Faropenem sodium granules
A technology of faropenem sodium and faropenem, which is applied in the field of pharmaceutical preparations, can solve the problems that affect the clinical use of faropenem, such as drug adaptability, poor preparation stability, and increased substances, and achieve good water solubility, good stability, and long-lasting effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
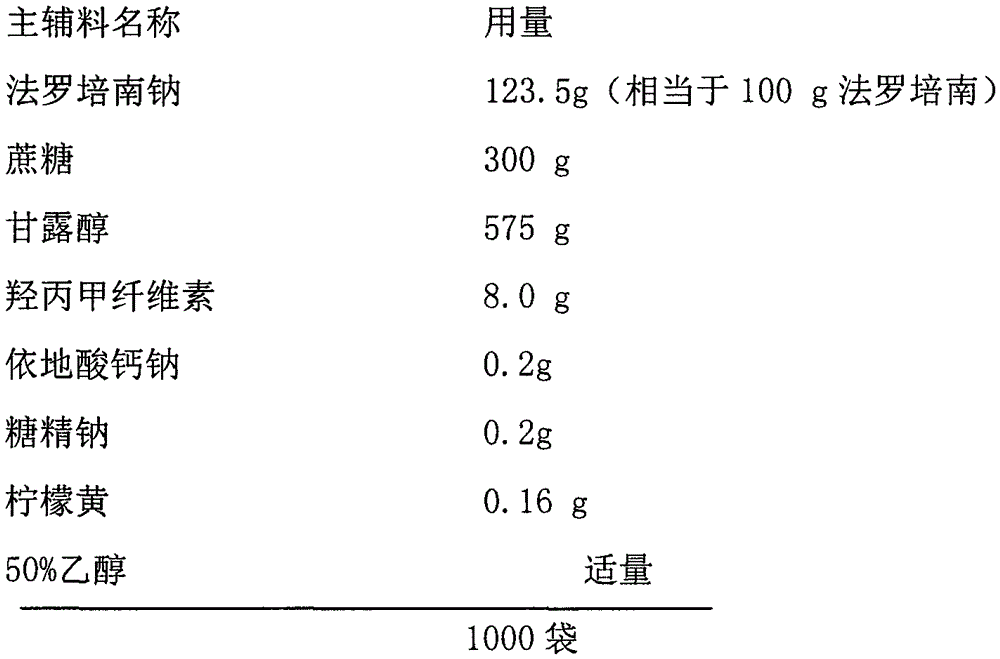
[0021] Prescription (specification: 0.1g):
[0022]
[0023] Preparation Process:
[0024] (1) The faropenem sodium is pulverized and sieved, and the sucrose, mannitol, saccharin sodium and edetate calcium sodium are all sieved;
[0025] (2), step (1) component is mixed homogeneously;
[0026] (3), hypromellose and tartrazine are dissolved in 50% ethanol to form a solution;
[0027] (4), adding the material obtained in step (2) to the solution prepared in step (3), mixing evenly and making wet granules;
[0028] (5) The wet granules prepared in step (4) are dried under reduced pressure at 35° C. to 40° C., sized, and packed.
Embodiment 2
[0030] Prescription (specification: 0.1g):
[0031]
[0032] Preparation Process:
[0033] (1) Crush and sieve faropenem sodium, sieve sucrose, mannitol, sodium saccharin, edetate calcium sodium, and orange essence;
[0034] (2), step (1) component is mixed homogeneously;
[0035] (3), hypromellose and sunset yellow are dissolved in 50% ethanol to form a solution;
[0036] (4), adding the material obtained in step (2) to the solution prepared in step (3), mixing evenly and making wet granules;
[0037] (5) The wet granules prepared in step (4) are dried under reduced pressure at 35° C. to 40° C., sized, and packed.
Embodiment 3
[0039] Prescription (specification: 0.1g):
[0040]
[0041] Preparation Process:
[0042] (1) The faropenem sodium is pulverized and sieved, and the sucrose, mannitol, aspartame and edetate calcium sodium are all sieved;
[0043] (2), step (1) component is mixed homogeneously;
[0044] (3), hypromellose and tartrazine are dissolved in 50% ethanol to form a solution;
[0045] (4), adding the material obtained in step (2) to the solution prepared in step (3), mixing evenly and making wet granules;
[0046] (5) The wet granules prepared in step (4) are dried under reduced pressure at 35° C. to 40° C., sized, and packed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com