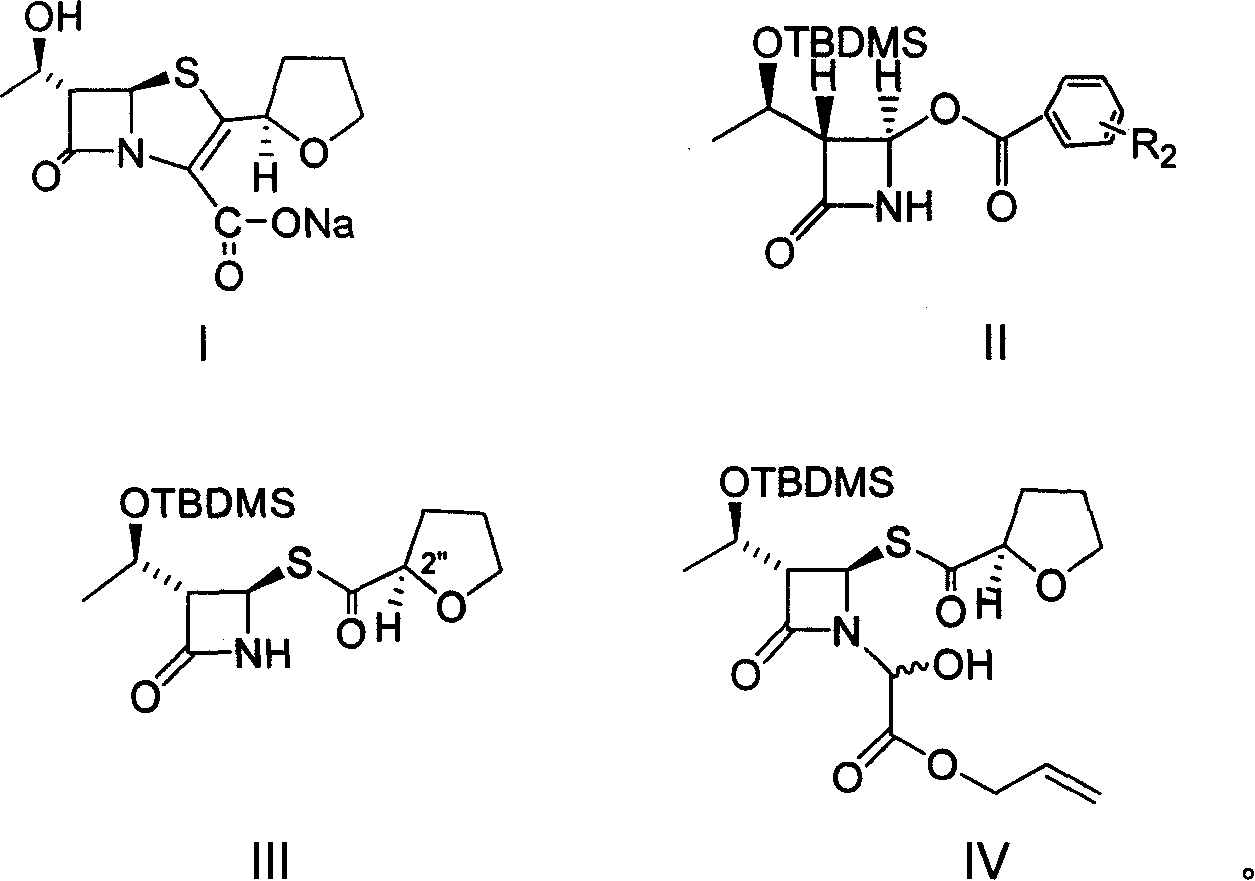
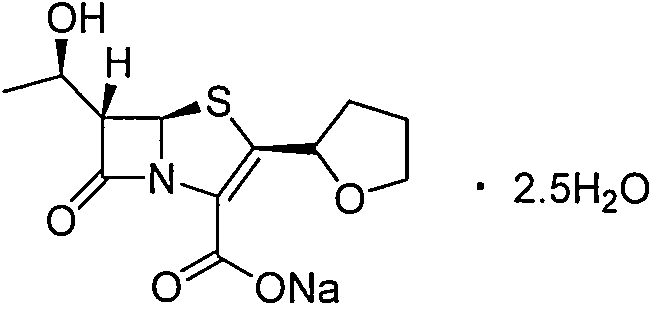
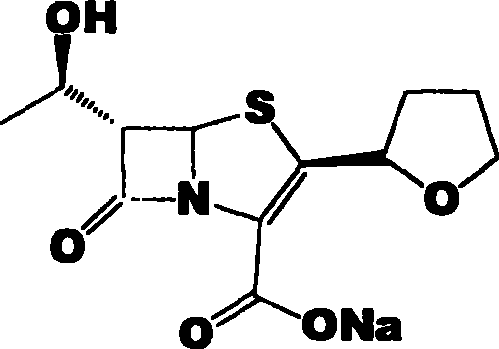
