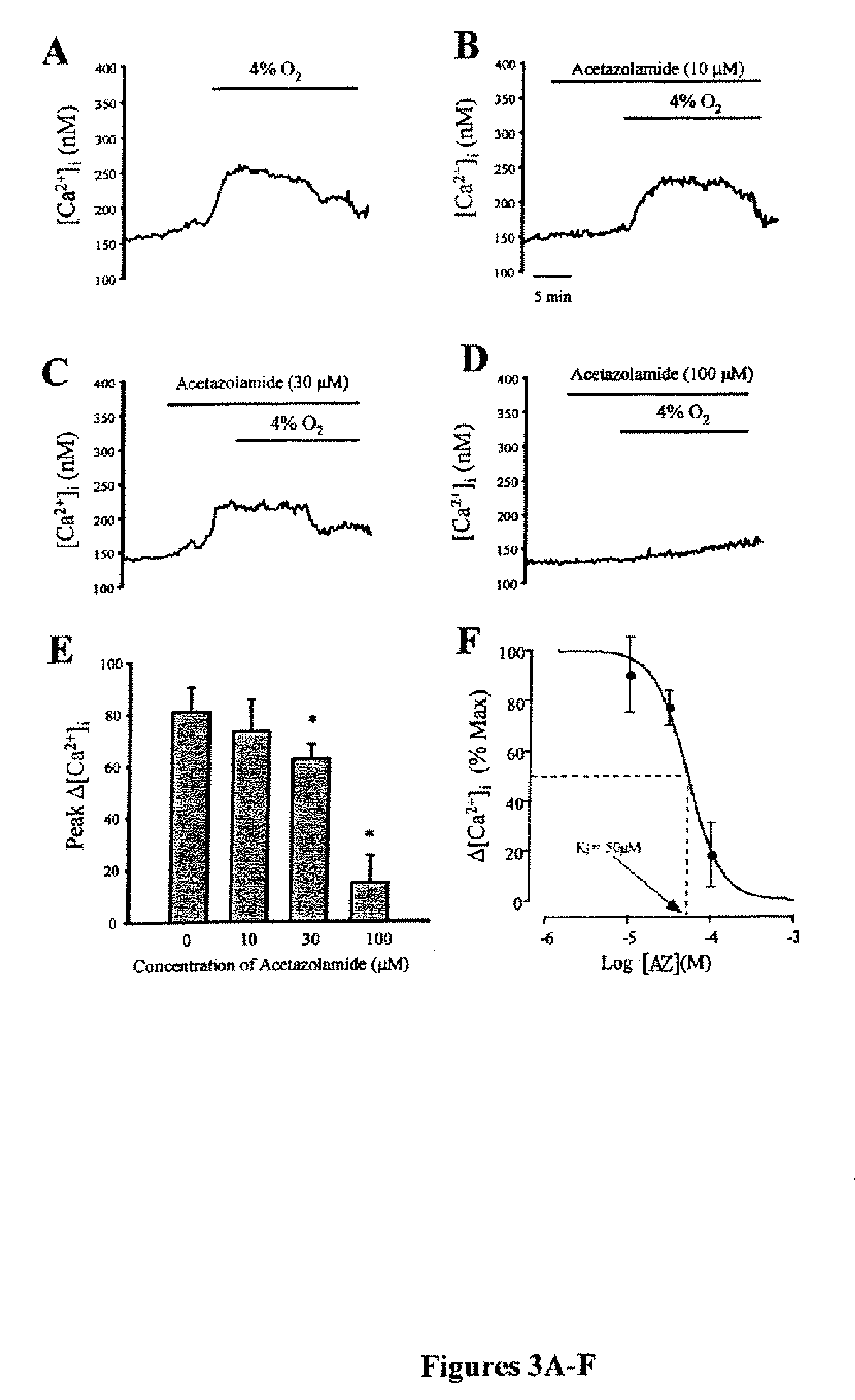
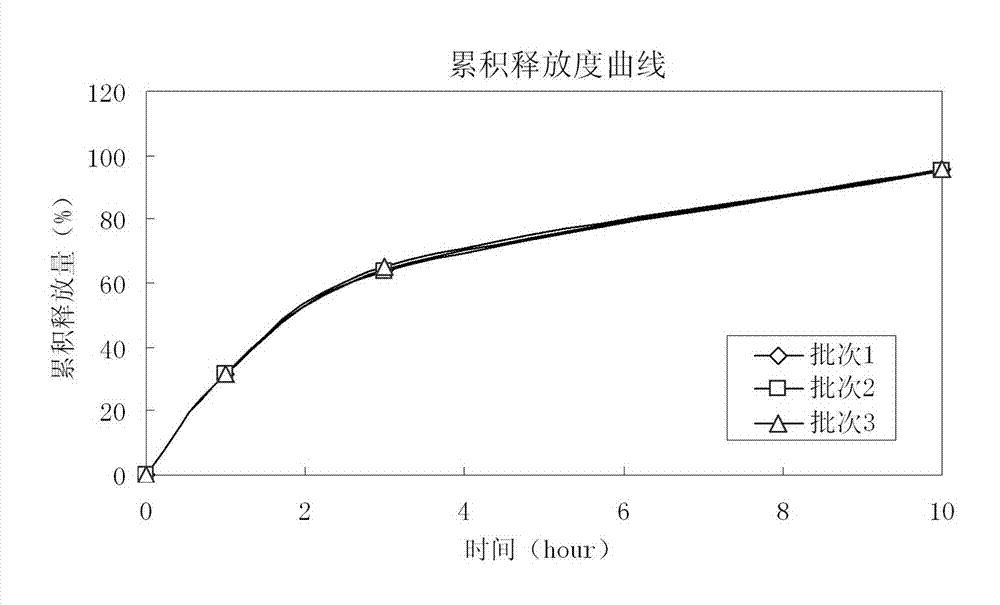
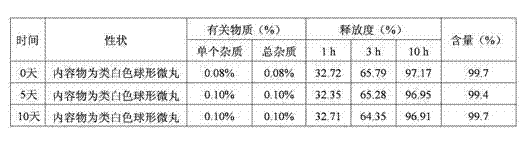
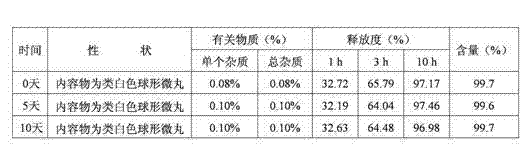
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
60 results about "Acetazolamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acetazolamide is used to prevent and reduce the symptoms of altitude sickness.
Medicine-carrying contact lens and preparation method thereof
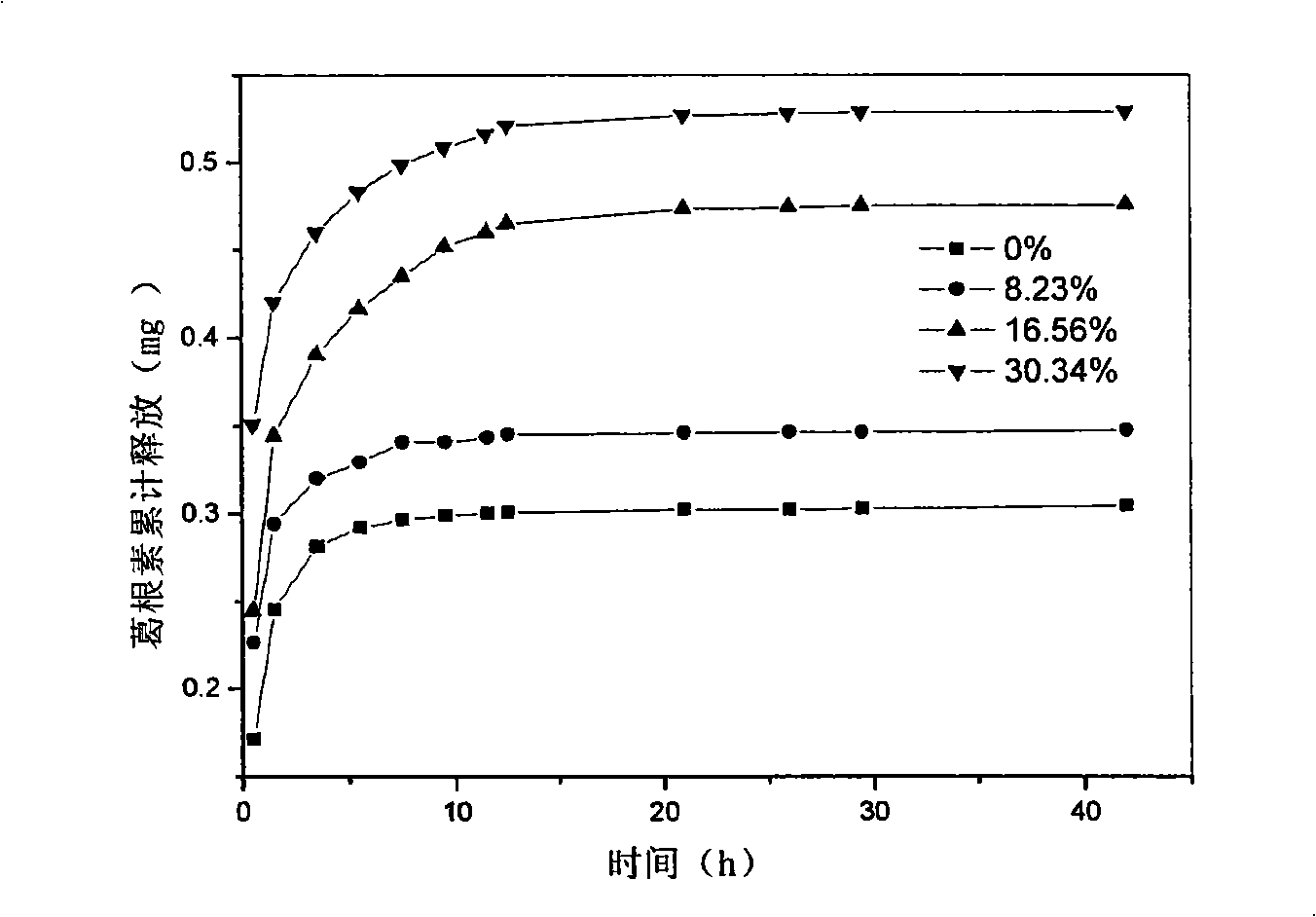
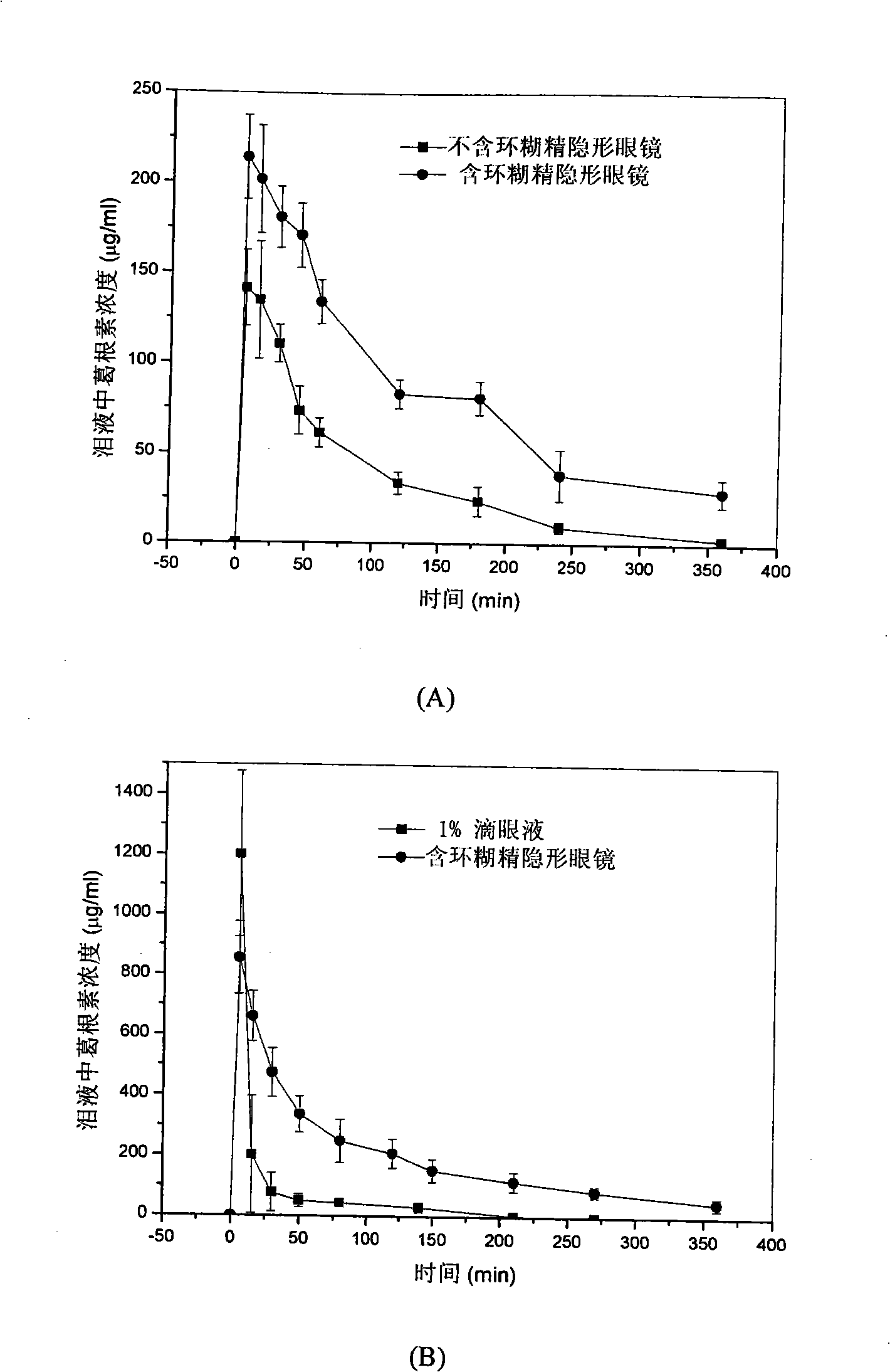
InactiveCN101344648AEasy to fixChange the amount addedOrganic active ingredientsSenses disorderPuerarinDrug release
The invention relates to a drug-loaded contact lenses and a corresponding preparation method. The drug-loaded contact lenses are obtained firstly through UV-curing or heat-curing and then through drug-liquid immersion. The drug-loaded contact lenses can be particularly defined as a combination of a contact lenses material containing cyclodextrin and drug molecules and comprise the contact lenses formed by combining a crosslinked polymer hydrogel formed by copolymerizing mono-substituted cyclodextrin monomers, polymerization monomers and crosslinkers with one kind or a plurality of acetazolamide, methazolamide, puerarin or prostaglandin. The characteristic of forming inclusion compounds between the cyclodextrin molecules and drug molecules is adopted to increase the quantity of loaded drugs so as to reduce the stimulation to eyes by drugs and control the drug release speed for eye diseases of curing glaucoma, etc.
