Calcium dobesilate medical composition with high drug loading capacity
A technology of calcium dobesilate and high drug loading, which is applied in drug combination, drug delivery, anhydride/acid/halide active ingredients, etc. It can solve the problems of large tablets, reduced drug compliance, and slow onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
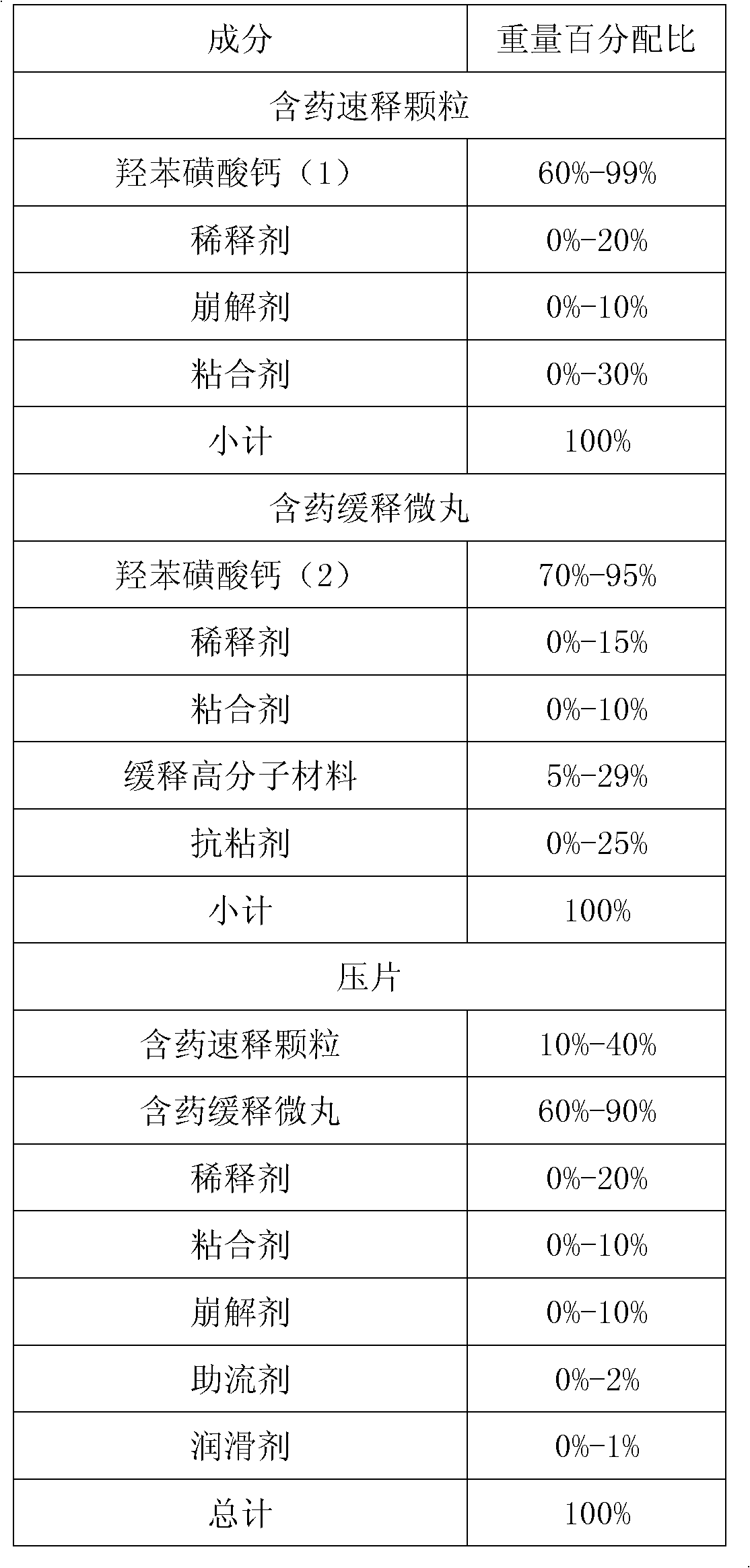
Embodiment 1
[0069] formula:
[0070]
[0071]
[0072] Preparation: According to the prescription of the sustained release part, 25 grams of hypromellose was made into a 10% aqueous solution, 450 grams of calcium dobesilate and 25 grams of microcrystalline cellulose were mixed evenly, and the aqueous solution of hypromellose E3 was added to the The wet material is prepared in a high-speed stirring granulator, and the wet material is extruded and spheronized by an extrusion spheronizer to obtain drug-containing pellets (extrusion port screen 1.0mm), and the drug-containing pellets are dried at 50°C for 2-4 After drying, collect the pellets between 0.4mm-1.4mm as quick-release pellets; in addition, 167 grams of polyacrylic resin NE30D (30% solid content) and 50 gram talcum powder are made into the coating of 20% solid content water dispersion, coating the collected drug-containing pellets to obtain sustained-release coated pellets.