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Faropenem" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
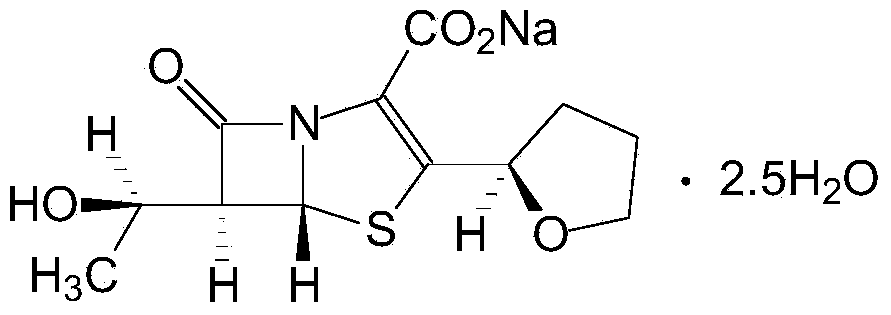
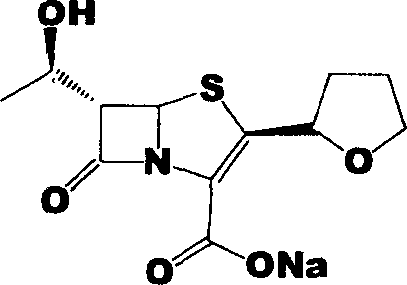
Faropenem is an orally active beta-lactam antibiotic belonging to the