Owner:SOUTHEAST UNIV
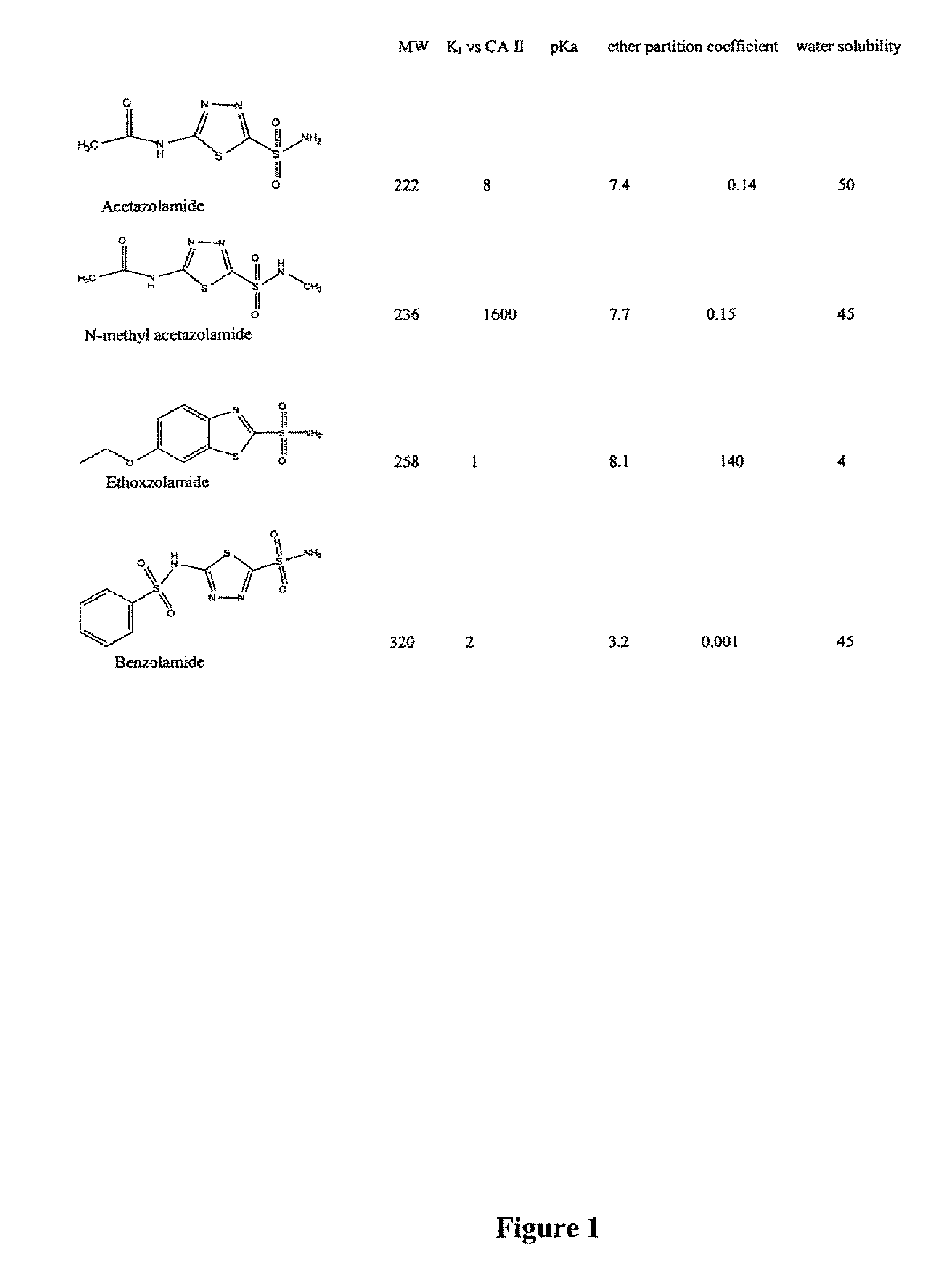
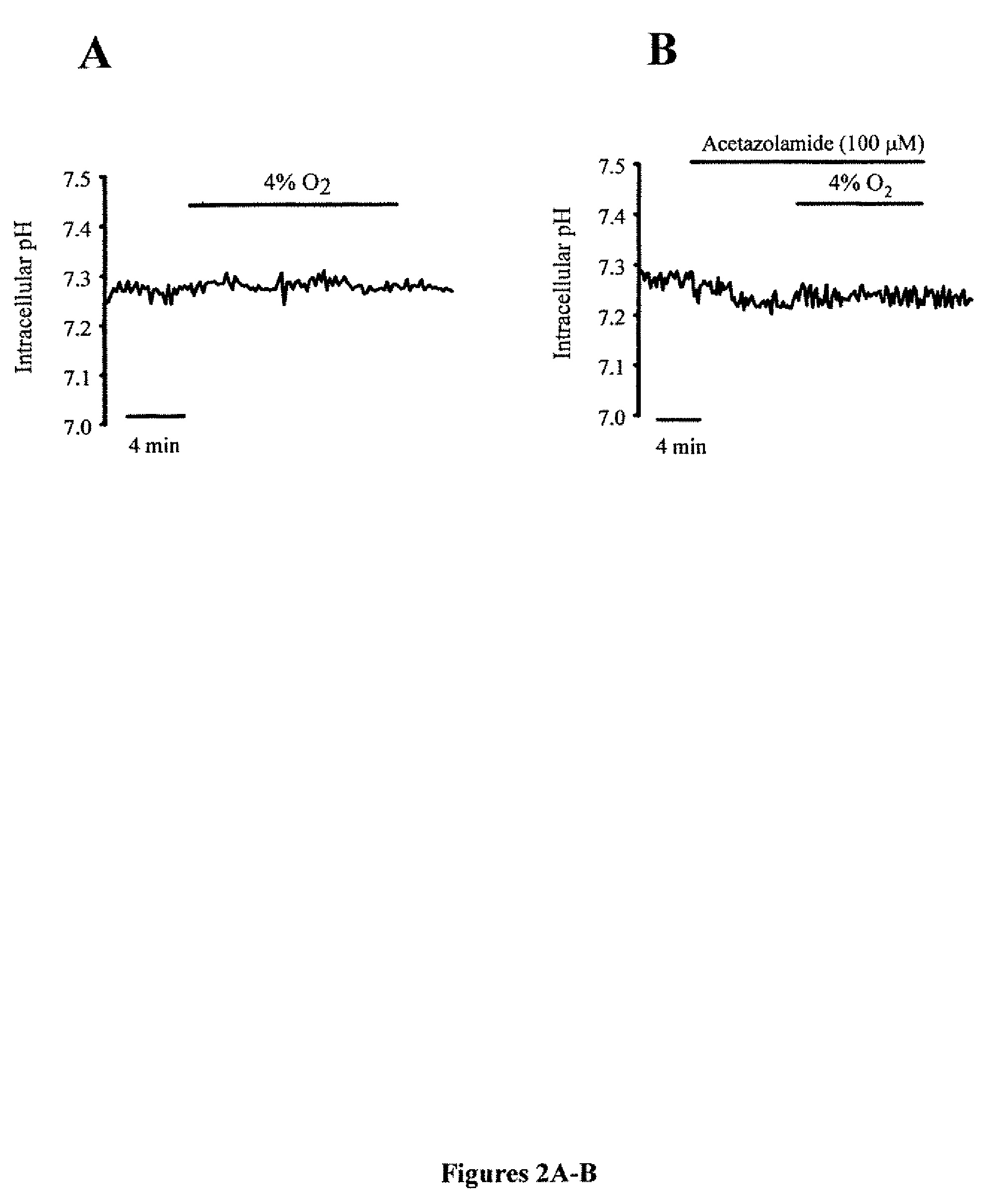
Methods of treating pulmonary disease using acetazolamide and structurally related derivatives
The present invention is directed to a method of treating a subject for a pulmonary disease by administering a therapeutically effective amount of a compound of the formula:wherein R1, R2 or R3 are each independently a C1 to C6 alkyl, a halogen, a sulfate, or a phosphate. The pulmonary disease in the subject can be hypoxic pulmonary vasoconstriction, pulmonary edema, pulmonary hypertension, asthma, chronic obstructive pulmonary disease, cystic fibrosis, interstitial fibrosis, high altitude residence, sleep apnea syndrome, atrial septal defects, and pulmonary diseases associated with other conditions. If this same compound is modified so that R1, R2 or R3 each independently is a C1 to C6 alkyl and the compound is not a carbonic acid inhibitor, it can be administered to a subject to block hypoxic pulmonary vasoconstriction and / or prevent high altitude pulmonary edema. Additional aspects of the present invention include an inhalable composition comprising the compound of the above formula without modification and an inhalable carrier, as well as the above modified compound.
