Calcium dobesilate dispersible tablet and preparation method thereof
A technology of calcium dobesilate and dispersible tablets, which is applied in the direction of medical preparations of non-active ingredients, anhydride/acid/halide active ingredients, pharmaceutical formulations, etc., and can solve the problem of low bioavailability of calcium dobesilate, hydroxyl Slow disintegration of calcium benzenesulfonate, slow drug dissolution rate and other problems, to achieve the effect of improving bioavailability, reducing the difficulty of taking medicine, and rapid drug dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
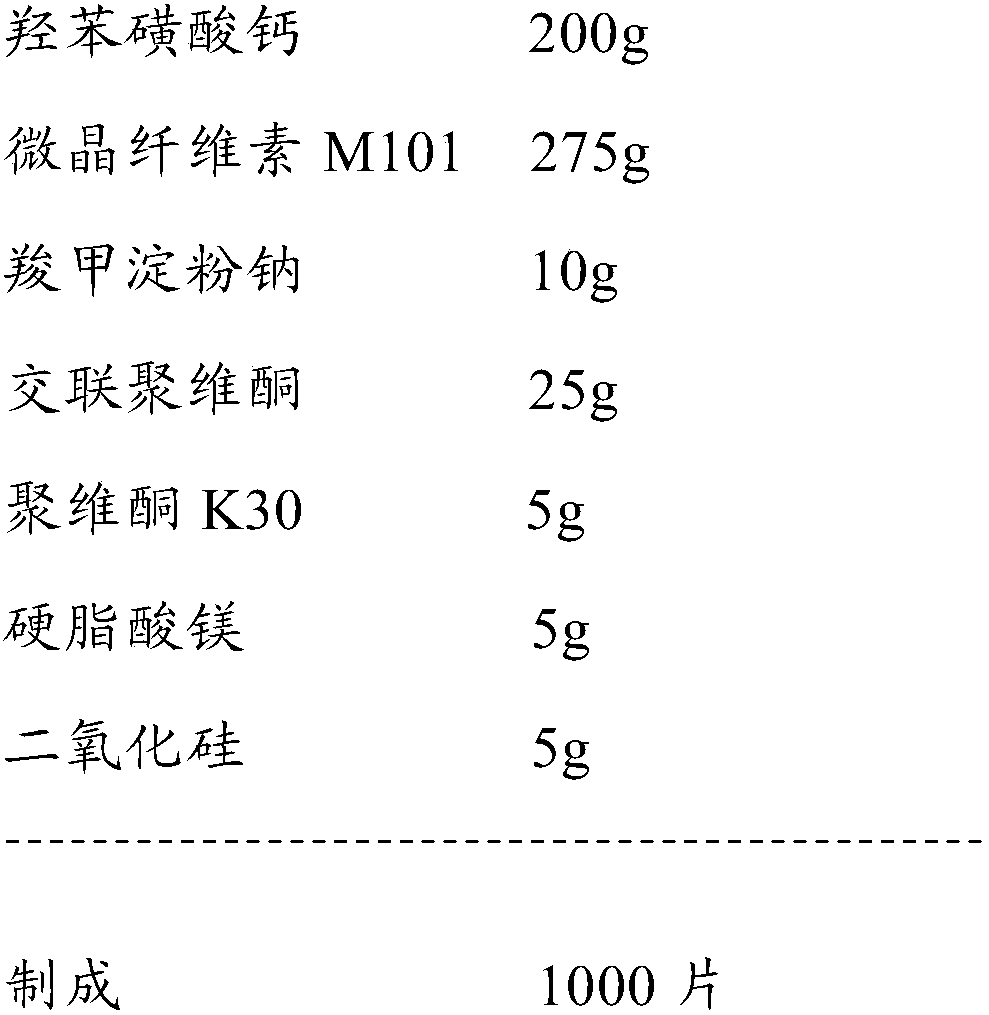
[0066] Raw material prescription:
[0067]
[0068] Preparation Process:
[0069] Step A1: Grinding the calcium dobesilate with an ultrafine pulverizer, and passing the pulverized calcium dobesilate through a 200-mesh sieve;
[0070] Step B1: Weigh Povidone K30 according to the prescription amount, add the weighed Povidone K30 into 80% ethanol until the total weight of the solution reaches 60kg, and make an adhesive;
[0071] Step C1: take by weighing the calcium dobesilate sieved in step A1 and the microcrystalline cellulose M101 of the prescription amount according to the prescription amount, and weigh 8kg sodium carboxymethyl starch and 6kg croscarone;
[0072] Step D1: Mix the components weighed in step C1 in a wet granulator for 5 minutes, add the binder made in step B1 during the mixing process, and then use the wet granulator to start granulation, control the humidity during the granulation process The mixing paddle rotating speed of French granulator is 15r / s, and...
Embodiment 2
[0085] Raw material prescription:
[0086] Identical with the raw material prescription that embodiment 1 provides;
[0087] Preparation Process:
[0088] The preparation technology that provides with embodiment 1 is substantially the same, and difference is:
[0089] Mix 12.8g carboxymethyl starch sodium and 10.8g crospovidone with the prescription amount of calcium dobesilate, the prescription amount of microcrystalline cellulose M101 and binder to prepare the intermediate granules, and then 6g carboxymethyl starch Sodium starch and 3 g of crospovidone are mixed with the dried intermediate granules and compressed into tablets.
[0090] Dispersion uniformity detection test and dissolution contrast test adopt the method that embodiment 1 provides to carry out, and test result shows that the dispersibility and dissolution of the calcium dobesilate dispersible tablet that embodiment 2 makes meet standard regulation.
Embodiment 3
[0092] Raw material prescription:
[0093] Identical with the raw material prescription that embodiment 1 provides;
[0094] Preparation Process:
[0095] The preparation technology that provides with embodiment 1 is substantially the same, and difference is:
[0096] 10g carboxymethyl starch sodium and 8g crospovidone are mixed with the calcium dobesilate of prescription quantity, the microcrystalline cellulose M101 of prescription quantity and binding agent, to prepare intermediate granular thing, then 8.8g carboxymethyl starch Sodium and 5.8 g of crospovidone were mixed with the dried intermediate granules and compressed into tablets.
[0097] The dispersion uniformity detection test and the dissolution rate comparative test are carried out by the method provided in Example 1, and the test results show that the dispersibility and dissolution rate of the calcium dobesilate dispersible tablets made in Example 3 meet the standard requirements.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com