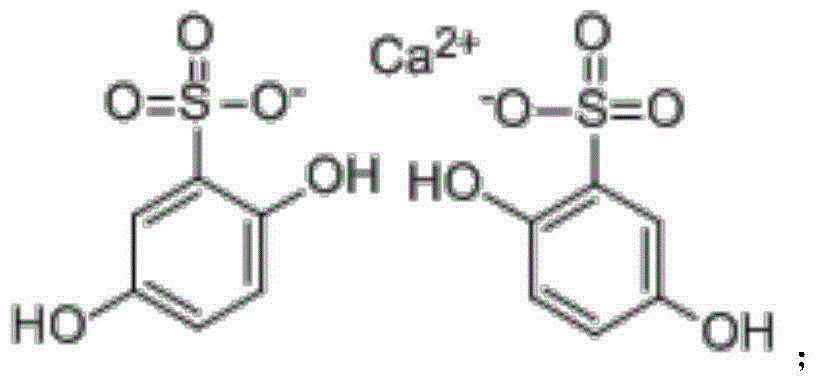
Calcium dobesilate tablet and preparation method thereof
A calcium dobesilate tablet, calcium dobesilate technology, applied in the field of medicine, can solve the problems of low purity, low stability of calcium dobesilate raw materials, poor fluidity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of calcium dobesilate described in embodiment 1 of the present invention
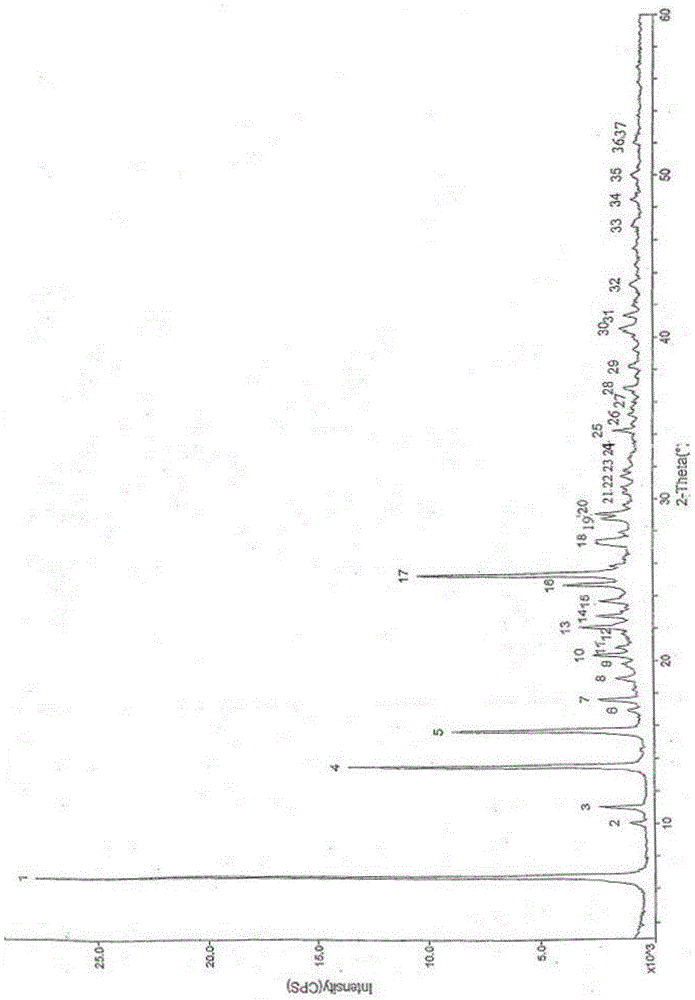
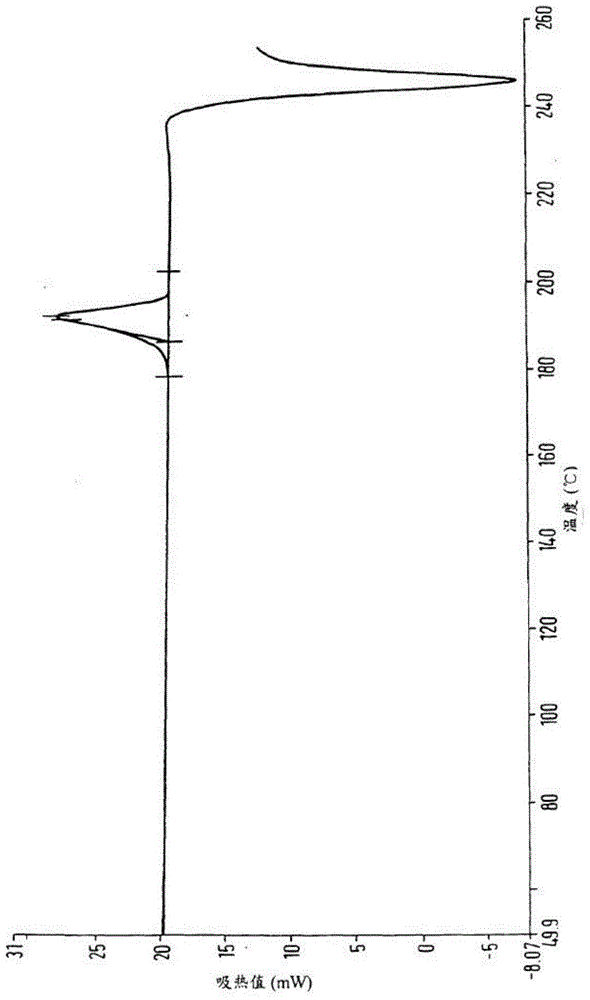
[0030] Add 200ml of methyl carbamate and 500ml of methyl tert-butyl ether to 100g of crude calcium dobesilate, heat to 60°C to dissolve, cool to room temperature, then add 210mL of water to the solution, stir and mix evenly, and then cool down to 3 ℃, standing for crystallization for 3 hours, filtering, and drying to obtain 97.7 g of calcium dobesilate, with a yield of 97.7% and a purity of 99.82% by HPLC.
Embodiment 2
[0031] The preparation of embodiment 2 calcium dobesilate of the present invention
[0032] Add 83ml of methyl carbamate and 417ml of methyl tert-butyl ether to 100g of crude calcium dobesilate, heat to 55°C to dissolve, cool to room temperature, then add 50mL of water to the solution, stir and mix evenly, and then cool down to 0 ℃, standing for crystallization for 5 hours, filtering, and drying to obtain 95.1 g of calcium dobesilate, with a yield of 95.1% and an HPLC purity of 99.52%.
Embodiment 3
[0033] The preparation of calcium dobesilate described in embodiment 3 of the present invention
[0034] Add 375ml of methyl carbamate and 625ml of methyl tert-butyl ether to 100g of crude calcium dobesilate, heat to 65°C to dissolve, cool to room temperature, then add 500mL of water to the solution, stir and mix evenly, and then cool down to 5 ℃, standing for crystallization for 1 h, filtering, and drying to obtain 98.4 g of calcium dobesilate, with a yield of 98.4% and an HPLC purity of 99.43%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com