Owner:UNIV OF WASHINGTON
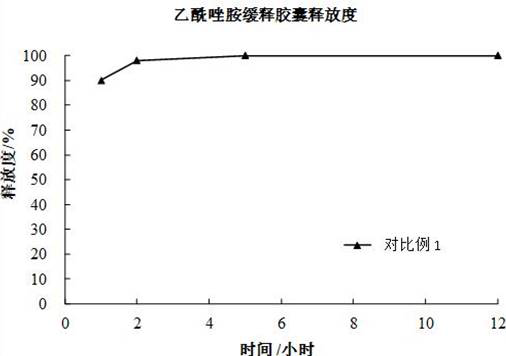
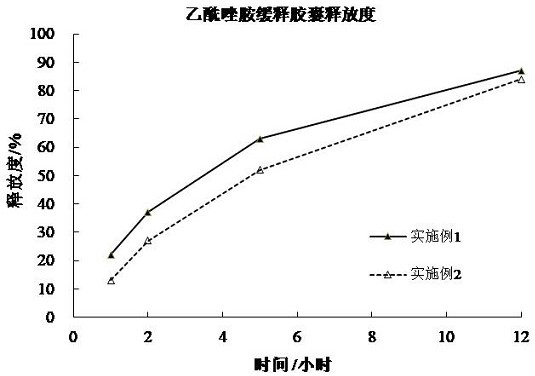
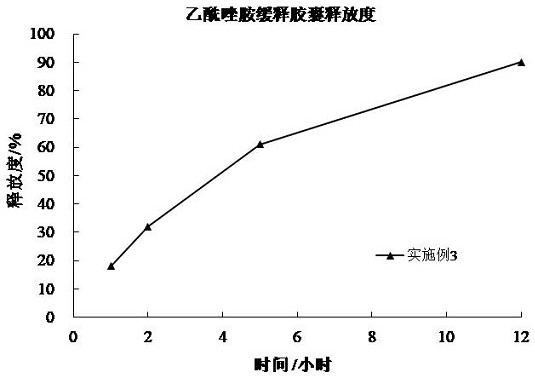
Acetazolamide sustained-release capsule and preparation method thereof
ActiveCN102949377AImprove stabilitySimple manufacturing processOrganic active ingredientsSenses disorderSustained release pelletsAdjuvant
The invention discloses an acetazolamide sustained-release capsule and a preparation method thereof. The acetazolamide sustained-release capsule is mainly composed of the following raw materials in accordance with parts by weight: 65 to 85 parts of acetazolamide, 15 to 35 parts of microcrystalline cellulose and 0.1 to 0.5 part of sodium dodecyl sulfate, and the acetazolamide sustained-release capsule further contains pharmaceutically acceptable pharmaceutic adjuvants. The preparation method comprises the following steps that the required raw materials and the adjuvants are smashed and uniformly mixed, and then the raw materials and adjuvants are prepared into soft materials after being uniformly mixed; a low-temperature refrigerator is turned on, the soft materials are put into an extrusion rolling machine to be extruded and rolled, and then drug containing micro pills with uniform particle sizes are formed; after the drug containing micro pills are dried, acetazolamide sustained-release micro pills are obtained; and the acetazolamide sustained-release micro pills are encapsulated to obtain the acetazolamide sustained-release capsule. The acetazolamide sustained-release capsule prepared by using the preparation method has little side effect and is convenient for patients to treat for a long time, and the drug compliance is improved. When the acetazolamide sustained-release capsule is taken once a day, the concentration of effective drugs in vivo can be guaranteed for 24 hours, and the toxic and side effects caused by a higher peak value of the blood drug concentration due to normal preparations can be reduced.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Acetazolamide medicine composition and medical application thereof
InactiveCN105859717AImprove the effect of prevention and controlHighlight substantiveOrganic active ingredientsNervous disorderState of artNatural product
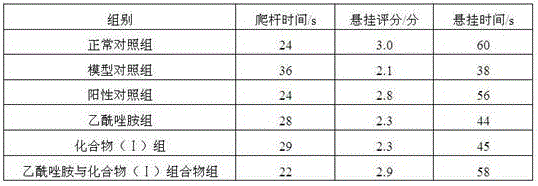
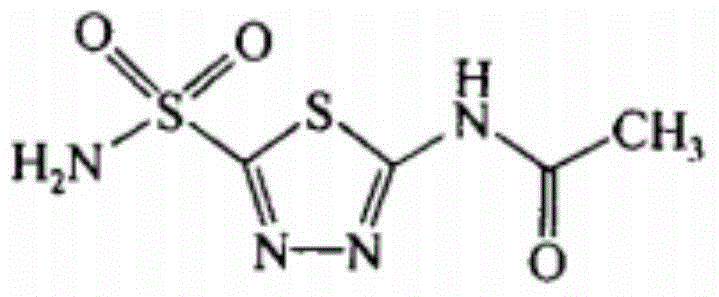
The invention discloses a pharmaceutical composition of acetazolamide and its medical application. The pharmaceutical composition of acetazolamide provided by the invention contains acetazolamide and a natural acetazolamide with a novel structure isolated from the dried root of Polygonum cuspidatum. When the product compound (I), acetazolamide and the natural product compound (I) act alone, the prevention and treatment effect on Parkinson's disease is weak; when the two act together, the prevention and treatment effect on Parkinson's disease is significantly improved, and can be developed The medicine for preventing and treating Parkinson's disease has outstanding substantive features and remarkable progress compared with the prior art.
Owner:SUZHOU BINUOJIA PHARMA CO LTD
Dispersible tablet of acetazolamide and its preparation method
InactiveCN1704054AGood dissolution uniformityQuick effectOrganic active ingredientsSenses disorderCelluloseMicroparticle
Disclosed is a dispersible tablet of acetazolamide where the preparation comprises, disintegrating acetazolamide, crystalline cellulose, sodium carboxymethylstarch, magnesium stearate, passing through 100 mesh sieve, charging polyvinylpyrrolidone, sodium dodecanesulphonate, mixing and disintegrating into particles, then charging magnesium stearate and mixing, adjusting sheet weight, tabletting so as to obtain the dispersible tablets. The dispersible tablets can be crumbled quickly, the crumbling time limit is less than 2 minutes, the other advantages include faster medicament dissolving and high biological availability.
Owner:马晶
Acetazolamide composition lyophilized tablet and preparation method thereof
InactiveCN104434837AGood molding effectHigh dissolution rateOrganic active ingredientsSenses disorderMedicineCurative effect
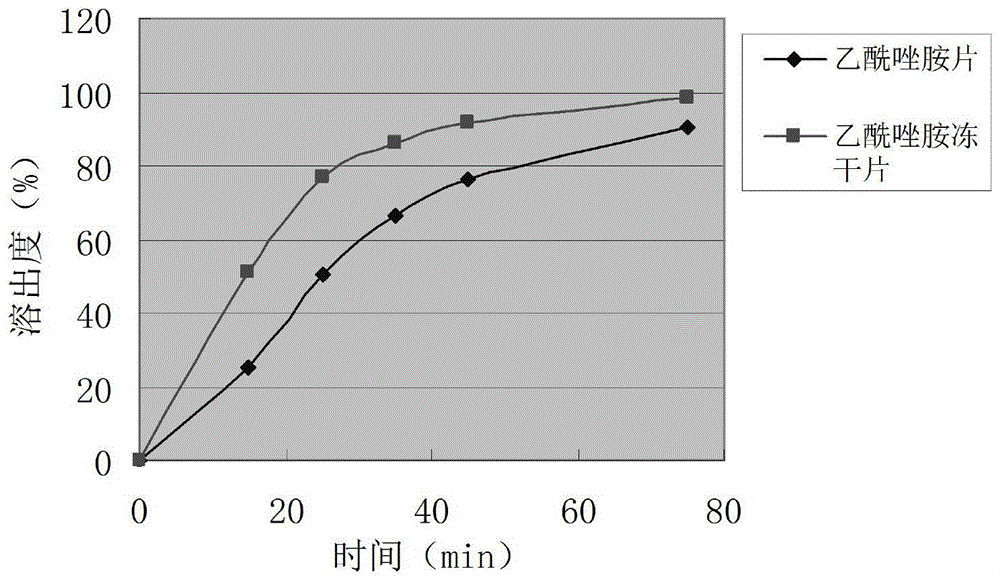
The invention provides an acetazolamide composition lyophilized tablet and a preparation method thereof and relates to the technical fields of medicines and medicine production. The acetazolamide composition lyophilized tablet comprises acetazolamide, starch and cane sugar. By taking starch and cane sugar as auxiliary materials, heating process is carried out on common corn starch, so that the bonding and disintegrating effects of starch in the tablet can be improved and the formability of the tablet is improved. The acetazolamide composition lyophilized tablet just needs two auxiliary materials: starch and cane sugar. According to the acetazolamide composition lyophilized tablet, by adopting a lyophilizing process of increasing and decreasing the temperature twice, the formability of the tablet is good due to temperature increase and decrease twice, so that the dissolution rate of the tablet is improved, thereby improving the bioavailability of the tablet. The tablet is used for overcoming the defects of common acetazolamide tablet and reducing the types and use level of auxiliary materials in the acetazolamide tablet, is good in dissolution rate and high in bioavailability and can be used for ensuring the curative effect and safety of clinical medication.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
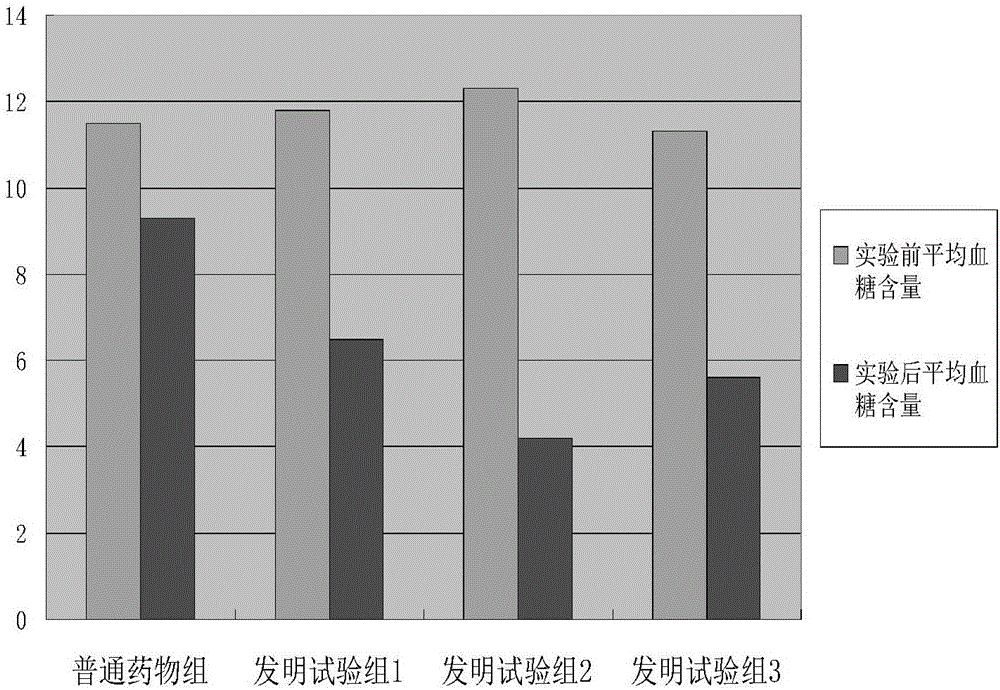
Hypoglycemic composition containing pioglitazone and preparation method of hypolipidemic composition
InactiveCN106110310AReduce resistanceReduce outputHydroxy compound active ingredientsPeptide/protein ingredientsBenzoic acidVitamin B12
The invention relates to a hypoglycemic composition containing pioglitazone and a preparation method of the hypoglycemic composition. The composition consists of the following components in parts by weight: 0.05-0.15 parts of pioglitazone, 0.5-2.8 parts of acetazolamide, 1.2-3.4 parts of vitamin A, 0.4-0.8 parts of arabinose, 0.6-2.0 parts of folic acid, 0.5-1.5 parts of benzoic acid, 0.3-0.9 parts of taurine, 0.8-1.4 parts of acesulfame, 0.2-0.6 parts of lentinan , 0.7-1.0 part of vitamin B12, 0.4-0.8 parts of proline, 0.6-1.2 parts of calcium citrate, 0.2-0.8 parts of xylitol, 0.05-0.08 parts of complex enzyme, 0.9-2.5 parts of magnesium stearate, 2-6 parts of carboxymethyl starch and 5-40 parts of a binding agent. The hypoglycemic composition disclosed by the invention, which is added with adjuvant materials, can be prepared into tablets or capsules by virtue of a conventional process; the hypoglycemic composition is simple in using method; and upon clinical verification, the hypoglycemic composition is good in hypoglycemic and diuretic effects.
