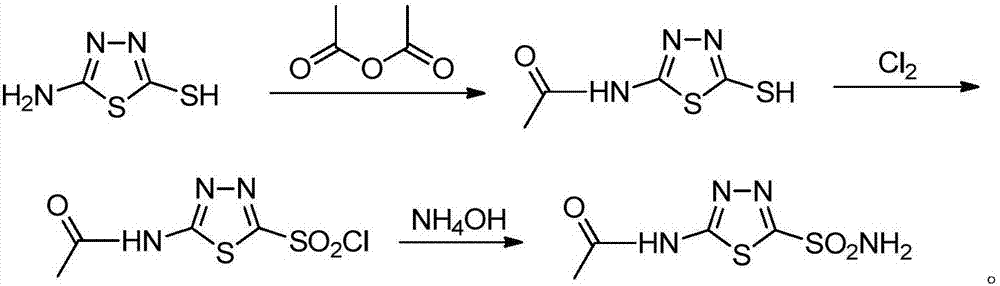
Preparation process for intermediate of acetazolamide
A technology for acetazolamide and intermediates, which is applied in the field of preparing acetazolamide intermediates, can solve the problems of low actual yield, failure to reach the yield value, low product yield, etc., and achieve mature technology and operability Strong, solve the effect of low product qualification rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 1.5L of pyridine, 300mL of piperidine, 100g of thiosemicarbazide and 132.6mL of carbon disulfide in a 3L three-necked flask, stir, heat to a temperature of 80°C in the bottle, and react for 5 hours. After the reaction, distill off the solvent and excess carbon disulfide. Gained solid adds 350mL sodium hydroxide solution (solute mass fraction is 20%) to dissolve, adds 2.9g active carbon decolorization 0.5 hour under 60 ℃, decolouring finishes, filters, and adding 135mL solute mass fraction in the gained filtrate is after the hydrochloric acid solution of 37%. A large amount of solids precipitated, stirred for 0.5 hours and filtered, the filter cake was washed three times with water, and dried under reduced pressure at 60°C for 4 hours to obtain 110 g of 2-amino-5-mercapto-1,3,4-thiadiazole with a purity of 99.7% .
[0020] After testing: the melting point of the product is 231-231.5°C; elemental analysis: theoretical value (C 18.03%, H 2.27%, N31.55%, S 48.15%), meas...
Embodiment 2
[0025] Add 1.5L of pyridine, 300mL of piperidine, 100g of thiosemicarbazide and 149.2mL of carbon disulfide in a 3L three-necked flask, stir, heat to a temperature of 80°C in the bottle, and react for 5 hours. After the reaction, distill off the solvent and excess carbon disulfide. Gained solid adds 350mL sodium hydroxide solution (solute mass fraction is 20%) to dissolve, adds 2.9g active carbon decolorization 0.5 hour under 60 ℃, decolouring finishes, filters, and adding 135mL solute mass fraction in the gained filtrate is after the hydrochloric acid solution of 37%. A large number of solids precipitated, stirred for 0.5 hours, filtered, washed the filter cake three times, and dried under reduced pressure at 60°C for 4 hours to obtain 118.2 g of 2-amino-5-mercapto-1,3,4-thiadiazole with a purity of 99.6 %.
[0026] After testing: the melting point of the product is 230-231°C; elemental analysis: theoretical value (C 18.03%, H 2.27%, N31.55%, S 48.15%), measured value (C 18.0...
Embodiment 3
[0031] Add 1.5L of pyridine, 300mL of piperidine, 100g of thiosemicarbazide and 165.8mL of carbon disulfide in a 3L three-necked flask, stir, heat to a temperature of 80°C in the bottle, and react for 5 hours. After the reaction, distill off the solvent and excess carbon disulfide. Gained solid adds 350mL sodium hydroxide solution (solute mass fraction is 20%) to dissolve, adds 2.9g active carbon decolorization 0.5 hour under 60 ℃, decolouring finishes, filters, and adding 135mL solute mass fraction in the gained filtrate is after the hydrochloric acid solution of 37%. A large amount of solids precipitated, stirred for 0.5 hours and filtered, the filter cake was washed three times with water, and dried under reduced pressure at 60°C for 4 hours to obtain 117.1 g of 2-amino-5-mercapto-1,3,4-thiadiazole with a purity of 99.7 %.
[0032] After testing: the melting point of the product is 231-231.5°C; elemental analysis: theoretical value (C 18.03%, H 2.27%, N31.55%, S 48.15%), me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com