penem group. It is resistant to some forms of extended-spectrum beta-lactamase. It is available for oral use.
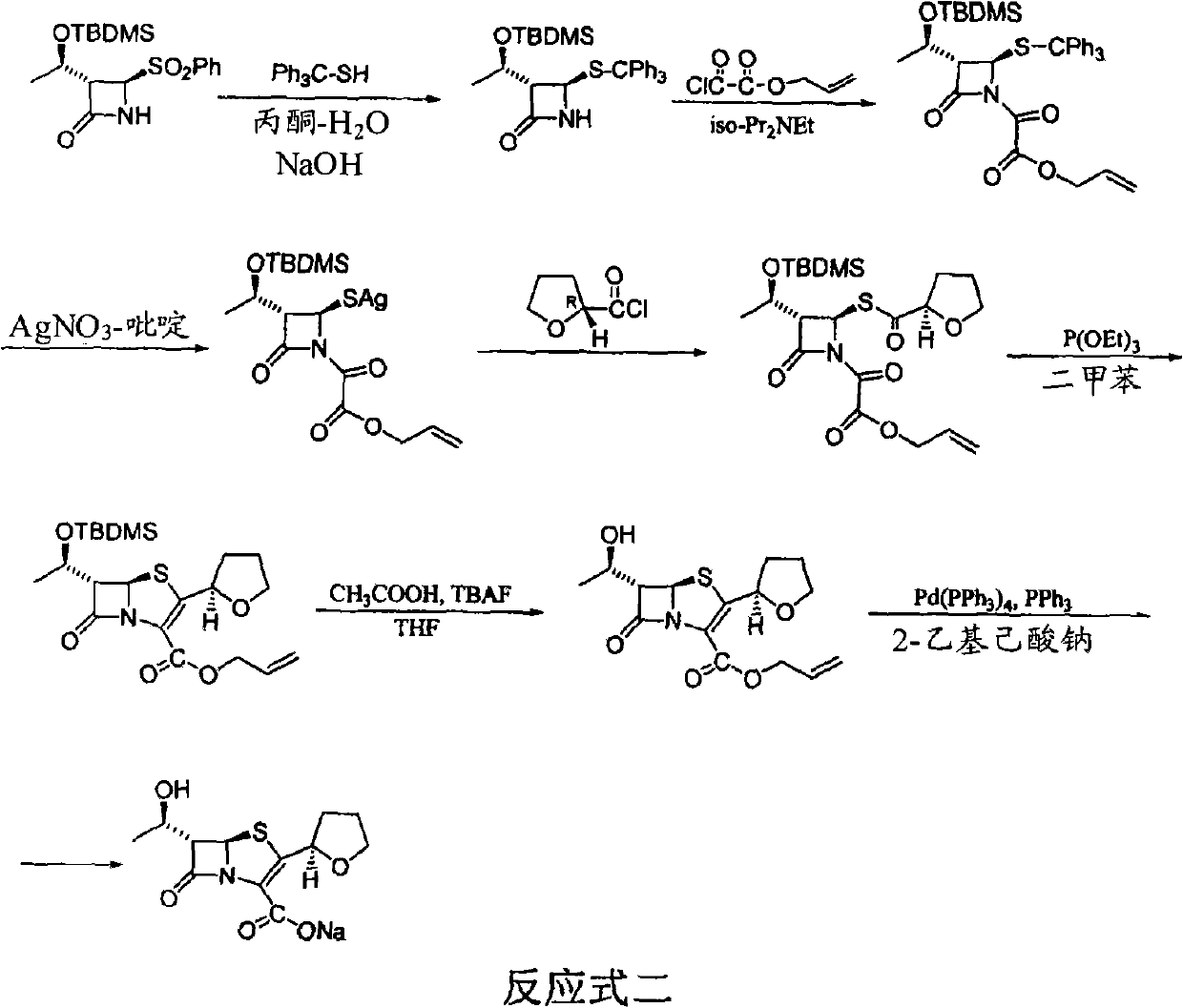
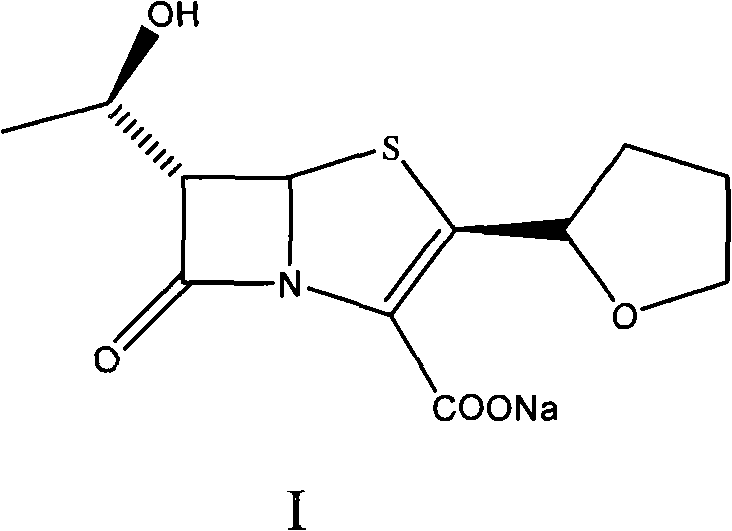
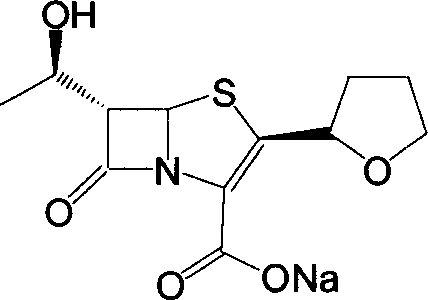
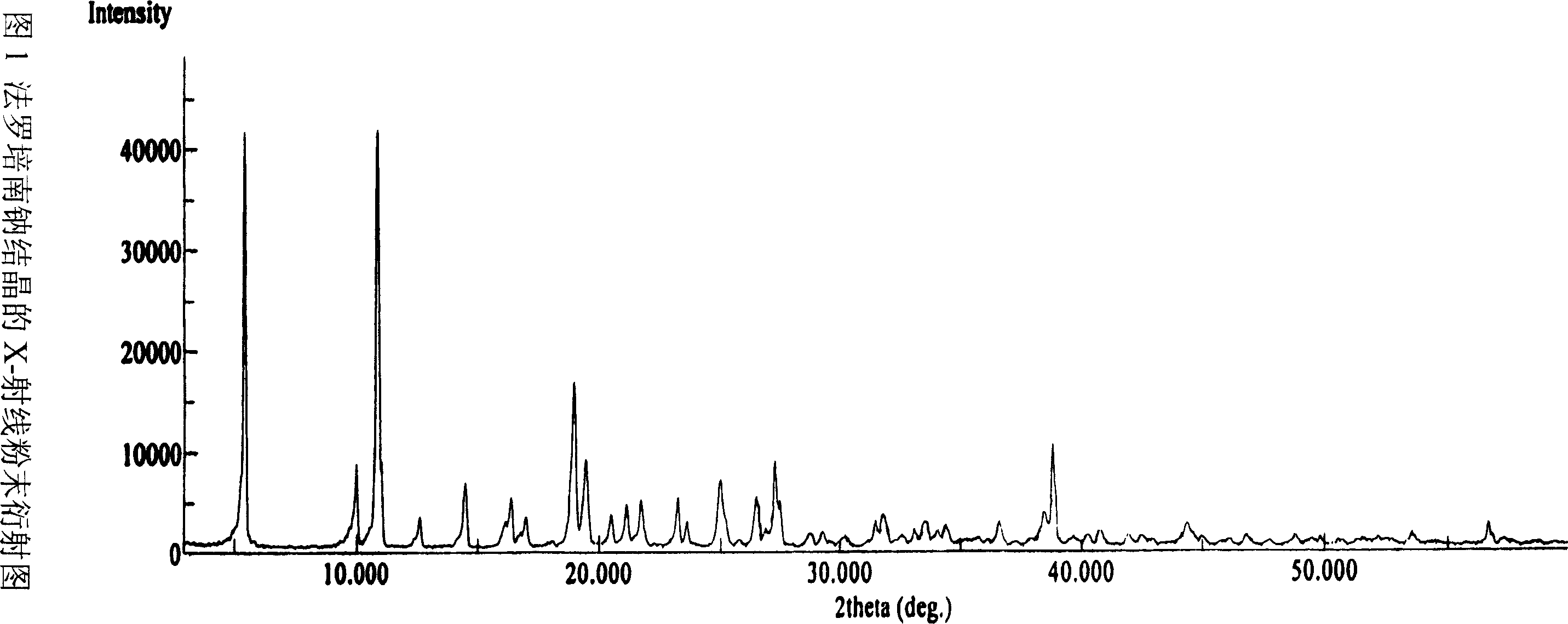
