Method for preparing medicinal high-purity calcium dobesilate
A calcium dobesilate, high-purity technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problem of difficult removal of hydroquinone impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add 35.2g (0.320mol) of hydroquinone to a 100ml reaction bottle, slowly add 38ml of concentrated H 2 SO 4 (including H 2 SO 4 0.563mol), controlled the temperature at 80°C for 1hr, then added 300ml of 75% ethanol aqueous solution and stirred to dissolve. Slowly add 70.4g (0.704mol) of powdered CaCO in batches 3 Neutralize excess H 2 SO 4 , and adjust the pH to 2.5-5.0. After suction filtration, the filtrate was extracted twice with 280 mL of a mixed solvent of acetone and ethyl acetate at a ratio of 5:4. The aqueous layer was separated, concentrated under reduced pressure, and the concentrated solution was placed for crystallization. After suction filtration, the filter cake was washed twice with the above aqueous ethanol solution to obtain 48.2 g of crude calcium dobesilate.
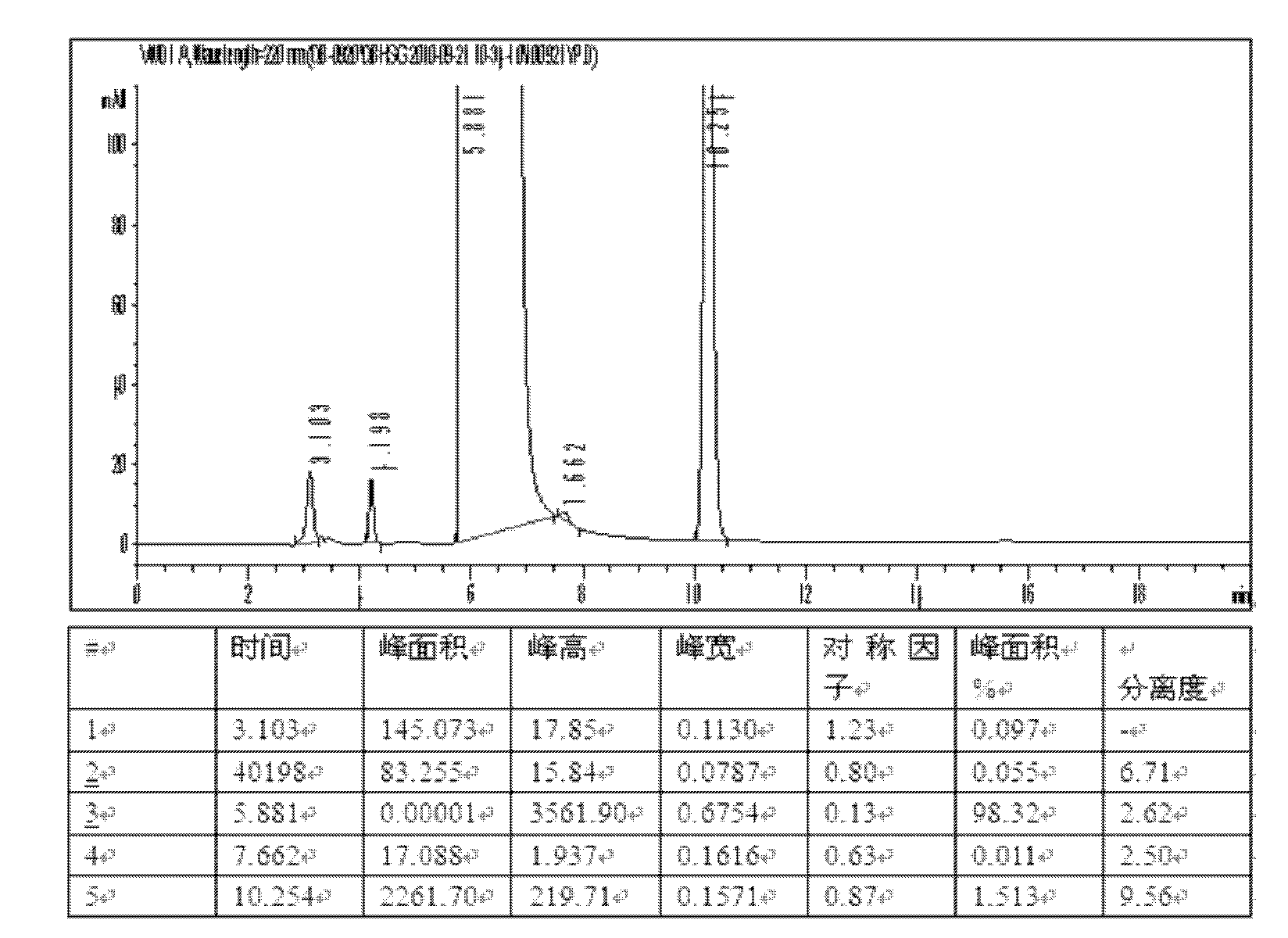
[0045] Dissolve the above crude product in 50 mL of water, add 50 mL of 75% ethanol aqueous solution, stir evenly, concentrate under reduced pressure, and stand for crystallization. Suc...