Owner:卢连伟
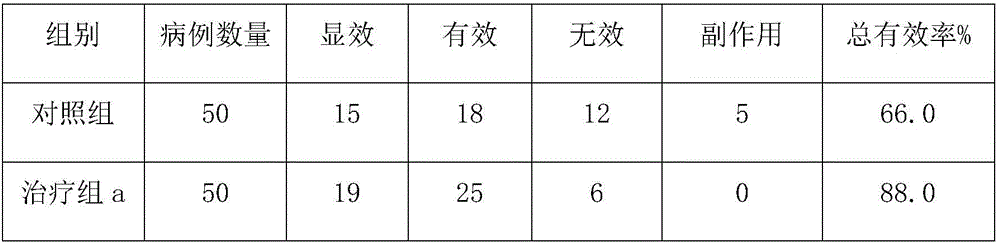
Compsn. of medication for treating high blood pressure
InactiveCN1695738ALower blood pressureOrganic active ingredientsCardiovascular disorderBlood pressure fallBumetanide
A composite medicine for treating hypertension contains the diuretic chosen from errolon, ethacrynic acid, bumetanide, chlorothiazinde, hydrochlorothiazinde, etc a Ca-contained component chosen from shell, inorganic Ca salt, organic Ca salt, etc, and the pharmacologically acceptable additives.
Owner:苏金平
Application of 5,6,7,8- trihydroxy-8-methoxyflavone in preparing anti-hypoxic drug
ActiveCN103877074ASignificant anti-hypoxic effectGood effectOrganic active ingredientsAntinoxious agentsDiseaseCerebral ischaemia
The invention discloses an application of 5,6,7,8- trihydroxy-8-methoxyflavone in preparing an anti-hypoxic drug which is the drug for preventing and treating hypoxic injury diseases; the hypoxic injury diseases are high-altitude low-pressure oxygen deficit-caused acute high altitude diseases, cerebral ischemia hypoxic diseases or myocardial ischemia hypoxic diseases. The application has beneficial effects as follows: an experiment proves that the 5,6,7,8- trihydroxy-8-methoxyflavone has a remarkable anti-hypoxic effect, and an effect tending to be better than that of acetazolamide; the anti-hypoxic drug prepared by applying the 5,6,7,8- trihydroxy-8-methoxyflavone as an active ingredient can be used for preventing and treating hypoxic injuries or treating various hypoxic pathological states caused by oxygen content drop in external environment, also can be used for preventing or treating hypoxic pathological states of heart, brain, respiratory system, and the like as external normal oxygen cannot sufficiently get to a body due to diseases, and the like, and further can be used for preventing or treating the pathological state that relative oxygen supply is insufficient as oxygen consumption needed for activities of the body exceeds physiological mobilization ability.
Owner:中国人民解放军联勤保障部队第九四〇医院
High-temperature-resistant and ageing-resistant cable sheath material and preparing method thereof
InactiveCN105623007AExtended service lifeImprove high temperature resistanceHydrogenated Palm Kernel OilChlornitrofen
The invention discloses a high-temperature-resistant and aging-resistant cable sheath material. The high-temperature-resistant and aging-resistant cable sheath material is prepared from, by weight, 60-80 parts of nitrile butadiene rubber, 25-35 parts of methyl vinyl silicone rubber, 3-8 parts of zinc oxide, 2-7 parts of magnesium stearate, 12-18 parts of diethylene glycol monoethyl ether acetate, 10-16 parts of 2,2,2-trichloroacetamide benzyl ester, 11-18 parts of ethopabate, 4-10 parts of acetazolamide, 3-7 parts of magnalium zirconium oxide, 4-7 parts of zirconocene dichloride, 1-4 parts of hydrogen-chlorine ferrocene, 10-15 parts of bifenox and 12-18 parts of hydrogenated palm kernel oil. According to the high-temperature-resistant and aging-resistant cable sheath material, at the high temperature of 150 DEG C, changes of all performances are small, aging resistance is high, and the performance is stable.
Owner:SUZHOU KEMAO ELECTRONICS MATERIALS TECH
Application of chrysin in preparing anti-hypoxic drug
ActiveCN103877075ASignificant anti-hypoxic effectGood effectOrganic active ingredientsAntinoxious agentsDiseaseCerebral ischaemia
The invention discloses an application of chrysin in preparing an anti-hypoxic drug. The anti-hypoxic drug is a drug for preventing and treating hypoxic injury diseases which are high-altitude low-pressure oxygen deficit-caused acute high altitude diseases, cerebral ischemia hypoxic diseases or myocardial ischemia hypoxic diseases. The application has beneficial effects as follows: experiments prove that the chrysin has a remarkable anti-hypoxic effect, and effect tending to be better than that of acetazolamide; the anti-hypoxic drug prepared by applying the chrysin as an active ingredient can be used for preventing and treating hypoxic injuries or treating various hypoxic pathological states caused by oxygen content drop in external environment, also can be used for preventing or treating hypoxic pathological states of heart, brain, respiratory system, and the like as external normal oxygen cannot sufficiently get to a body due to diseases, and the like, and further can be used for preventing or treating the pathological state that relative oxygen supply is insufficient as oxygen consumption needed for activities of the body exceeds physiological mobilization ability.
Owner:中国人民解放军联勤保障部队第九四〇医院
Application of pyrroloquinoline quinone in preparation of medicine for preventing and treating acute altitude sickness and acute high altitude hypoxic injury
InactiveCN110870866AToxic and side effectsIncreased toxicityOrganic active ingredientsOrganic chemistrySide effectPyrrovinyquinium
The invention relates to an application of pyrroloquinoline quinone (PQQ) in preparation of a medicine for preventing and treating acute altitude sickness and acute high altitude hypoxic injury. The pyrroloquinoline quinine provided by the invention has the effect of preventing and treating the acute high altitude hypoxic injury, the efficacy is equivalent to that of acetazolamide, but the acetazolamide has large toxic and side effects, and the pyrroloquinoline quinone has the advantages of low toxicity and easy acceptance by patients as a coenzyme; and in addition, through exhaustive swimmingexperiments of mice under hypoxic exposure conditions, results show that the PQQ has the characteristics of improving the physical working ability at altitude, but the acetazamide is not found to have the effect.
Owner:ZHE JIANG MEDICINE CO LTD XINCHANG PHARMA FAB
Capsule for subsequent filainous uveitis after cataract surgery
InactiveCN106038603APrevent relapseImprove circulatory disordersSenses disorderHydroxy compound active ingredientsUveitisVitamin C
The invention discloses a capsule for subsequent filainous uveitis after cataract surgery. The capsule is mainly prepared from the following components: acetazolamide, taurine, aucubin, hyperoside, L-fucose, wogonin, water-soluble nutrient pigment, caffeic acid, beta-sitosterol, baicalein, pearl powder, ursolic acid, D-mannitol, vitamin A, vitamin C, diluent, lubricant, glidant, disintegrant and a wetting agent. According to the capsule, ocular circulatory disturbance can be improved, inflammatory response can be controlled, and retinal metabolism and immunoregulation functions can be enhanced, so that absorption of postoperative hyperplastic lesions can be promoted, inflammatory fibrous exudation can be inhibited, and vision recovery after cataract surgery can be promoted. The capsule has a remarkable curative effect, and is suitable for popularization.
Owner:钟志敏
Methods of treating pulmonary disease using acetazolamide and structurally related derivatives
The present invention is directed to a method of treating a subject for a pulmonary disease by administering a therapeutically effective amount of a compound of the formula:wherein R1, R2 or R3 are each independently a C1 to C6 alkyl, a halogen, a sulfate, or a phosphate. The pulmonary disease in the subject can be hypoxic pulmonary vasoconstriction, pulmonary edema, pulmonary hypertension, asthma, chronic obstructive pulmonary disease, cystic fibrosis, interstitial fibrosis, high altitude residence, sleep apnea syndrome, atrial septal defects, and pulmonary diseases associated with other conditions. If this same compound is modified so that R1, R2 or R3 each independently is a C1 to C6 alkyl and the compound is not a carbonic acid inhibitor, it can be administered to a subject to block hypoxic pulmonary vasoconstriction and / or prevent high altitude pulmonary edema. Additional aspects of the present invention include an inhalable composition comprising the compound of the above formula without modification and an inhalable carrier, as well as the above modified compound.