[0073] According to the prescription of the immedi...
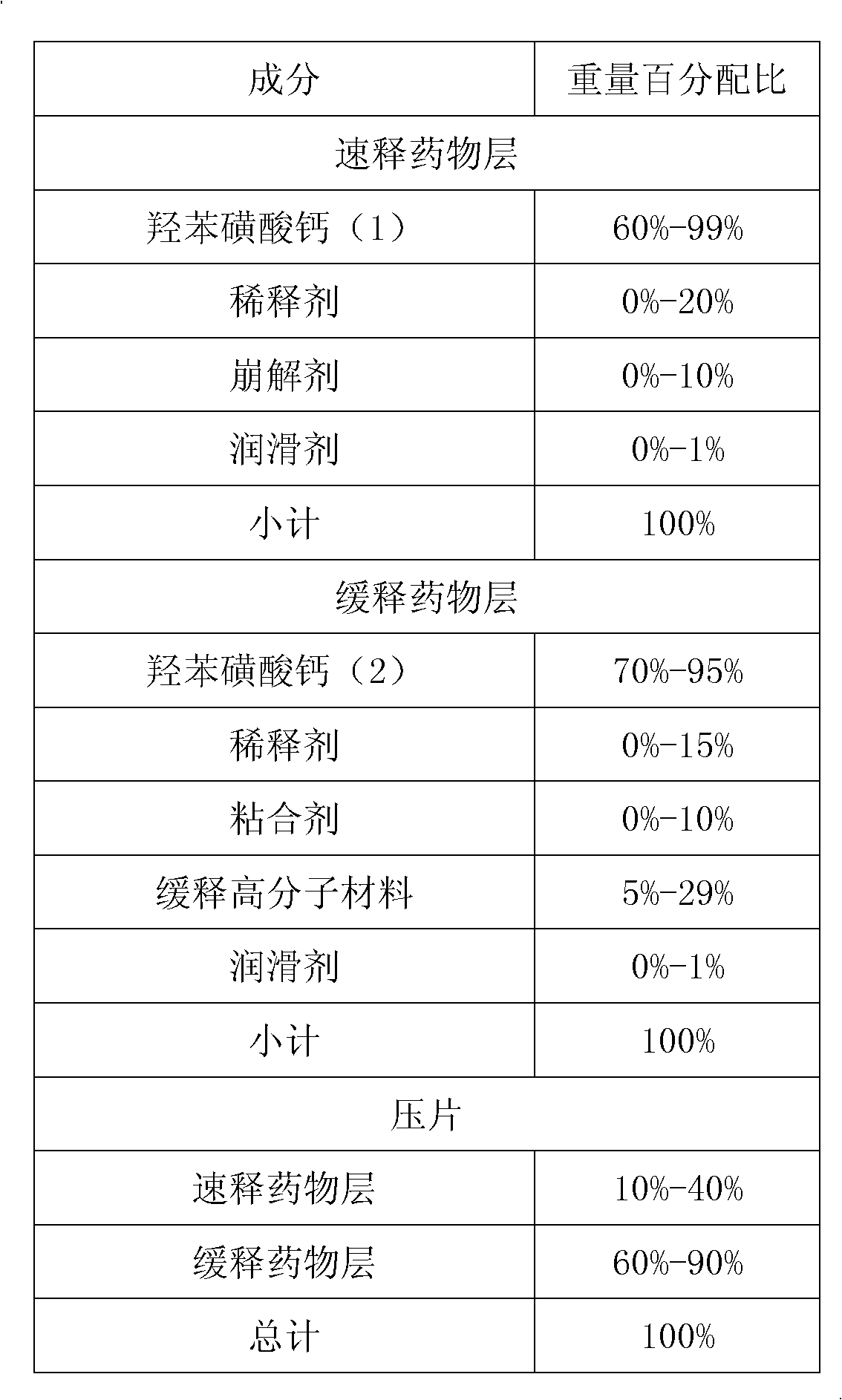
Embodiment 2
[0076] formula:
[0077]
[0078] Preparation: 15 grams of hypromellose E6 in the slow-release part was made into a 10% aqueous solution, and 300 grams of calcium dobesilate were granulated in a high-speed stirring granulator with the aqueous solution of hypromellose E6, at 50 Dry at ℃ for 2-4 hours, pass through a 1.4mm sieve after drying, mix the sieved dry granules, 100 grams of hypromellose K15M, 4 grams of silicon dioxide and 1 gram of magnesium stearate, and set aside.
[0079] Mix 10 grams of hypromellose E6 and 200 grams of calcium dobesilate in the immediate release part evenly, add 80 grams of purified water, wet granulate in a high-speed stirring granulator, and dry at 50°C for 2-4 hours. Pass through a 1.4mm sieve after drying, mix the sieved dry granules, 2 grams of silicon dioxide and 0.5 gram of magnesium stearate, and set aside.
[0080] The two-part granules are compressed into a double-layer tablet by using a double-layer tablet press machine, with the su...
