilate in the gel can be used for improving the blood circulation of skin and reducing the telangiectasis of the skin; and metronidazole, or tinidazole or ornidazole has an effect of exterminating worms and mites on the skin.
Owner:段亚东
Preparation method of stable calcium dobesilate
InactiveCN103271896AImprove stabilityGuaranteed validitySenses disorderAntipyreticParticulatesEffervescent tablet
The invention provides a preparation method of stable calcium dobesilate and overcomes the defect in the prior art. According to the invention, a dry granulating method is used for controlling water content in the preparation process, and obviously reducing degradation and increasing stability. Acceptable carrier or excipients and other pharmaceutic adjuvants are added into the prescription for preparing common tablet, oral disintegrating tablet, chewing tablet, film coated tablet, effervescent tablet, particulate agent, capsule, electuary, powder, suspension or sustained release agent.
Owner:南京先宇科技有限公司
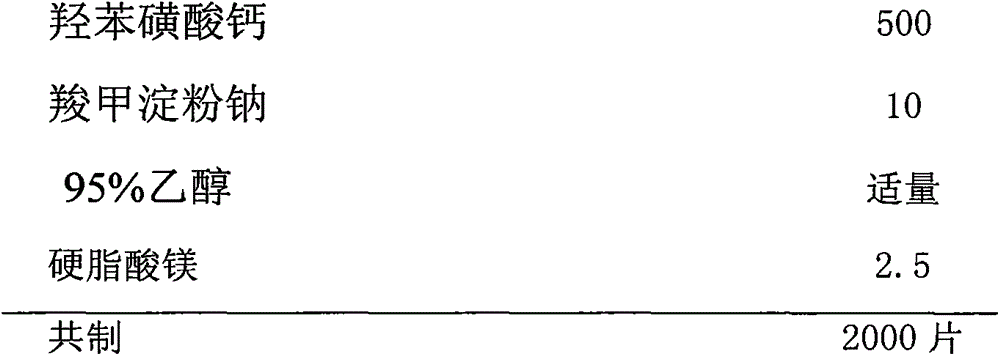
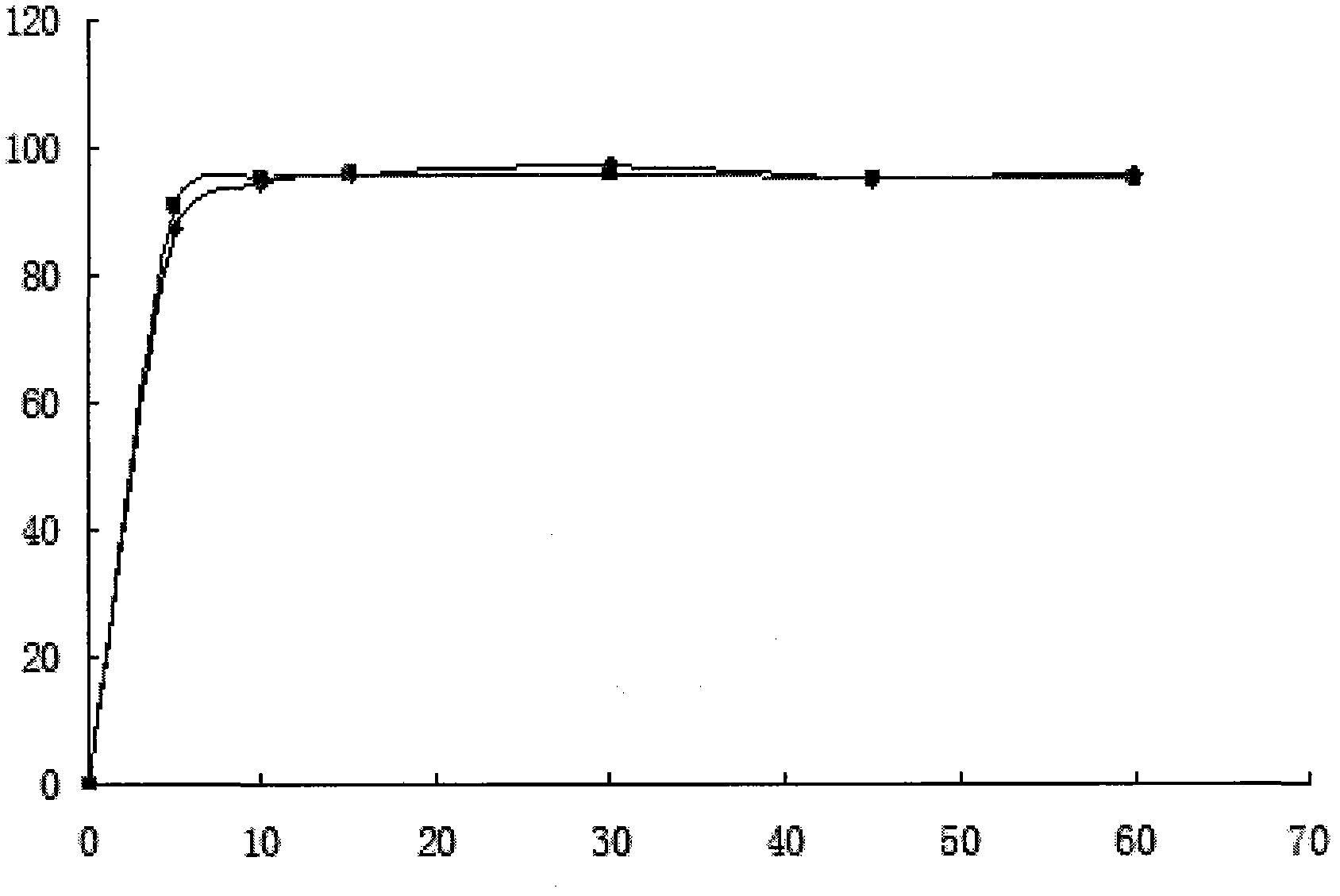
Calcium dobesilate dispersible tablet and preparation method thereof
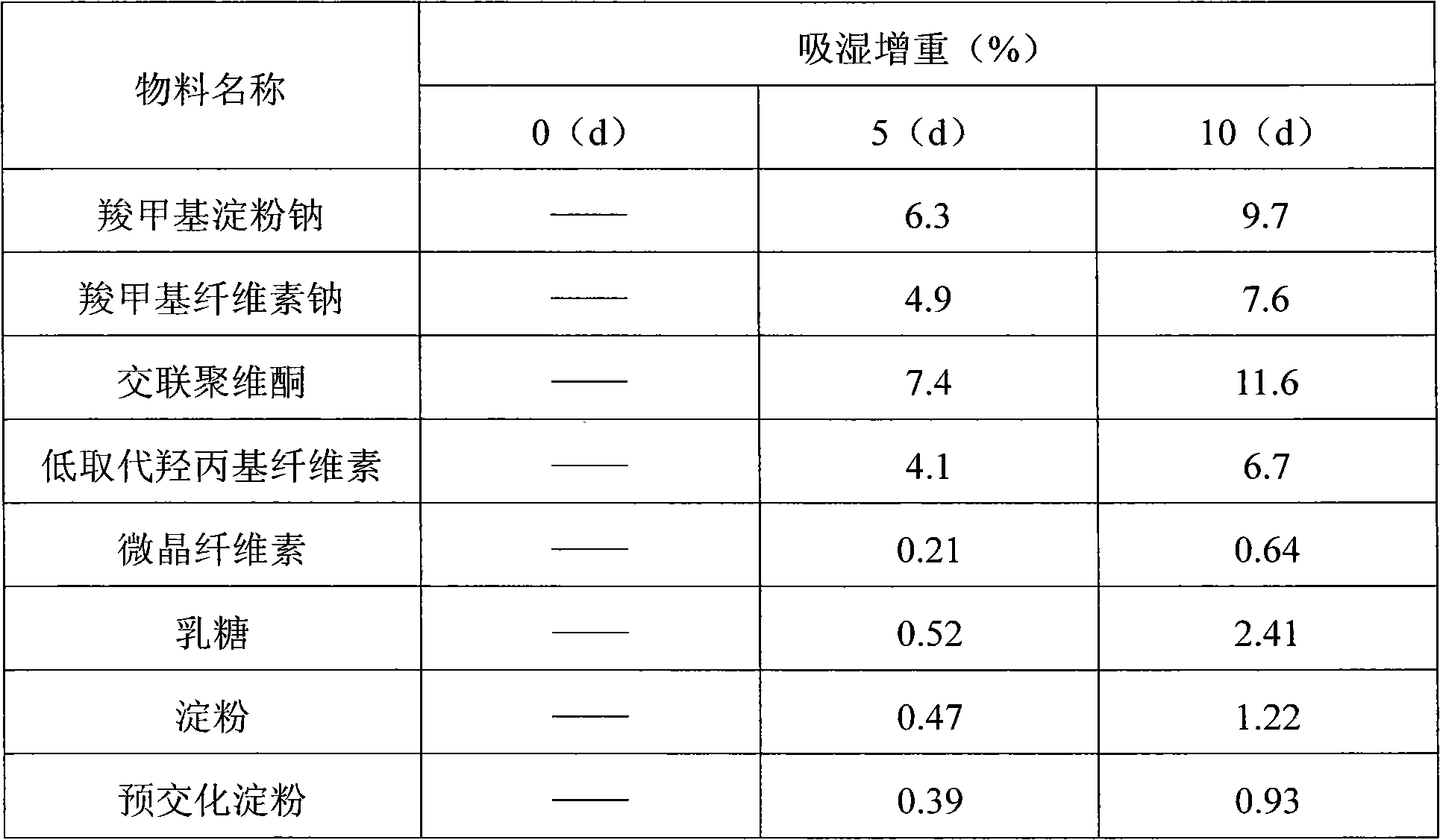
InactiveCN103877038AFlat shapeBright and cleanSenses disorderPill deliveryCross-linkMoisture absorption
The present invention discloses a calcium dobesilate dispersible tablet and a preparation method thereof. The calcium dobesilate dispersible tablet contains, by weight, a main drug calcium dobesilate, and auxiliary materials such as 1-42% of cross-linked povidone, 0.5-42% of microcrystalline cellulose, 2-44% of lactose and 0.01-15% of magnesium