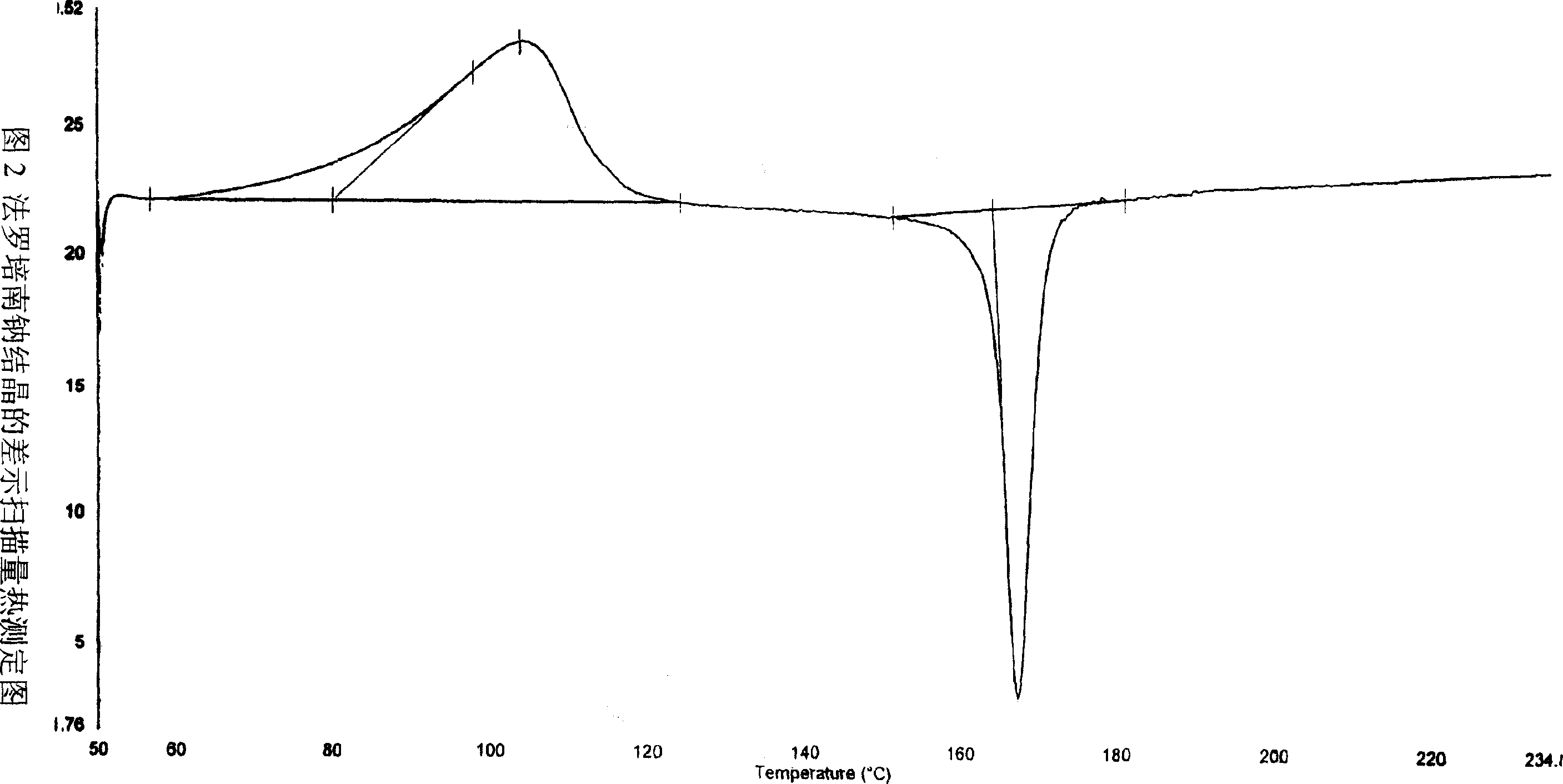
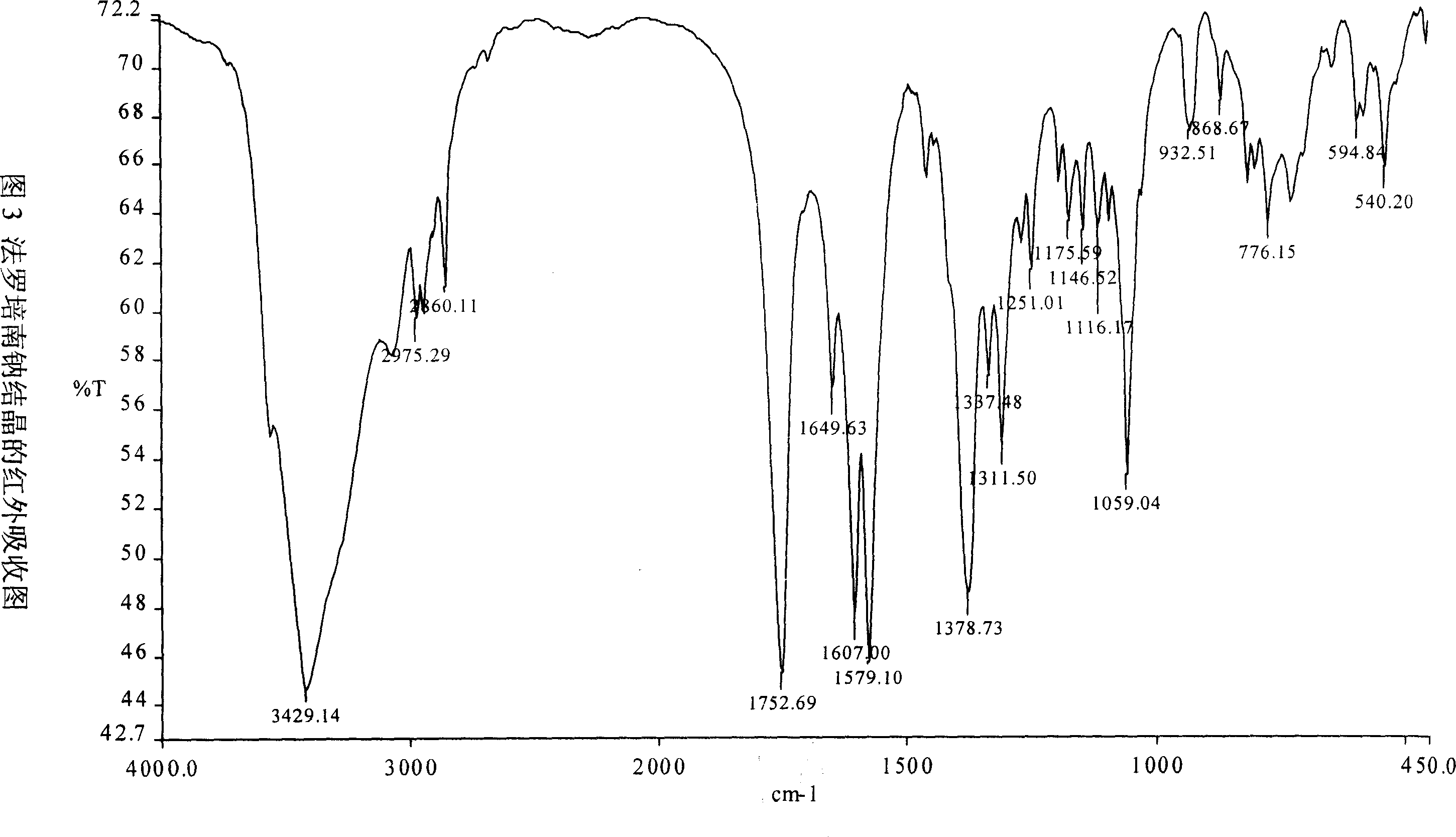
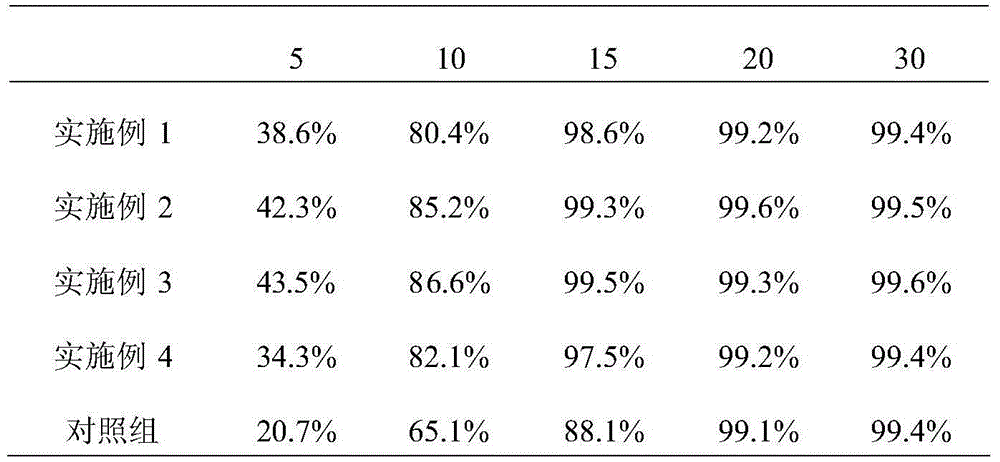