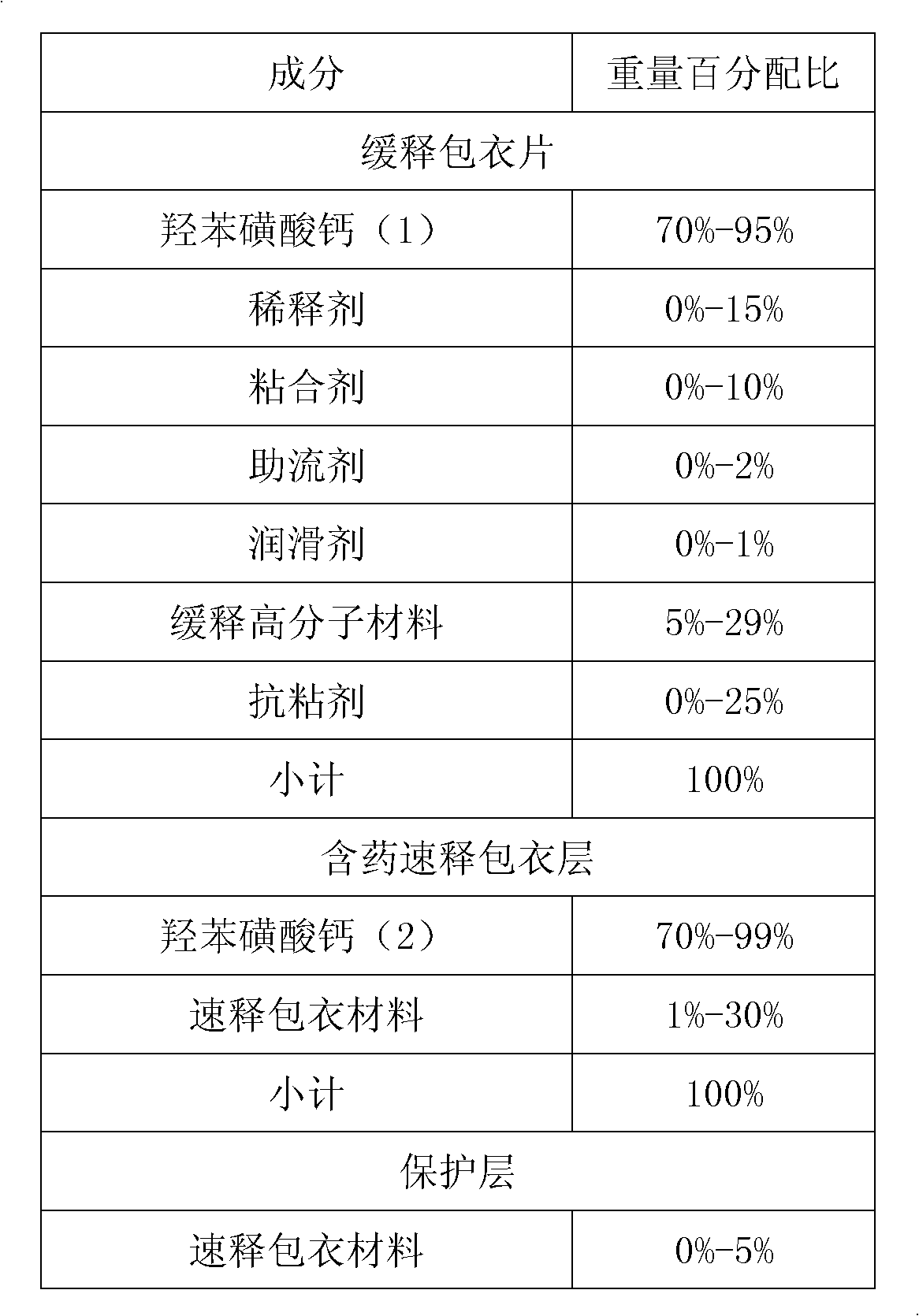
Embodiment 3
[0082] formula:
[0083]
[0084] Preparation: 25 grams of hypromellose E3 in the slow-release part was made into a 10% aqueous solution, 450 grams of calcium dobesilate and 45 grams of microcrystalline cellulose were mixed evenly, and the aqueous solution of hypromellose E3 was used at high speed The wet material was prepared in a stirring granulator, dried at 50°C for 2-4 hours, passed through a 1.4mm sieve after drying, mixed the sieved granules, 4 grams of silicon dioxide and 1 gram of magnesium stearate, and pressed piece. 167 grams of polyacrylic resin NE30D (30% solid content) and 50 grams of talcum powder of the slow-release part are made into the coating aqueous dispersion of 20% solid content, and the above-mentioned tablet core is coated to obtain a slow-release coating piece.
[0085] Disperse and dissolve 15 grams of polyacrylic acid resin E100 and 50 grams of calcium dobesilate in 800 grams of isopropanol for the immediate-release part, and coat the above-me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com