stearate. According to the present invention, a direct powder tabletting method is adopted to prepare the calcium dobesilate dispersible tablet; the method has characteristics of simple operation, time saving, reduction of times of calcium dobesilate exposed to air and light, and reduction of moisture absorption and light instability; the prepared calcium dobesilate dispersible tablet has a smooth surface morphology, and can be completely disintegrated within 2 min and screened with a 2# sieve, and the dissolution rate of the prepared calcium dobesilate dispersible tablet in water and a dilute hydrochloric acid solution within 5 min can achieve more than 90%; and the preparation method has characteristics of simple preparation process and time saving, and the prepared calcium dobesilate dispersible tablet has characteristics of smooth surface, good dispersion uniformity and high dissolution rate, and is suitable for industrial production.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Calcium dobesilate capsule and preparation method thereof
ActiveCN104622842AImprove long-term stabilityCapsule deliveryMacromolecular non-active ingredientsCarboxymethyl starchMedicine
The invention belongs to the technical field of medicine preparation, and in particular relates to a calcium dobesilate capsule composition. The calcium dobesilate capsule composition is characterized by being prepared from the following components in parts by weight: calcium dobesilate, carboxymethyl starch sodium, magnesium stearate, 90 percent of zein and a proper amount of ethanol solution. The long-term stability of the composition is obviously improved.