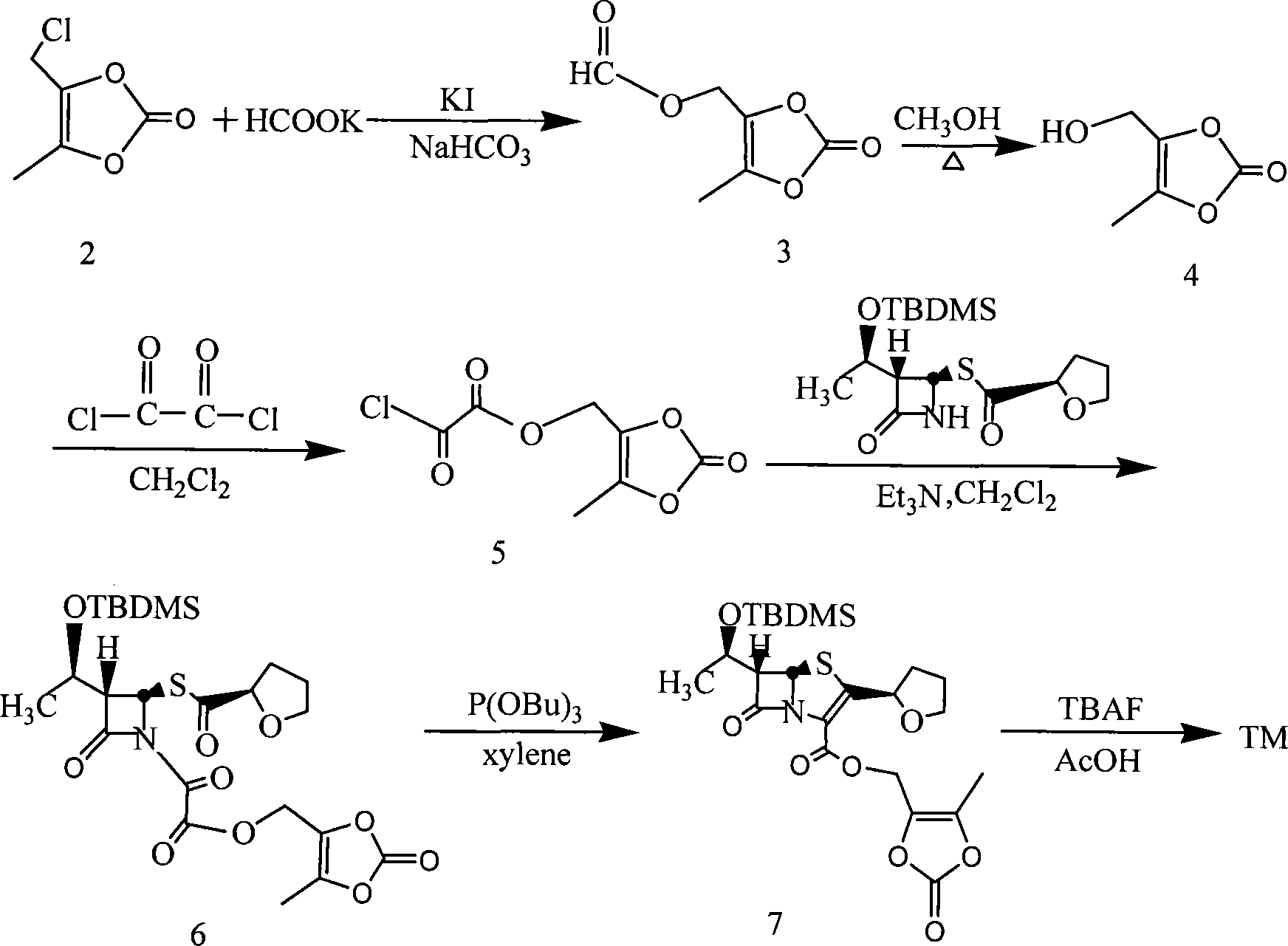
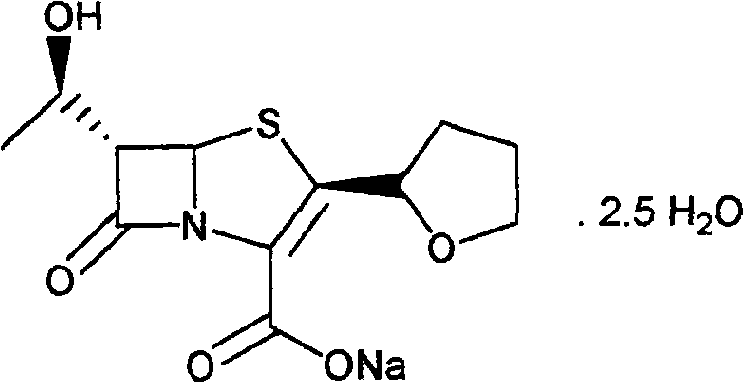
Process for the preparation of sodium faropenem