Owner:UNIV OF WASHINGTON
Culture medium for amplification of human umbilical cord mesenchymal stem cells and culture method
ActiveCN113151165APromote proliferationPromote secretionCulture processSkeletal/connective tissue cellsVitamin CPancreatic hormone
The invention discloses a culture medium for amplification of human umbilical cord mesenchymal stem cells. The culture medium is prepared from a basic culture medium and the following components added into the basic culture medium of glutathione, alpha-tocopherol, eudesmin, estradiol valerate, acetazolamide, sodium selenite, vitamin C and insulin. Exogenous serum is not added into the culture medium provided by the invention, the components are clear, and the components have a synergistic effect, so that the proliferation of the umbilical cord mesenchymal stem cells and the secretion of cell factors are promoted. The added ingredients such as eudesmin, estradiol valerate, acetazolamide and the like not only avoid adding serum into the culture medium to form ingredients with potential safety hazards, but also can improve the proliferation efficiency of cells, a large number of umbilical cord mesenchymal stem cells can be obtained within a short time, and the content of cell factors in the culture solution is increased. The invention also provides a culture method of the human umbilical cord mesenchymal stem cells, which adopts the culture medium provided by the invention, and is safe, efficient, simple and convenient in process and easy to operate.
Owner:山西北科生物科技有限公司
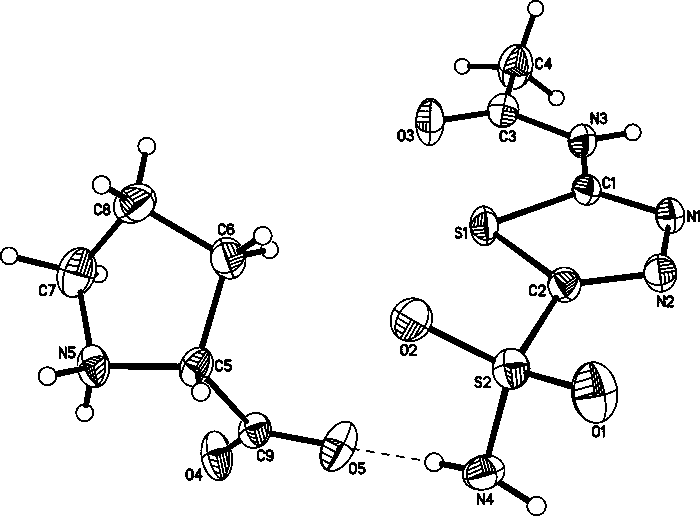
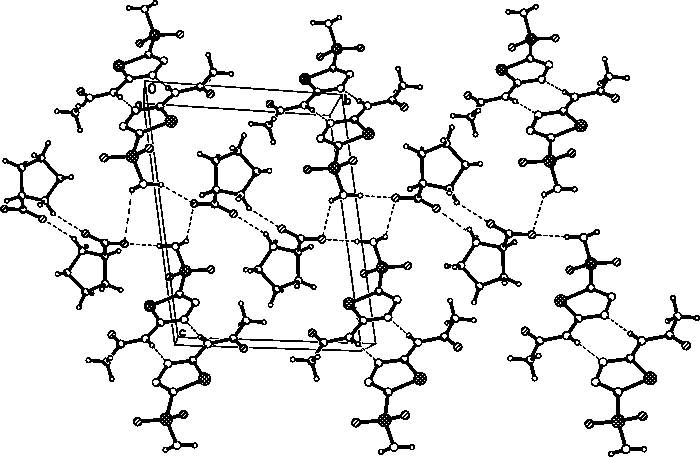
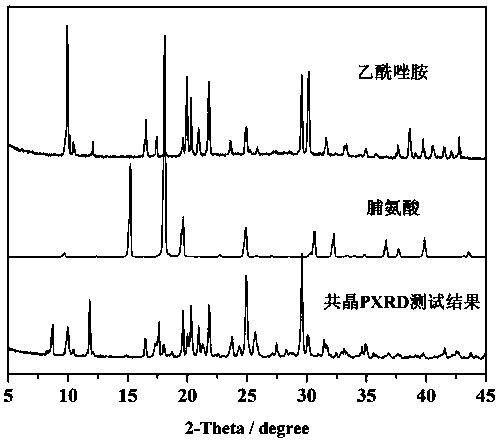
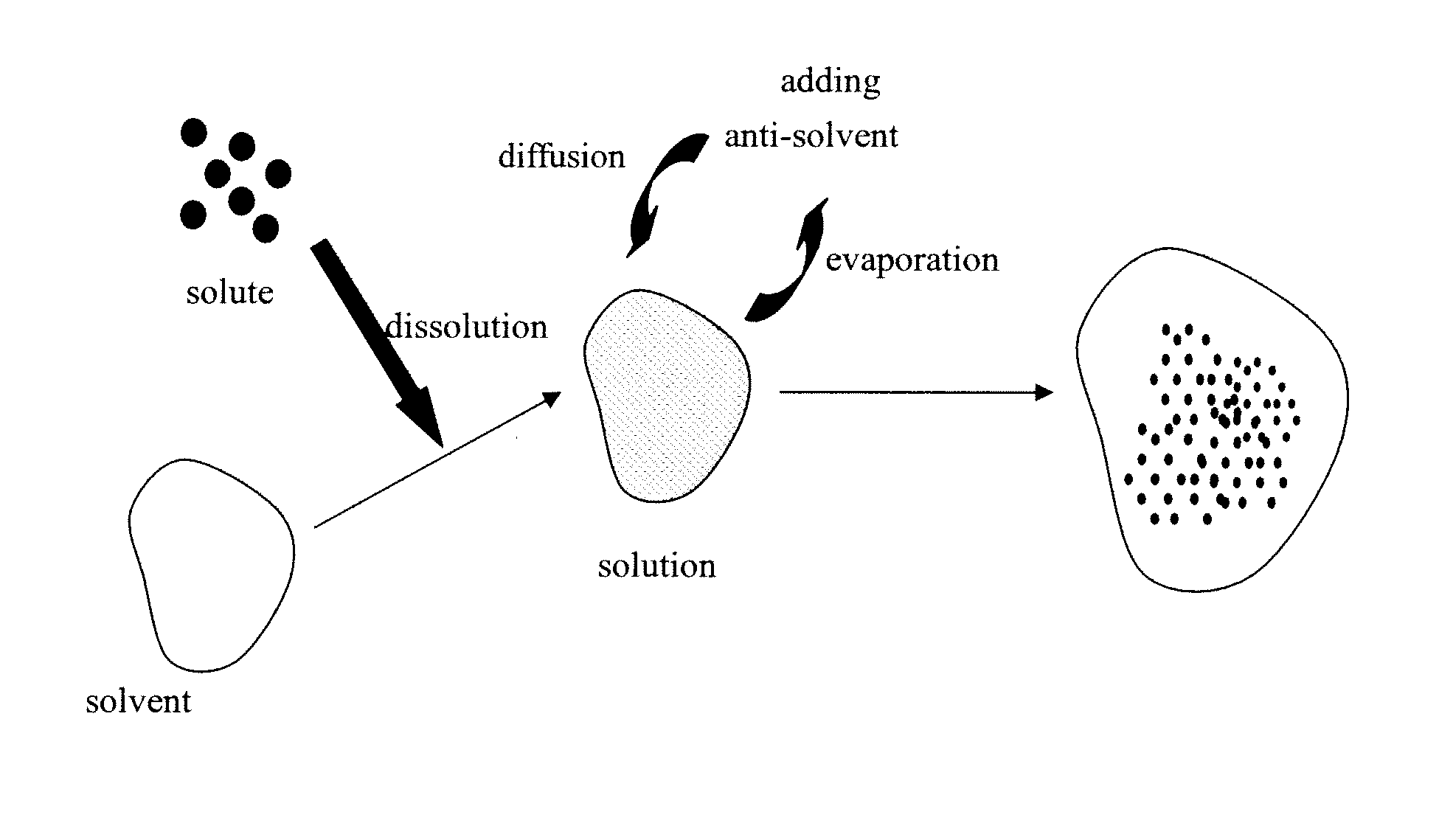
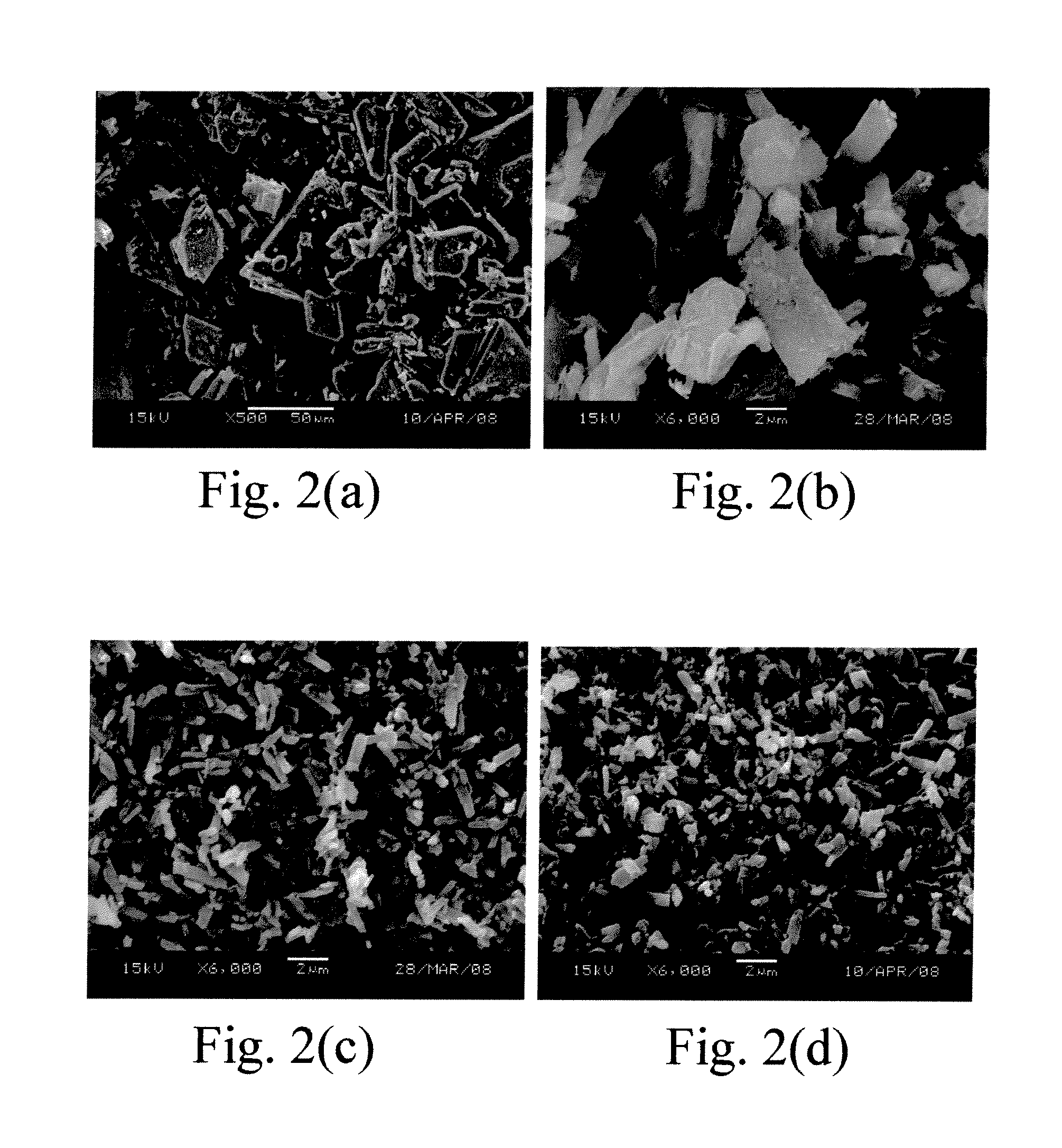
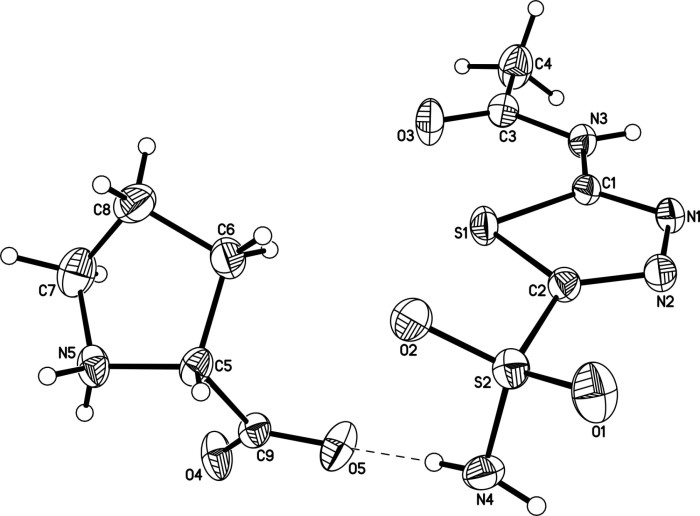
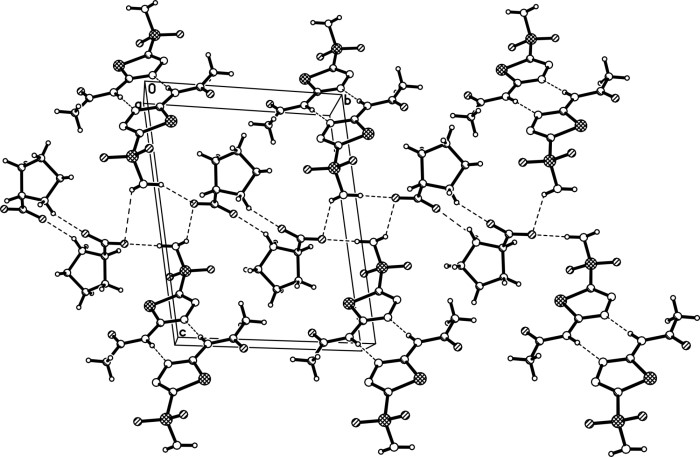
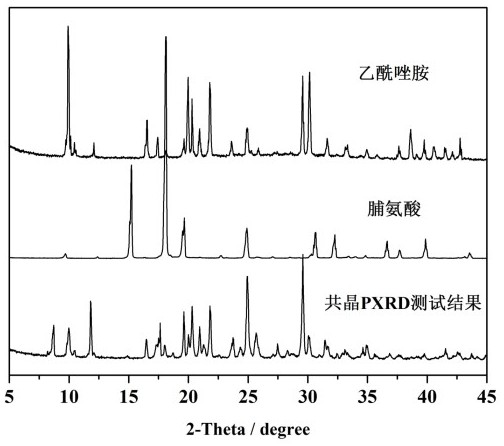
Eutectic crystal of acetazolamide and proline and preparation method of eutectic crystal
InactiveCN108558791ASimple processHigh yieldOrganic active ingredientsSenses disorderCrystal systemSolvent molecule
The invention provides a eutectic crystal of acetazolamide and proline and a preparation method of the eutectic crystal and relates to the field of medicine eutectic crystals. A molecular formula of the medicine eutectic crystal is [C4H6N4O3S2.C5H9NO2] and a basic structural unit is composed of an acetazolamide molecule and a proline molecule. The medicine eutectic crystal belongs to a triclinic crystal system and a space group is P-1. Acetazolamide and the proline are used as raw materials and the medicine eutectic crystal is prepared by adopting a cooling method and a solvent volatilizationmethod respectively. According to the medicine eutectic crystal provided by the invention, the solubility of acetazolamide is improved and the bioavailability of acetazolamide is easy to improve. A eutectic crystal structure does not contain any solvent molecule so that the eutectic crystal structure still can keep stable after being placed for a long time under a room-temperature condition. The preparation method and a technological route of the medicine eutectic crystal are simple and feasible and large-scale production is convenient to realize.
Owner:OCEAN UNIV OF CHINA
Acetazolamide Microparticle And Its Preparation Method And Use