Owner:BEIJING JINGFENG PHARMA GRP
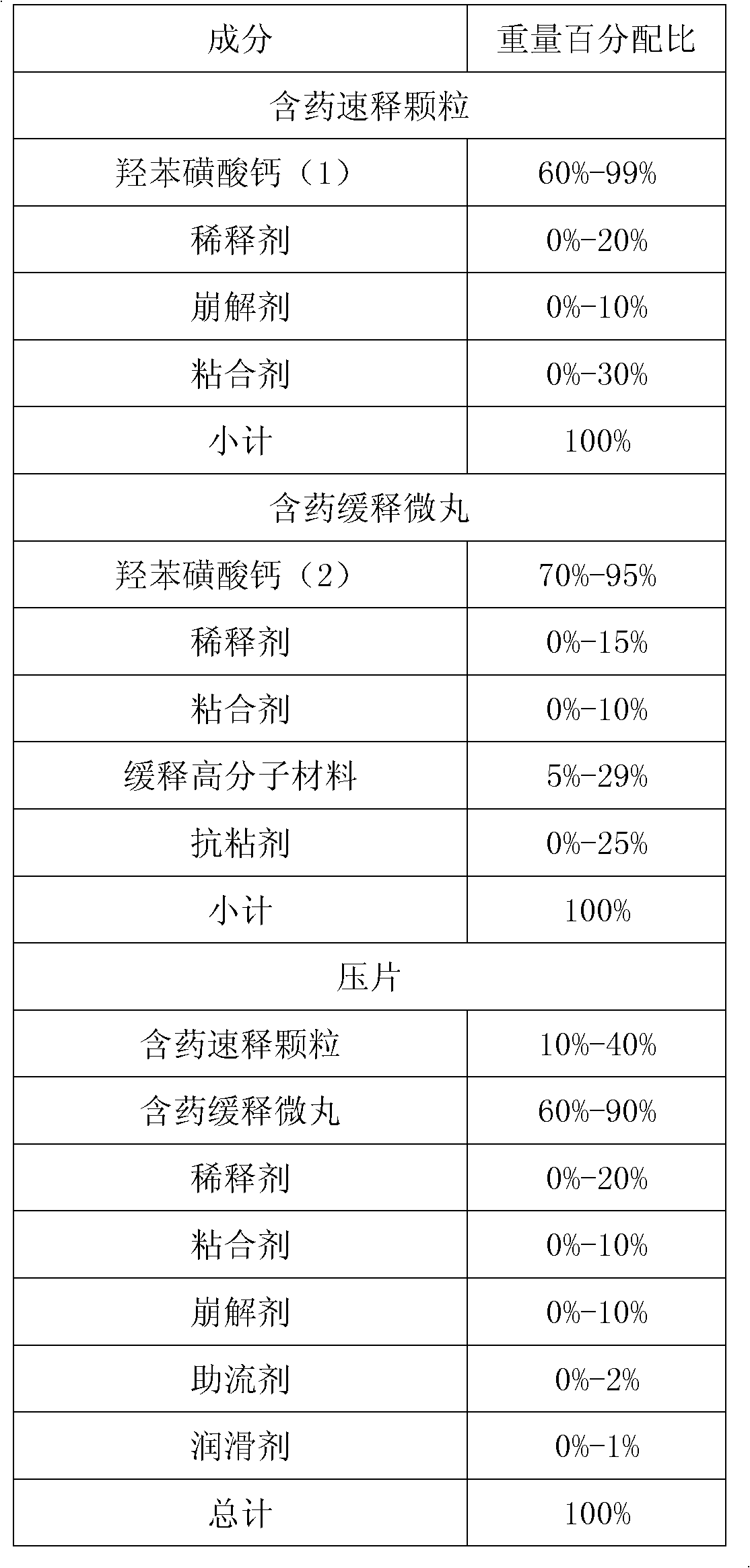
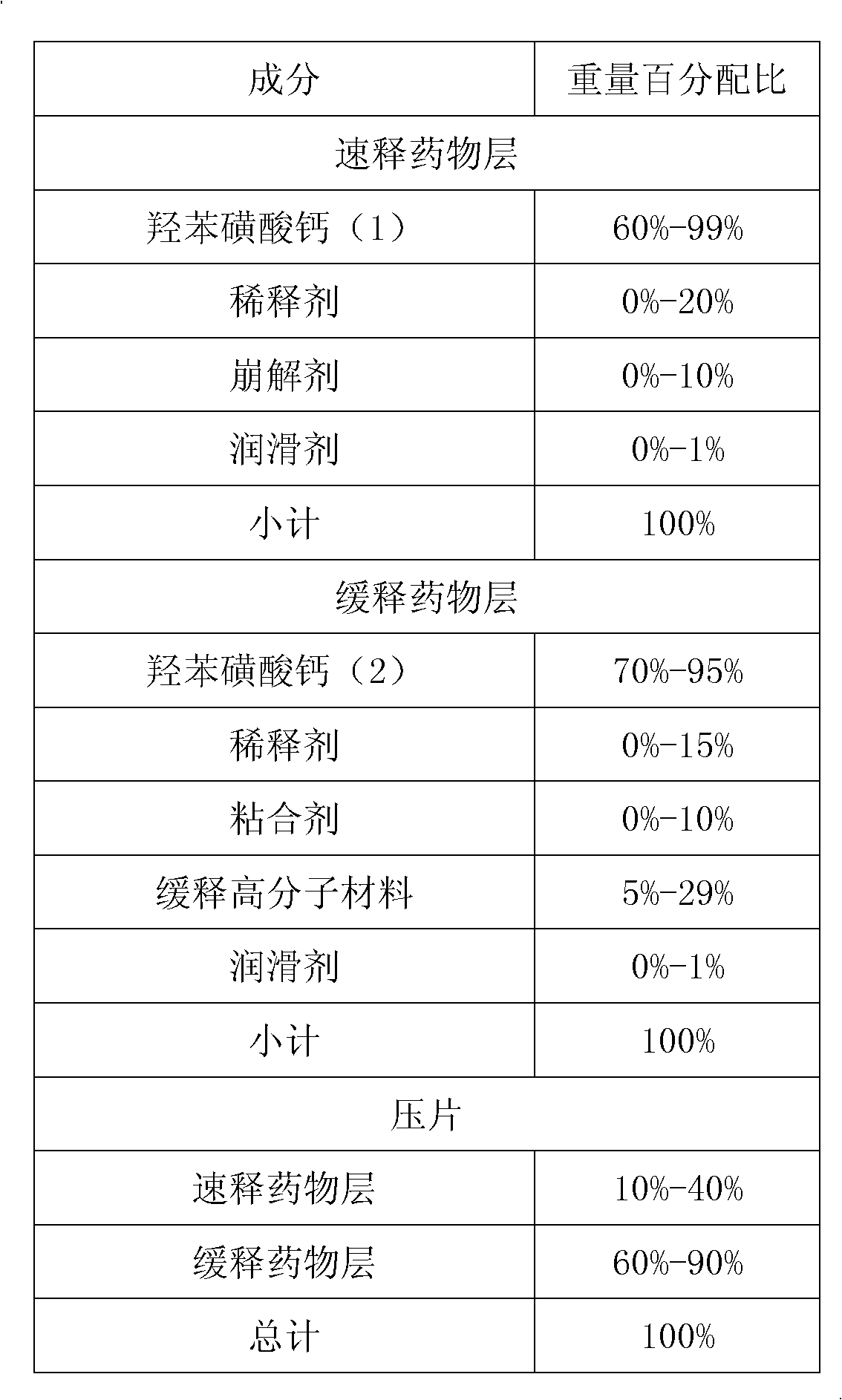
Calcium dobesilate medical composition with high drug loading capacity
InactiveCN103169679ASolve the problem of acting too slowlyHigh clinical application valuePill deliveryAnhydride/acid/halide active ingredientsOral phase dysphagiaSlurry
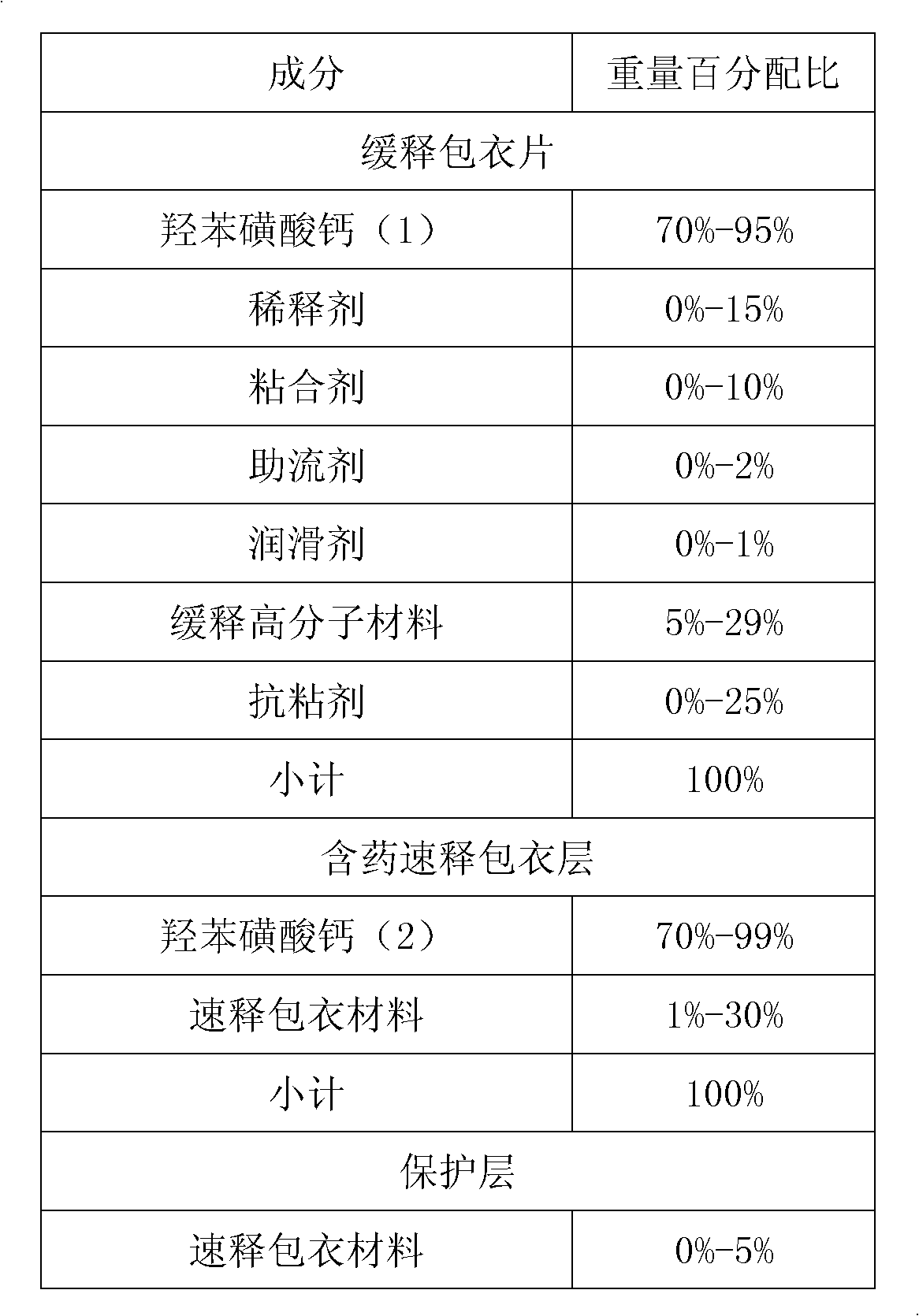
The invention provides a calcium dobesilate medical composition with high drug loading capacity. The composition comprises a quick release part and a slow release part and is combined by the two parts in a single unit or multiple units. The calcium dobesilate in the quick release part accounts for 10-40% of total weight of calcium dobesilate, while the calcium dobesilate in the slow release part accounts for 60-90% of total weight of calcium dobesilate. The single dose of the composition comprises 500-100mg of calcium dobesilate. According to Chinese pharmacopoeia, in pH6.8 buffer liquid, the quick release part of the calcium dobesilate medical composition is quickly released within 30 minutes by a dissolution rate detection slurry method, and the total release amount of calcium dobesilate reaches over 80% of the total weight in 12-16 hours. According to the invention, not only is medicine in initial period relatively more released to solve the problem of over-slow effect of the medicine, but also good slow release effect can be obtained just by relatively less amount of slow release auxiliary materials because calcium dobesilate in the slow release part is reduced, thereby avoiding the problem that the preparation in unit dosage is overlarge and hard to swallow. The composition has greater clinical application value. The composition provided by the invention is simple and accessible and is suitable for industrialized production.
