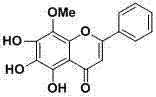
Application of 5,6,7,8- trihydroxy-8-methoxyflavone in preparing anti-hypoxic drug
A technology of methoxyflavonoids and anti-hypoxia drugs, applied in the field of medicine, can solve unseen and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] tablet:
[0059] 5,6,7-trihydroxy-8-methoxyflavone (purity greater than 95%) 50.0g,
[0060] Starch (Shaanxi Aoke Pharmaceutical Excipients Co., Ltd.) 130.0g,
[0061] Microcrystalline cellulose (MCC, Shaanxi Aoke Pharmaceutical Excipients Co., Ltd.) 10.0g,
[0062] Methylcellulose (Anhui Shanhe Pharmaceutical Excipients Co., Ltd.) 6.0g,
[0063] Magnesium stearate (Shaanxi Aoke Pharmaceutical Excipients Co., Ltd.) 4.0g,
[0064] 200.0g total.
[0065] Prepared according to the conventional tablet method, a total of 1000 tablets were made, and each tablet contained 50 mg of 5,6,7-trihydroxy-8-methoxyflavone.
Embodiment 2
[0067] Capsules:
[0068] 5,6,7-trihydroxy-8-methoxyflavone (purity greater than 95%) 50.0g,
[0069] Starch 135.0g,
[0070] Methylcellulose 5.0g,
[0071] Magnesium Stearate 10.0g,
[0072] 200.0g total.
[0073] Prepared according to the conventional method of capsules, 1000 capsules are prepared in total, and each capsule contains 50 mg of 5,6,7-trihydroxy-8-methoxyflavone.
Embodiment 3
[0075] Dispersible tablets:
[0076] 5,6,7-trihydroxy-8-methoxyflavone (purity greater than 95%) 50.0g,
[0077] Microcrystalline cellulose (MCC) 130.0g,
[0078] Croscarmellose sodium (cCMC-Na) 20.0g,
[0079] 200.0g total.
[0080] Prepared according to the conventional method for dispersible tablets, a total of 1000 tablets were made, each containing 50 mg of 5,6,7-trihydroxy-8-methoxyflavone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com