A method for preparing an acetazolamide microparticle having a mean particle size ranged between 0.36 μm and 18 μm is provided. The method includes steps of dissolving an acetazolamide in a solvent to form an acetazolamide solution; and mixing the acetazolamide solution with a supercritical fluid at a temperature and a pressure above a critical point of the supercritical fluid for forming the acetazolamide microparticle, wherein the solvent is miscible with the supercritical fluid.
Owner:NAT TAIWAN UNIV
Acetazolamide derivative, preparation method thereof and application thereof in preparation of medicine for treating coronary heart disease
ActiveCN111961043AAvoid drug resistanceIncrease vitalityOrganic chemistryCardiovascular disorderIndometacinDisease
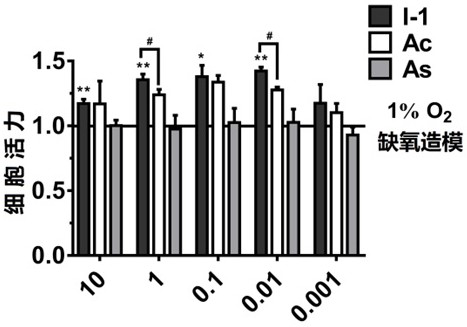
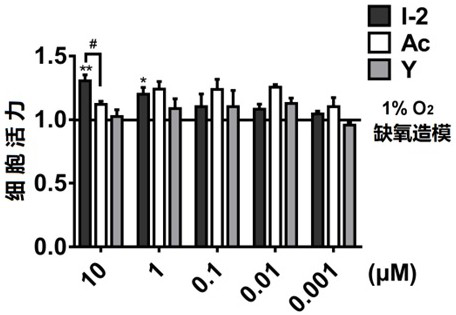
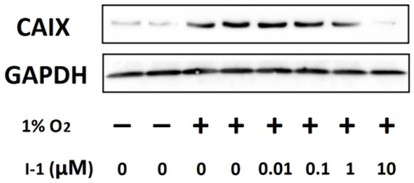
The invention relates to an acetazolamide derivative, a preparation method thereof and an application of the acetazolamide derivative in preparation of a medicine for treating coronary heart disease.The acetazolamide derivative is an acetazolamide derivative I obtained by bonding a carbonic anhydrase inhibitor acetazolamide Ac and a non-steroidal anti-inflammatory drug which is represented by aspirin As and has a carboxylic acid group through a linking group, and the structural general formula is shown as a formula 1 shown in the specification, wherein in the formula 1, OOC-NSAID represents anon-steroidal anti-inflammatory drug in which one carboxylic acid proton is lost, representative are acetazolamide derivatives containing aspirin or indomethacin, and the acetazolamide derivative I has good activity of inhibiting carbonic anhydrase 9, can effectively improve myocardial anoxia injury in an anoxia microenvironment, and is applied to preparation of the medicine for treating coronaryheart disease.
Owner:SOUTHEAST UNIV
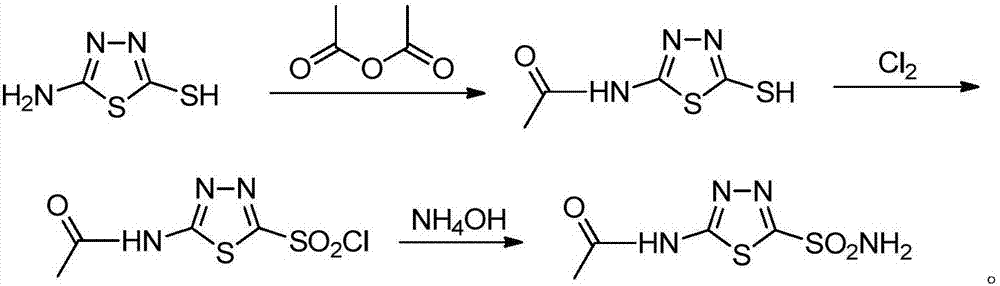
Preparation method of non-chlorine gas of acetazolamide key intermediate 2-acetamido-5-chlorosulfonyl-1, 3, 4-thiadiazole
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Preparation process for intermediate of acetazolamide
ActiveCN107129472ASolve the problem of low pass rate and unstable qualityHigh yieldOrganic chemistryPyridineAcetazolamide
The invention especially relates to a preparation process for an intermediate of acetazolamide, belonging to the field of medicine synthesis. The process comprises the following steps: with thiosemicarbazide and carbon disulfide as reactants and a mixture of pyridine and piperidine as a solvent, carrying out a reaction; and then successively carrying out purification and drying so as to obtain 2-amino-5-mercapto-1,3,4-thiadiazole, i.e., the intermediate of acetazolamide.
Owner:常州金澄医药化工有限公司
Acetazolamide eye droplet and its application method in treating glaucoma
InactiveCN1557304AImprove solubilityImprove stabilityOrganic active ingredientsSenses disorderSolubilitySide effect
The present invention relates to acetazolamide eye drop and its application in treating glaucoma, is the recipe of acetazolamide solution for local use to treat glaucoma. The recipe of the acetazolamide eye drop consists of acetazolamide, poloxamer as polyoxyethylene polyoxypropylene block copolymer with solubilizing, effect-prolonging, moistening and local absorption promoting effects, and synergistic solubilizing liquid state carrier system. Into the recipe, buffering system, isotonic regulator and / or preservative may be added. The recipe has increased acetazolamide solubility, raised acetazolamide stability, and strengthened cornea penetrability of acetazolamide. The local application of the recipe can avoid the side effect of acetazolamide on cardio vascular system, breath system, etc.