Owner:SHANGHAI FOSUN PHARMA DEV CO LTD
Kit for measuring total cholesterol and method
The invention relates to a kit for measuring total cholesterol and a method. The kit comprises a reagent group 1 and a reagent group 2, wherein the reagent group 1 comprises chromogen and cholesterol esterase; the reagent group 2 comprises 4-aminoantipyrine, peroxidase and cholesterol oxidase; the chromogen comprises N-ethyl-N-(2-hydroxyl-3-sulfopropyl)-3, 5-dimethoxyaniline. The kit and the method can reduce interference caused by calcium dobesilate and / or etamsylate in samples during total cholesterol detection.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
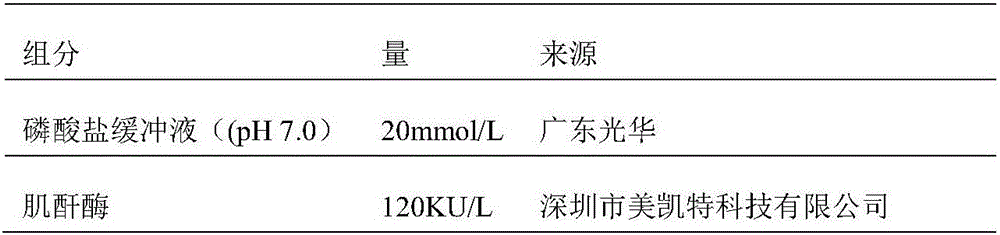
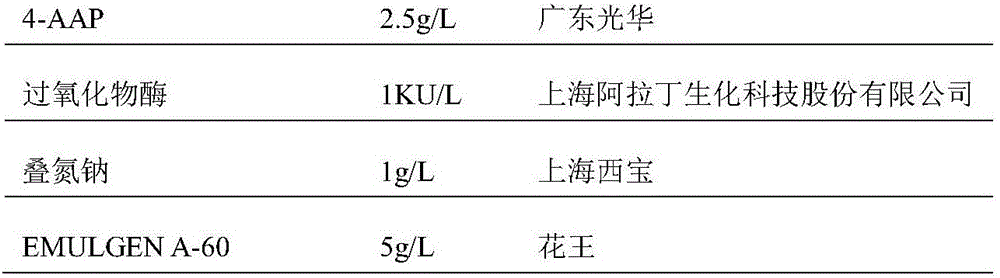
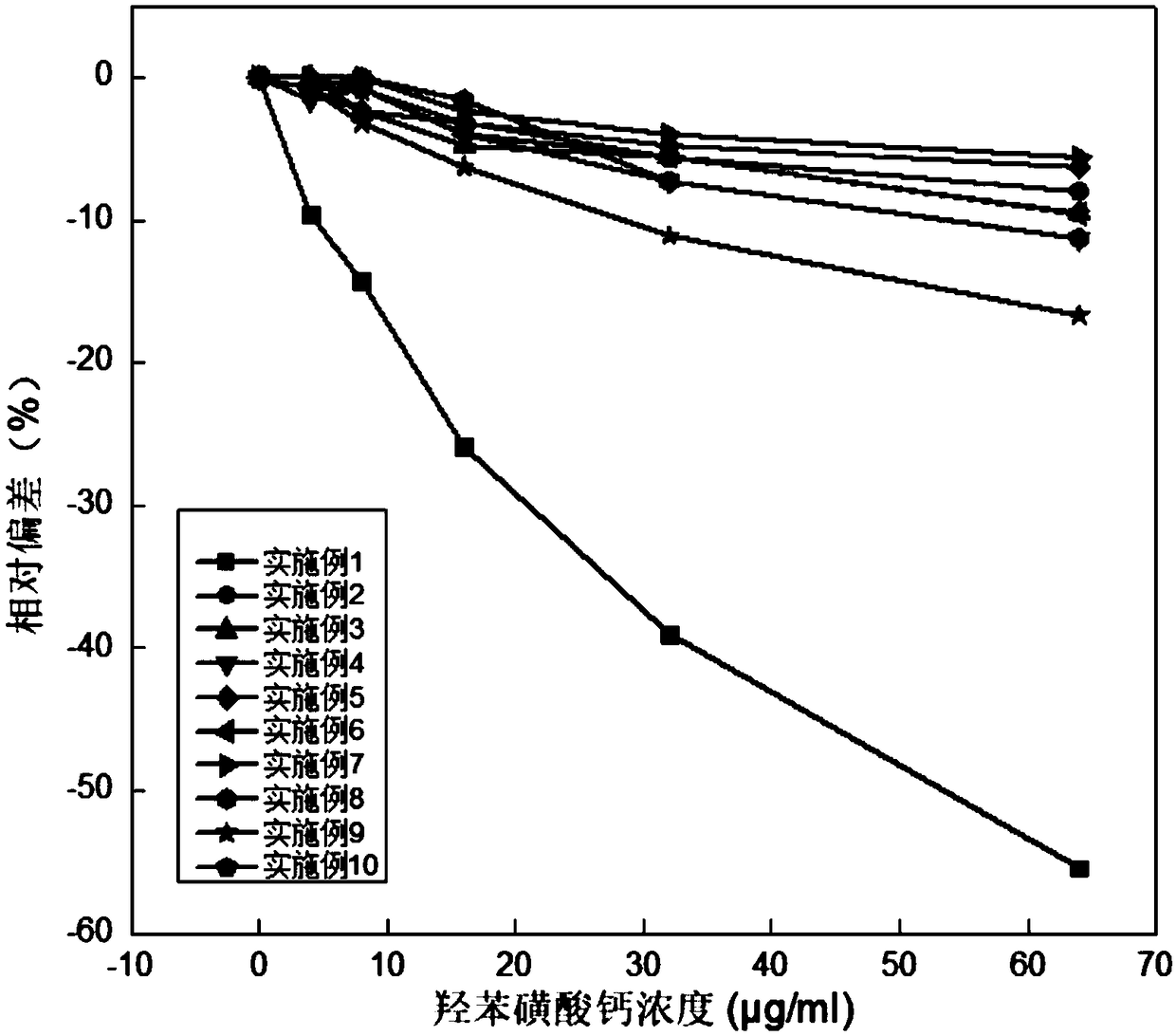
Creatinine kit for eliminating calcium dobesilate and etamsylate and preparation method thereof
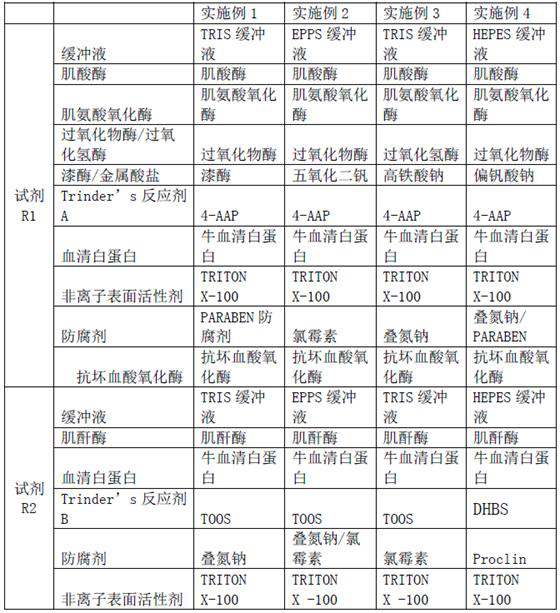
ActiveCN111733208AImprove stabilityEasy to operateMicrobiological testing/measurementBiological material analysisActive agentPeroxidase
The invention relates to a creatinine kit capable of eliminating negative deviation interference in a creatinine determination caused by calcium dobesilate and etamsylate drugs in serum and a preparation method thereof. Key points of a technical scheme of the invention are that the creatinine kit includes a reagent R1 and a reagent R2, wherein the reagent R1 includes buffer, creatinase, sarcosineoxidase, ascorbic acid oxidase, peroxidase or catalase, serum albumin, nonionic surfactant, preservative, Trinder's reagent A, laccase or metalic acid salt; and the reagent R2 includes buffer, serum albumin, creatinine enzyme, preservative and Trinder's reagent B. The invention overcomes a defect of inaccurate creatinine determination due to the presence of the negative deviation caused by calciumdobesilate and etamsylat in serum samples when the clinical tests of creatinine determination are being carried out in various hospitals, and the invention is suitable for the detection of serum creatinine by an automatic biochemical analyzer.
Owner:ZHONGSHAN BGH BIOTECH CO LTD
Calcium dobesilate dispersible tablet and preparation method thereof
InactiveCN108272763AGood conditionShort dispersion timeMetabolism disorderPharmaceutical non-active ingredientsAdhesiveCalcium dobesilate