Embodiment 2
[0047] Add 5.00Kg (45.4mol) of hydroquinone to the 50L reactor, slowly add 4L concentrated H 2 SO 4 (including H 2SO 4 75.1 mol), the temperature was controlled at 80° C. for 1 hr, and then 40 L of 75% ethanol aqueous solution was added and stirred to dissolve. Slowly add 10.0Kg (100mol) powdered CaCO in batches 3 Neutralize excess H 2 SO 4 , and adjust the pH to 2.5-5.0. After suction filtration, the filtrate was extracted twice with 40 L of mixed solvent of acetone and ethyl acetate at a ratio of 1:4. The aqueous layer was separated, concentrated under reduced pressure, and the concentrated solution was placed for crystallization. After suction filtration, the filter cake was washed twice with the above ethanol aqueous solution to obtain 7.63 kg of crude calcium dobesilate.
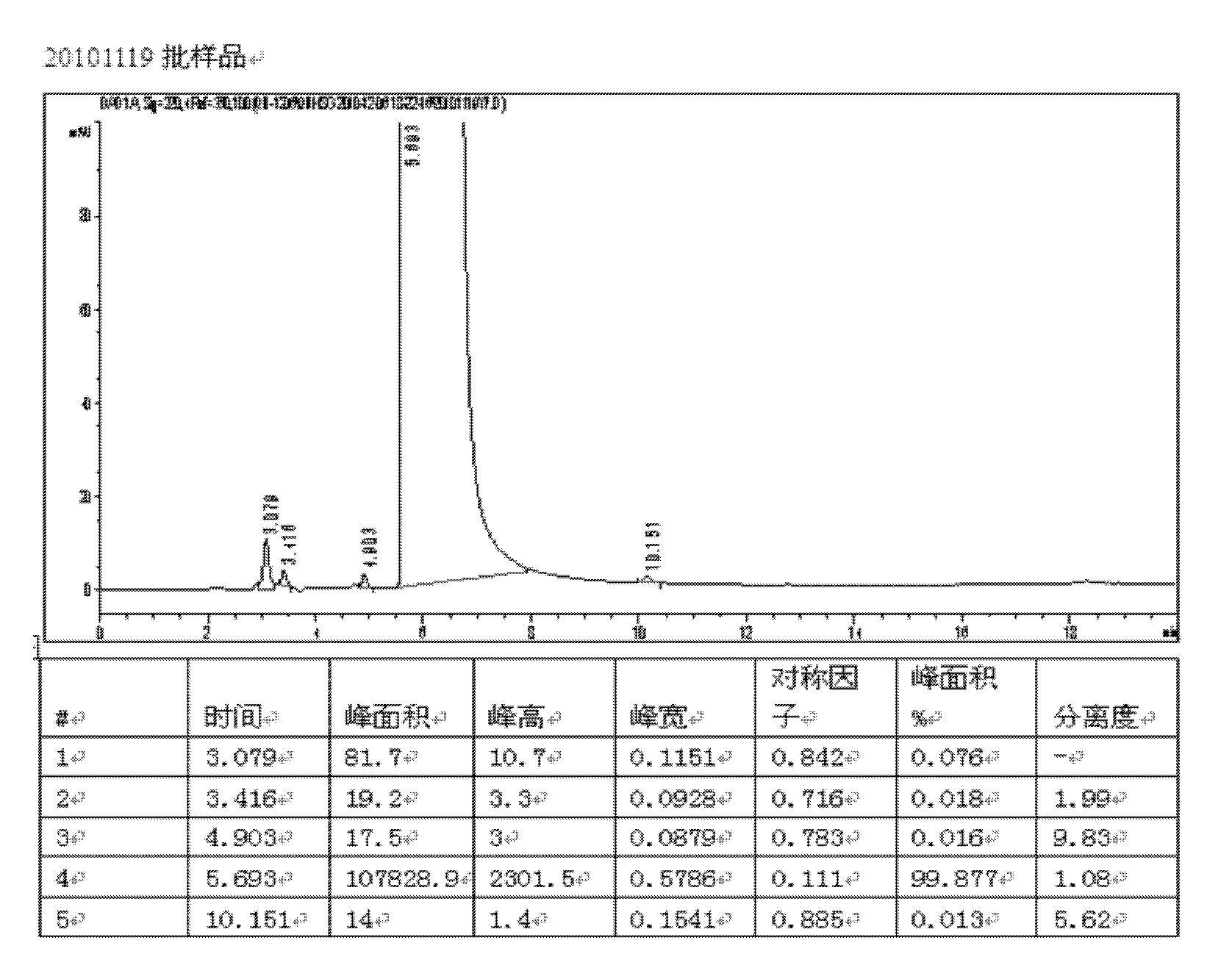
[0048] The above crude product was dissolved in 8 L of water, and 8 L of the above ethanol aqueous solution was added to stir evenly, concentrated under reduced pressure, and left for crystalli...