Owner:JC (WUXI) CO INC
Application Of Pyrroloquinoline Quinone In Preparation Of Medicament Used For Preventing And Treating Acute Altitude Sickness And Acute Altitude Hypoxia Injury
PendingUS20220023289A1Preventing and treating acute altitude hypoxia injuryLow toxicityOrganic active ingredientsOrganic chemistryHigh altitude hypoxiaSide effect
The present invention relates to an application of pyrroloquinoline quinone (PQQ) in the preparation of a medicament used for preventing and treating acute altitude sickness and acute altitude hypoxia injury. Pyrroloquinoline quinone has the effect of preventing and treating acute high altitude hypoxia injury, and as a drug for the prevention and treatment of acute altitude sickness, the efficacy thereof is equivalent to that of acetazolamide, however acetazolamide has many toxic side effects; meanwhile, as a coenzyme, pyrroloquinoline quinone has the advantages of low toxicity and is easily acceptance by patients. In addition, by means of exhaustive swimming experiments of mice under the conditions of hypoxic exposure, PQQ has been shown to have the feature characteristics of improving the working capabilities of a subject at a high altitude, however acetazolamide has not been found to have said effect.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
New compound and method for preparing same
ActiveCN101113144AOrganic chemistryHeterocyclic compound active ingredientsChemical compositionCoronary heart disease
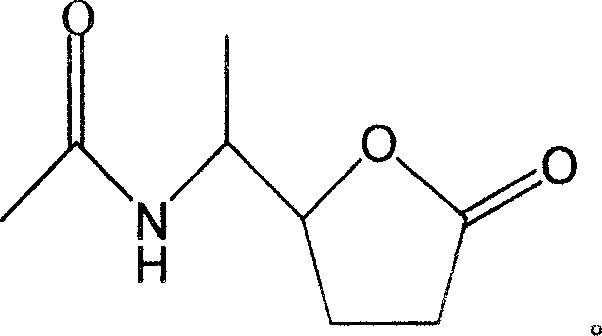
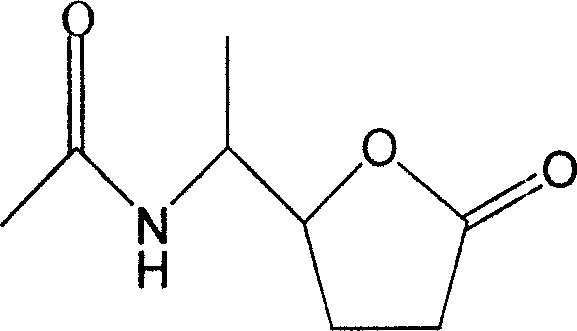
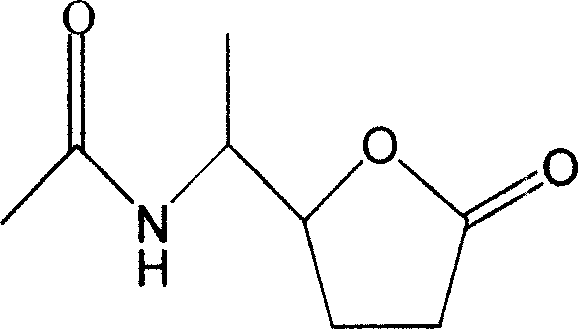
The invention discloses a novel compound and preparation thereof. Researches prove that, Xuezhikang special made Hongqu can obviously decrease the occurrence rate of coronary heart disease attack and the general death rate. The chemical components of Xuezhikang are researched and ten components are separated from the Xuezhikang and evaluated, wherein, N-(1-(tetrahydro-5-oxyfuran-2)-ethyl) acetazoamide is a new compound.
Owner:BEIJING WBL PEKING UNIV BIOTECH
A kind of acetazolamide sustained-release capsule and preparation method thereof
ActiveCN113855648BIssues that affect release uniformityRelease stabilityOrganic active ingredientsSenses disorderSustained release pelletsSustained Release Capsule
The invention provides an acetazolamide sustained-release capsule and a preparation method thereof. The content of the acetazolamide sustained-release capsule is acetazolamide sustained-release pellets, and waxy materials are used in the sustained-release pellets. Granules – Extrusion spheronization to obtain wet pellets and drying at temperatures close to the melting point of the waxy material to impart sustained release properties to the micropellet particles. The obtained acetazolamide sustained-release capsule is a sustained-release preparation taken once a day, and compared with the immediate-release preparation, it reduces the number of daily doses and side effects, and improves patient compliance.
Owner:北京联嘉医药科技开发有限公司
A kind of co-crystal of acetazolamide and proline and preparation method thereof
InactiveCN108558791BSimple processHigh yieldOrganic active ingredientsSenses disorderSolvent moleculeCrystal system