The invention provides a calcium dobesilate dispersible tablet and a preparation method thereof. The calcium dobesilate dispersible tablet comprises the following raw materials: calcium dobesilate, afilling agent, a disintegrating agent, an adhesive and a lubricating agent, wherein the weight parts of the calcium dobesilate, the filling agent, the disintegrating agent, the adhesive and the lubricating agent account for 29 to 50 percent, 35 to 55 percent, 3 to 13 percent, 0.2 to 1 percent and 0.4 to 2 percent of the total weight of the calcium dobesilate dispersible tablet. According to the scheme, the bioavailability of the calcium dobesilate can be improved.
Owner:HAINAN LINHENG PHARMA
Kit and method for testing glucose
ActiveCN106319029AMicrobiological testing/measurementBiological material analysisGlucose detectionD-Glucose
The invention relates to a kit and method for testing glucose. The kit comprises a reagent group 1 and a reagent group 2, wherein the reagent group 1 comprises ascorbic acid oxidase and chromogen; the reagent group 2 comprises 4-amino-antipyrine, peroxidase and glucose oxidase; the chromogen comprises N-ethyl-N(2- hydroxyl-3-sulfopropyl)-3,5-dimethoxyaniline or sodium salts of the N-ethyl-N(2- hydroxyl-3-sulfopropyl)-3,5-dimethoxyaniline. The kit and the method provided by the invention can reduce the interference due to calcium dobesilate and / or etamsylate in a sample in the glucose determination.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Calcium dobesilate capsule composition
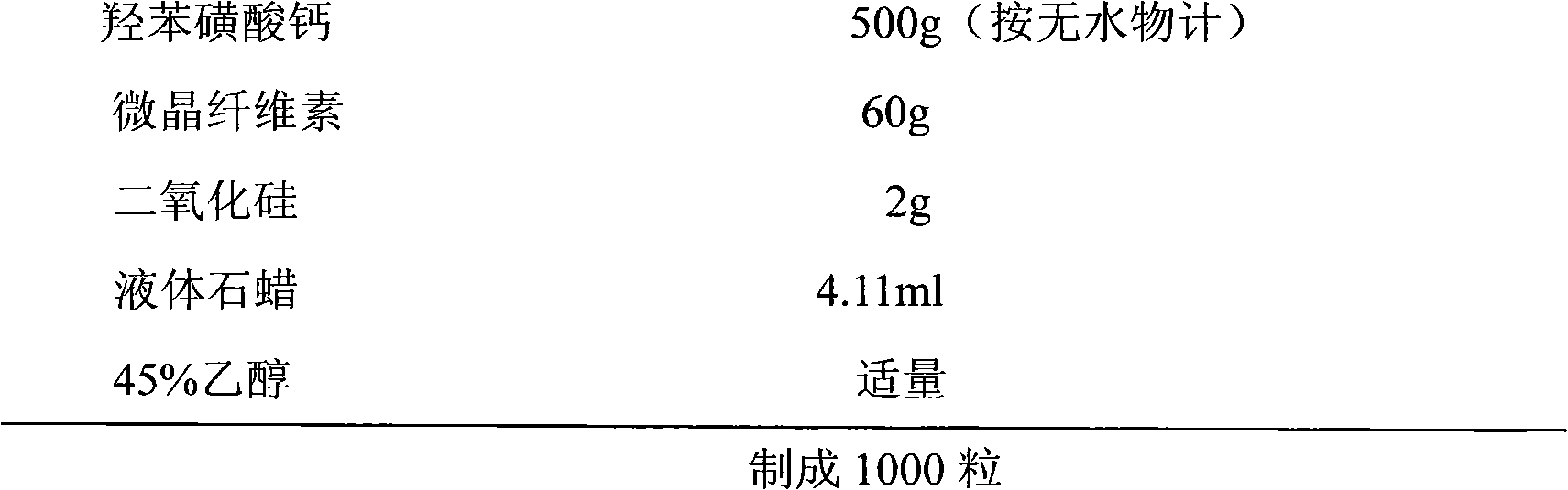
InactiveCN102579396AQuality assuranceReduce humidityCapsule deliveryOil/fats/waxes non-active ingredientsFiller ExcipientBULK ACTIVE INGREDIENT
The invention relates to a calcium dobesilate capsule composition, which comprises calcium dobesilate serving as an active ingredient, a filling agent and a lubricating agent, wherein the filling agent is microcrystalline cellulose preferably, the lubricating agent is a mixture of silicon dioxide and atoleine, and a weight ratio of the silicon dioxide to the atoleine is (1:1)-(1:3). According to the calcium dobesilate capsule composition, by optimizing a formula and a process, the problem of poor stability during long-term storage is solved well to ensure the quality of medicines.
