Calcium dobesilate dispersible tablet and preparation method thereof
A technology of calcium dobesilate and dispersible tablets, which is applied in the directions of anhydride/acid/halide active ingredients, pill delivery, cardiovascular system diseases, etc., can solve the problems of complex process, large human influence, and long time consuming granulation method. , to achieve the effect of simple method, reduced irradiation time, and small difference in film weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Raw material prescription:
[0032] Calcium dobesilate 25 g
[0033] Crospovidone 9 g
[0034] Microcrystalline cellulose 2.25 g
[0035] Lactose 8.525 g
[0036] Magnesium stearate 0.225 g
[0037]
[0038] Makes 100 pieces
[0039] Preparation process: Mix the prescribed amount of crospovidone, microcrystalline cellulose and lactose in equal increments, and then mix the prescribed amount of calcium dobesilate with the above-mentioned mixed powder in equal increments , and finally add the prescribed amount of magnesium stearate and mix well, determine the tablet weight and hardness, and directly compress the tablet to obtain the calcium dobesilate dispersible tablet. The whole preparation process is completed at 25°C.
[0040] Appearance: The calcium dobesilate dispersible tablet prepared by this method is off-white with smooth surface.
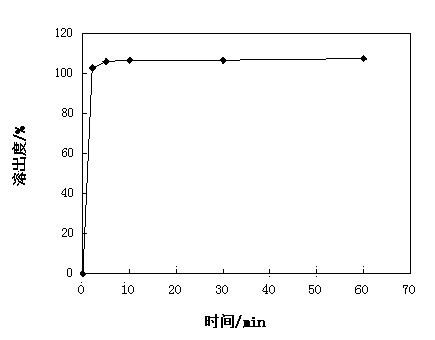
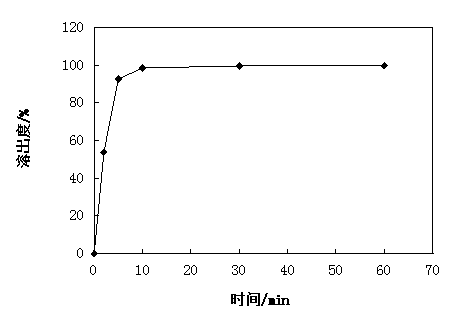
[0041] Uniformity of dispersion: Take 6 tablets of the prepared calcium dobesilate dispersible tablets, place them in 10...
Embodiment 2
[0044] Raw material prescription:
[0045] Calcium dobesilate 25 g
[0046] Crospovidone 9 g
[0047] Microcrystalline Cellulose 4.5 g
[0048] Lactose 6.275 g
[0049] Magnesium stearate 0.225 g
[0050]
[0051] Makes 100 pieces
[0052] Preparation process: Mix the prescription amount of crospovidone, microcrystalline cellulose and lactose in equal increments, then mix the prescription amount of calcium dobesilate with the above-mentioned mixed powder in equal increments Finally, add the prescribed amount of magnesium stearate and mix evenly, determine the tablet weight and hardness, and directly compress the tablet to obtain the calcium dobesilate dispersible tablet. The whole preparation process is completed at 25°C.
[0053] Appearance: The calcium dobesilate dispersible tablet prepared by this method is off-white with smooth surface.
[0054] Uniformity of dispersion: Take 6 tablets of the prepared calcium dobesilate dispersible tablets, place them in 100...
Embodiment 3
[0057] Raw material prescription:
[0058] Calcium dobesilate 25 g
[0059] Crospovidone 9 g
[0060] Microcrystalline cellulose 6.75 g
[0061] Lactose 4.025 g
[0062] Magnesium stearate 0.225 g
[0063]
[0064] Makes 100 pieces
[0065] Preparation process: Mix the prescription amount of crospovidone, microcrystalline cellulose and lactose in equal increments, then mix the prescription amount of calcium dobesilate with the above-mentioned mixed powder in equal increments Finally, add the prescribed amount of magnesium stearate and mix evenly, determine the tablet weight and hardness, and directly compress the tablet to obtain the calcium dobesilate dispersible tablet. The whole preparation process is completed at 25°C.
[0066] Appearance: The calcium dobesilate dispersible tablet prepared by this method is off-white with smooth surface.
[0067] Uniformity of dispersion: Take 6 tablets of the prepared calcium dobesilate dispersible tablets, place them in 100ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com