A co-crystal of acetazolamide and proline and a preparation method thereof, the invention relates to the field of drug co-crystals. The molecular formula of the drug co-crystal is [C 4 h 6 N 4 o 3 S 2 ·C 5 h 9 NO 2 ], the basic structural unit is composed of an acetazolamide molecule and a proline molecule. The drug cocrystal belongs to the triclinic crystal system, and the space group is P‑ 1. Using acetazolamide and proline as raw materials, the drug co-crystal is prepared by cooling method and solvent evaporation method respectively. The drug co-crystal of the invention improves the solubility of acetazolamide and is beneficial to improve the bioavailability of acetazolamide. Since there are no solvent molecules in the eutectic structure, it can remain stable for a long time at room temperature. The preparation method and process route of the drug co-crystal of the invention are simple and easy, and are convenient for large-scale production.
Owner:OCEAN UNIV OF CHINA
Composition of medication for treating high blood pressure
InactiveCN1695738BLower blood pressureOrganic active ingredientsCardiovascular disorderBumetanideHigh pressure
Owner:苏金平
Acetazolamide ophthalmic solution
The present invention provides a stable, ophthalmic aqueous composition for topical administration comprising acetazolamide and an aqueous liquid capable of forming a pharmaceutically acceptable gel in situ when applied topically to a patient, said composition has the pH of less than 4.5.
Owner:SHILPA MEDICARE LTD
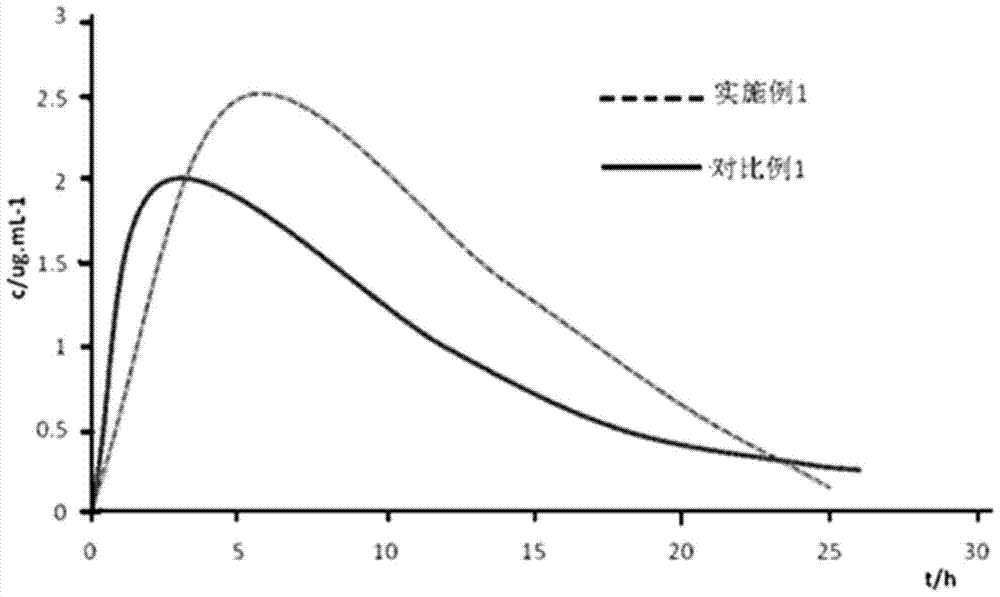
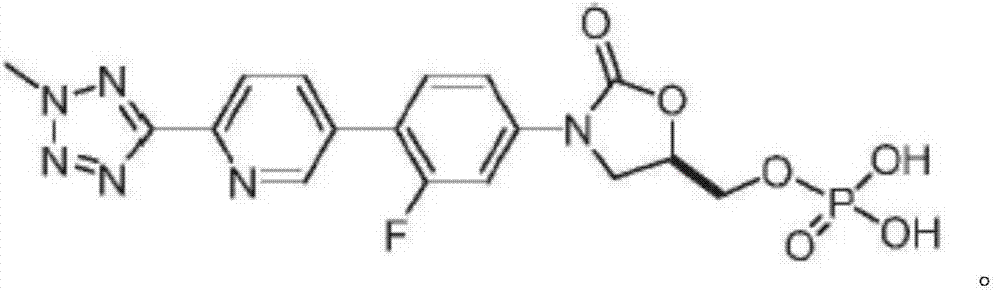
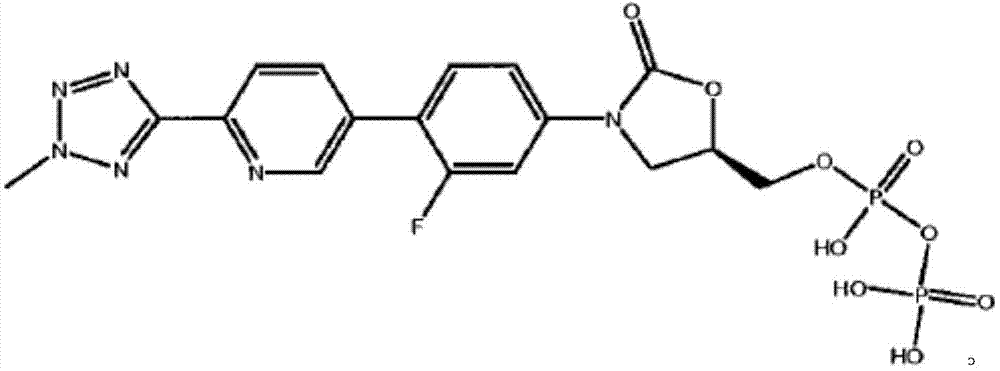
A kind of Tedizolid phosphate compound and preparation method thereof
ActiveCN105085570BReduce typesReduce moisture contentGroup 5/15 element organic compoundsPhosphodiesteraseSide effect
The invention relates to a tedizolid phosphate compound, which contains a trace amount of pyrophosphate, the content of the tedizolid phosphate is ≥98%, and the content of the pyrophosphate is ≤0.2%. The present invention also relates to the preparation method of the tedizolid phosphate compound. The tedizolid phosphate compound provided by the present invention has few types of impurities and low content, especially the dimer content is greatly reduced, the moisture content is small, and the stability is good. Surprisingly, the test found that compared with the prior art, the bioavailability of the compound was significantly improved, and the curative effect and adverse reactions were superior to the prior art, especially the antibacterial activity against MRSA was significantly improved.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Dispersible tablet of acetazolamide and its preparation method
InactiveCN1302778CGood dissolution uniformityQuick effectOrganic active ingredientsSenses disorderCelluloseMicroparticle
Owner:马晶
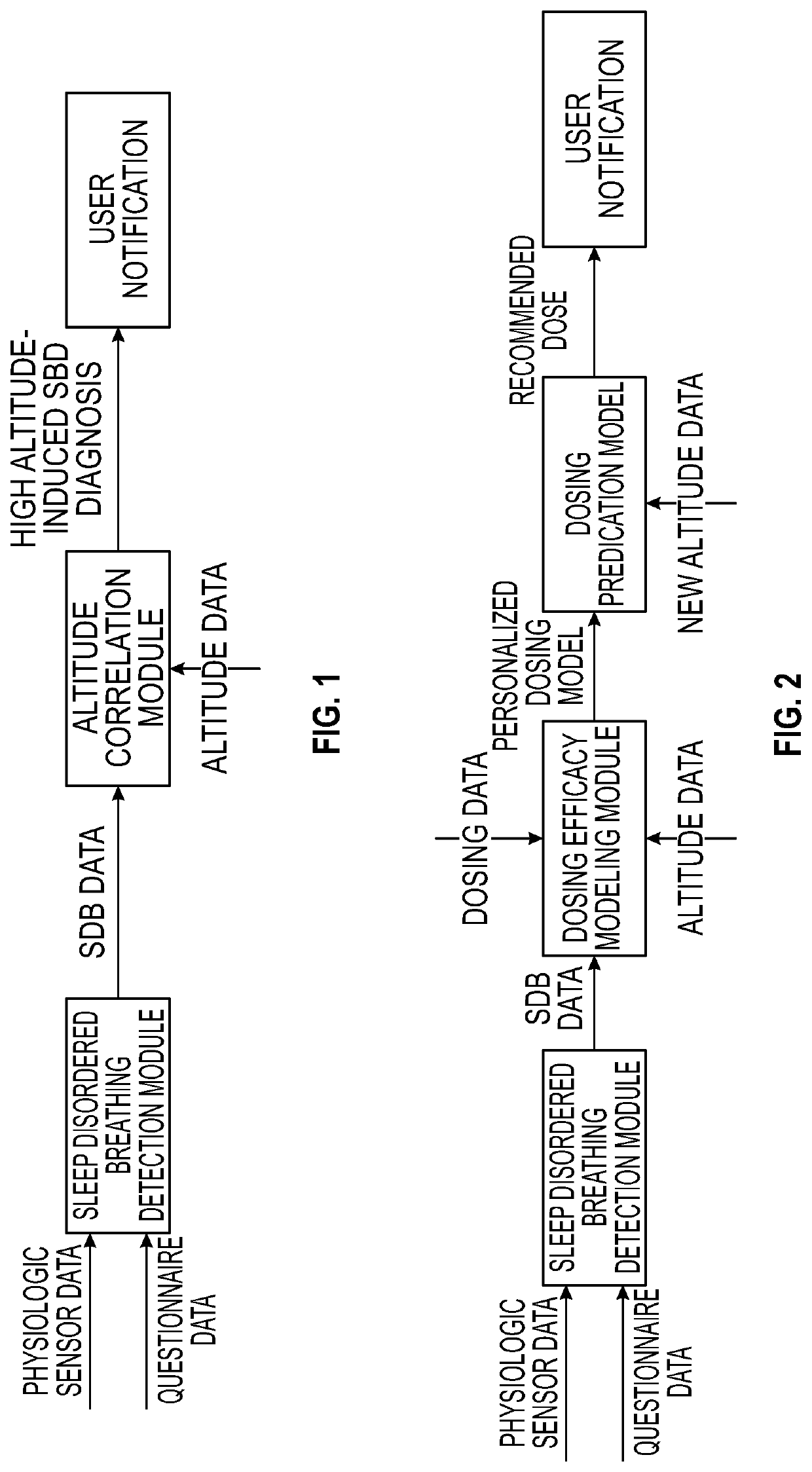
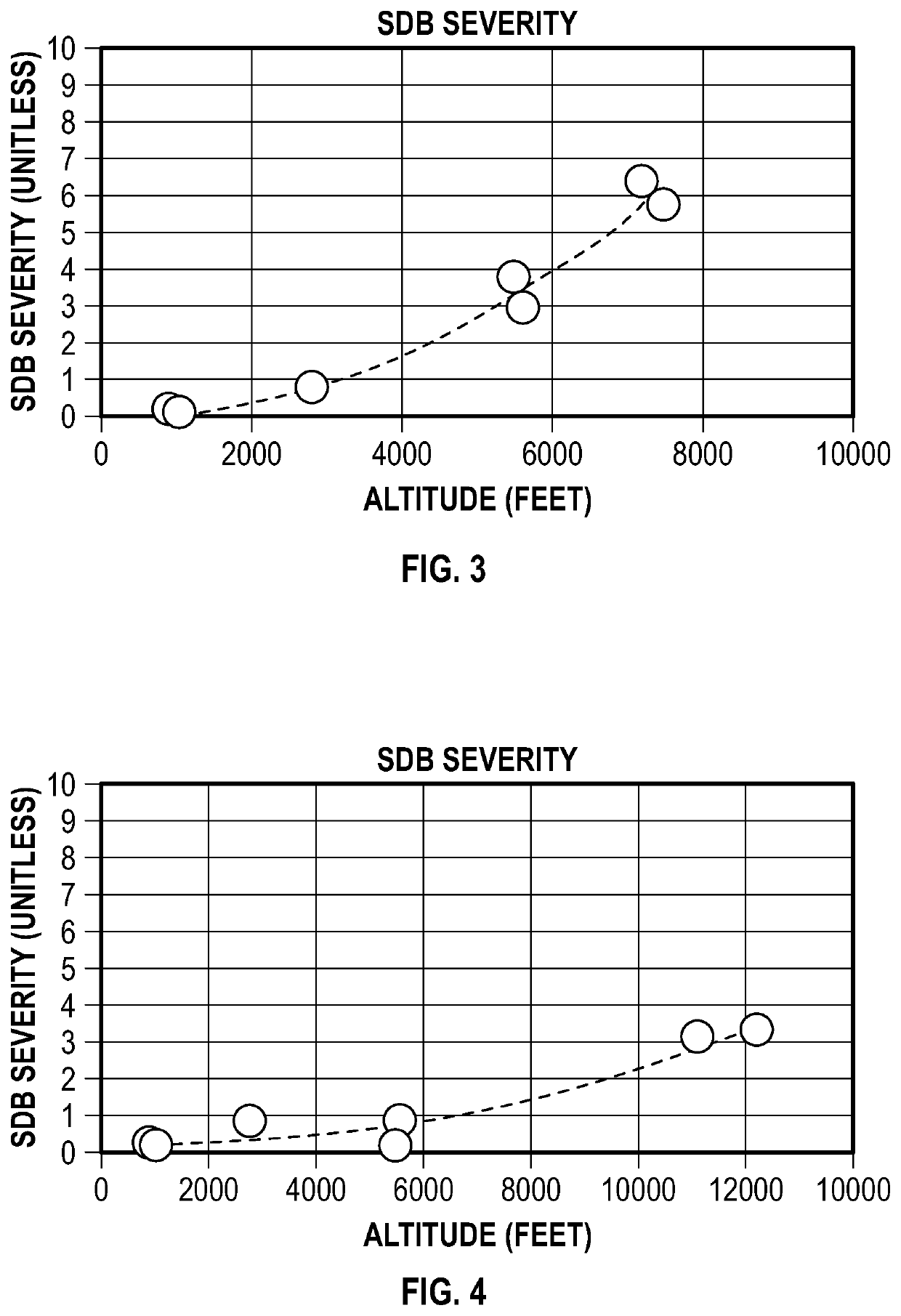
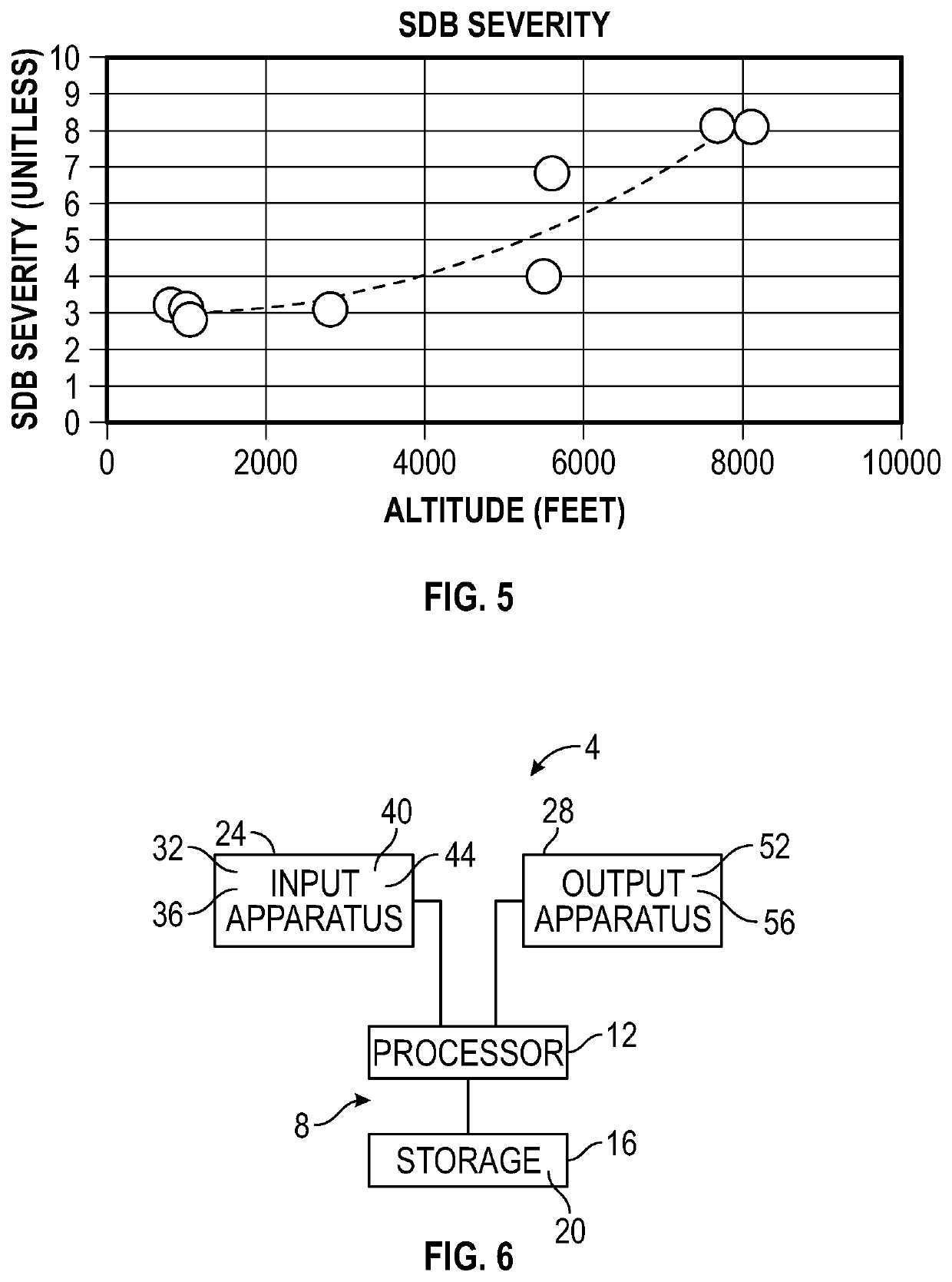
System and method to monitor and titrate treatment for high altitude-induced central sleep apnea (CSA)
PendingUS20220020501A1Easy to detectEasy to predictPhysical therapies and activitiesOrganic active ingredientsPhysical medicine and rehabilitationDrug dosing
An apparatus and method for employing data from the person and from the environment where the person is situated facilitate the detection and prediction of severity of high altitude-induced Sleep Disordered Breathing (SDB, and specifically Central Sleep Apnea (CSA)). Longitudinal tracking of local barometric pressure and severity of SDB symptoms (objective or subjective or both) allow for prediction of occurrence of SDB (per altitude). Some of all of the data can be obtained from one or more wearable devices that may be worn by the person. Additionally, personalized mapping for dosing of medication (e.g. acetazolamide, low-flow oxygen therapy) to treat high altitude-induced SDB are provided, with recommended dosing provided to the person per altitude.
Owner:KONINKLJIJKE PHILIPS NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com