Owner:KANGYA OF NINGXIA PHARMA
Antirust lubricating oil and a preparing method thereof
InactiveCN106635313AImprove wear resistanceImprove rust resistanceLubricant compositionMethacroleinDolomite
Antirust lubricating oil is disclosed. The lubricating oil includes, by weight, 65-80 parts of mineral oil, 12-16 parts of dicyclopentadiene, 7-13 parts of methacrolein, 5-9 parts of methacrylamide, 8-12 parts of 5-norbornene-2-methanol, 2-5 parts of calcium dobesilate, 1-4 parts of dolomite dust, 9-13 parts of ethopabate, 7-12 parts of sodium naphthalene-1-acetate, 5-10 parts of 5-hydroxytryptophan, 4-8 parts of zinc hypophosphite, 12-17 parts of 4-bromophenoxybenzene and 6-11 parts of acetazolamide. The lubricating oil has good antiwear, antirust and lubricating effects and a production process is simple.
Owner:苏州圣鑫莱新材料有限公司
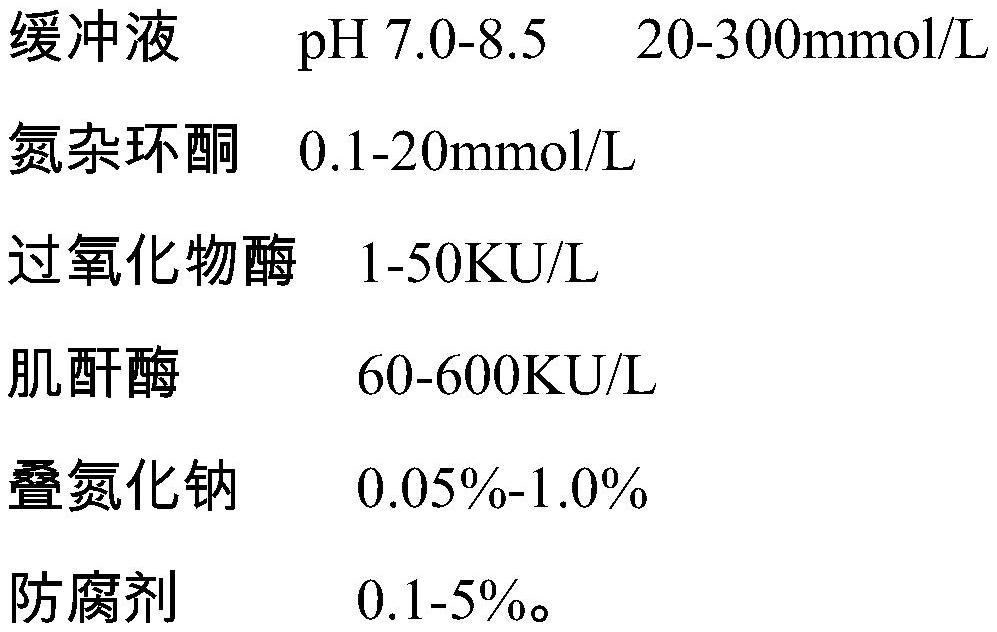
Uric acid test kit capable of eliminating drug interference of calcium dobesilate and etamsylate in serum and preparation method thereof
ActiveCN111733211AEliminate negative interferenceImprove stabilityMicrobiological testing/measurementBiological material analysisPeroxidaseActive agent
The invention discloses a uric acid test kit capable of eliminating drug interference of calcium dobesilate and etamsylate in serum and a preparation method thereof. According to the technical point,the test kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises a buffer solution, peroxidase, ascorbic acid oxidase, a preservative, a Trinder's reactant A, metallate or a nonionic surfactant; and the reagent R2 comprises a buffer solution, serum albumin, uricase, a preservative and a Trinder's reactant B or a nonionic surfactant. According to the uric acid test kit disclosed by the invention, one or more than one of high-redox-potential oxidized metallate, vanadium pentoxide and vanadium oxychloride is / are added into R1 to be mixed, and calcium dobesilate and etamsylate are oxidized by utilizing the metallate, H2O2 generated by uricase in decomposing uric acid after R2 is added is prevented from being consumed, and an accurate uric acid measured value is rapidly obtained.