Embodiment 3
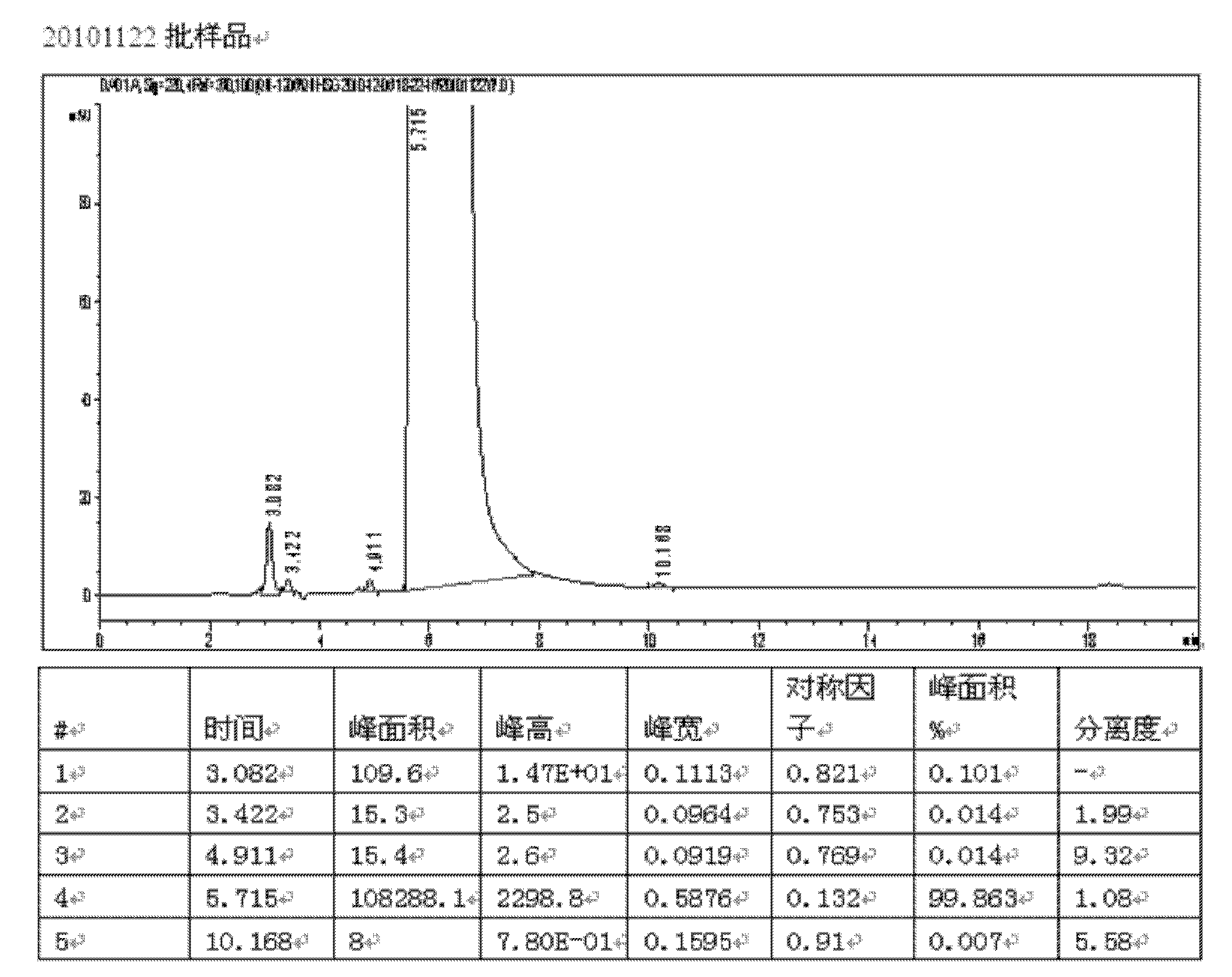
[0050] With embodiment 2 technique, drop into 5.00Kg (45.4mol) Hydroquinone, finally obtain the pure product 6.17Kg of pharmaceutically acceptable calcium dobesilate. The overall yield based on hydroquinone was 62.3%. HPLC purity 99.8%, hydroquinone impurity content 0.007%, total impurity content 0.136%. The test results are attached image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com