Owner:ZHONGSHAN BGH BIOTECH CO LTD
Kits and methods for measuring creatinine
ActiveCN106198509BMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementSarcosine oxidaseCreatininase
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
Composition for eliminating interference of calcium dobesilate drug on creatinine enzyme method detection
ActiveCN108627654BSolve the problem of serious negative interferenceDisease diagnosisBiological testingHigh concentrationCreatininase
The invention relates to a composition for eliminating the interference of calcium dobesilate medicine on creatinine enzymatic detection, comprising: R1 reagent, R2 reagent and polymer oxidant; the polymer oxidant is polymer peroxyacid, polymer One or more of selenium oxides, polymer chlorosulfides, N-imides, water-soluble tetrazole, and Dess Martin oxidants. The polymer oxidant in the composition disclosed in the present invention can interact with the calcium dobesilate in the sample to be tested, so that the calcium dobesilate loses the effect of interfering with the detection of creatinine by enzymatic method, and improves the detection by sarcosine oxidase method Accuracy, thus solving the problem of serious negative interference of high concentration of calcium dobesilate in the patient's blood to the test results of enzymatic creatinine during the period when the patient is taking calcium dobesilate.
Owner:WUHAN HANHAI NEW ENZYMES BIOLOGICAL TECH CO LTD
Calcium dobesilate capsule composition and preparation method thereof
InactiveCN103230383AReduce dissolutionCapsule deliveryAnhydride/acid/halide active ingredientsAdhesiveDiluent
The invention belongs to the technical field of medicines, and concretely discloses a calcium dobesilate capsule composition and a preparation method. Calcium dobesilate is used as a raw material, and the composition also comprises one or a plurality of pharmaceutically acceptable excipients. The preparation method of the composition comprises the following steps: mixing calcium dobesilate with magnesium stearate to obtain a mixture, mixing the mixture with a selectable diluent, adding a proper amount of an adhesive to prepare a soft material, sieving to prepare wet particles, drying the wet particles, sieving for granulation, adding a corresponding lubricant, mixing, and filling to a capsule. The composition has the advantages of simple preparation method, consistency of a dissolution curve with that of reference listed drugs, and guarantee of the effective plasma concentration and the safety. The composition can be used for the diabetic microangiopathy.
Owner:BEIJING WANQUAN SUNSHINE MEDICAL TECH CO LTD
Calcium dobesilate tablet and preparation method thereof
InactiveCN105213343AGood quality and stabilitySuitable for long term storageSenses disorderPill deliveryDry weightMagnesium stearate
The invention relates to a calcium dobesilate tablet and a preparation method thereof, and belongs to the technical field of medicine. The calcium dobesilate tablet is prepared from, by weight, 200-300 parts of calcium dobesilate, 5-15 parts of mannitol, 2-4 parts of sodium dihydrogen citrate anhydrous, 6-8 parts of sodium metabisulfite, 0.2-0.5 part of anhydrous citric acid, 30-50 parts of absolute ethyl alcohol and 20-40 parts of magnesium stearate. According to the prepared calcium dobesilate tablet, through 6-month accelerated test inspection, the characters all meet the specification, the dissolution rate is qualified, the dry weight loss change range does not exceed + / -0.5%, related substances have the little rising tendency, the total impurity increasing amplitude does not exceed 0.02%, and the content ranges from 98 percent to 101 percent; all the inspection indexes do not change obviously, the quality is stable, and therefore the calcium dobesilate tablet can be more suitable for clinical application.
Owner:SHIJIAZHUANG HUAXIN PHARMA
Application of traditional Chinese medicine composition in preparation of medicine for treatment of diabetic retinopathy
ActiveCN108079184AGood curative effectReduce generationSenses disorderMetabolism disorderDiabetes retinopathyCodonopsis
The invention discloses application of a traditional Chinese medicine composition in preparation of a medicine for treatment of diabetic retinopathy. The traditional Chinese medicine composition comprises the following components: rhizoma acori graminei, semen cassiae, cistanche, root of kudzu vine, feather cockscomb seed, radix codonopsis, fructus viticis, Chinese wolfberry, semen plantaginis, radix paeoniae alba, dogwood, licorice, dodder seed, rhizoma cimicifugae, hedge prinsepia nut, chrysanthemum, butterflybush flower, Rhizoma Chuanxiong, polygonati rhizoma processed with yellow wine, prepared rehmannia root, Phellodendron amurense and Radix Astragali. The traditional Chinese medicine composition has good effects on preventing and treating the diabetic retinopathy, and can significantly reduce the production of retinal microangioma of the diabetic retinopathy, and compared with commonly-used diabetic retinopathy therapeutic drug calcium dobesilate, the traditional Chinese medicinecomposition is more remarkable in curative effect.
Owner:GUANGZHOU BAIYUNSHAN ZHONGYI PHARMA COMPANY
Cosmetic composition and uses thereof for preparing cosmetic composition
The invention provides a cosmetic composition and uses thereof. The cosmetic composition comprises an active ingredient and a matrix, wherein the active ingredient is calcium dobesilate. The cosmetics can be made into solution, soap, cream, tincture, film, gel, shampoo and toothpaste type. The calcium dobesilate in the cosmetic composition has the effects of improving skin capillary circulation and reducing skin telangiectasia. The cosmetic composition can be used for preparing cosmetics for caring skin, preventing alopecia, promoting hair growth, protecting gums and strengthening teeth, removing facial blood streak and ecchymoses, and preventing and treating seborrheic dermatitis, rosacea and acne.
Owner:段亚东
Calcium dobesilate capsule and preparation method
InactiveCN106619564AReduce the problem of large variabilityImprove liquidityMetabolism disorderPharmaceutical non-active ingredientsPoor mobilityDissolution
The invention relates to a calcium dobesilate capsule and a preparation method, and belongs to the technical field of pharmaceutical preparations. According to the technical scheme, the calcium dobesilate capsule is prepared from a therapeutically effective dose of calcium dobesilate, filler and a lubricant. The capsule is characterized by being produced with a wet granulation and dry granulation combined process. The materials are subjected to wet granulation before the dry granulation process, the problem of poor mobility due to too large proportion of calcium dobesilate in a prescription is solved, and the problem of high variability of each sample in the early stage of the dissolution process is also solved.
Owner:北京满格医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com