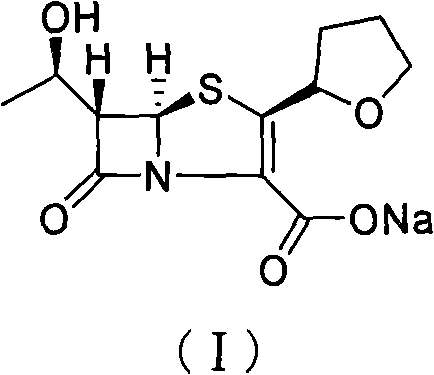
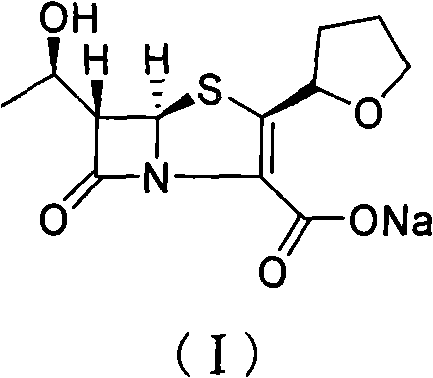
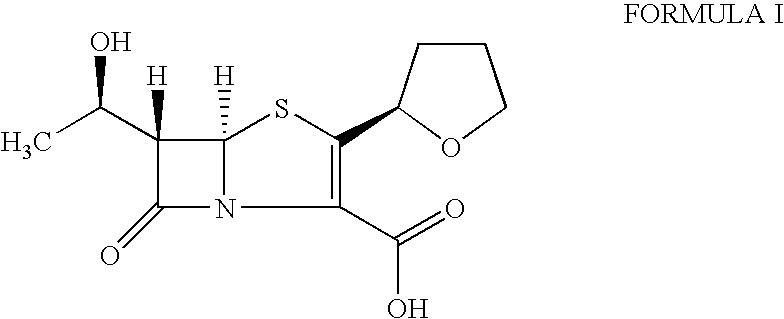
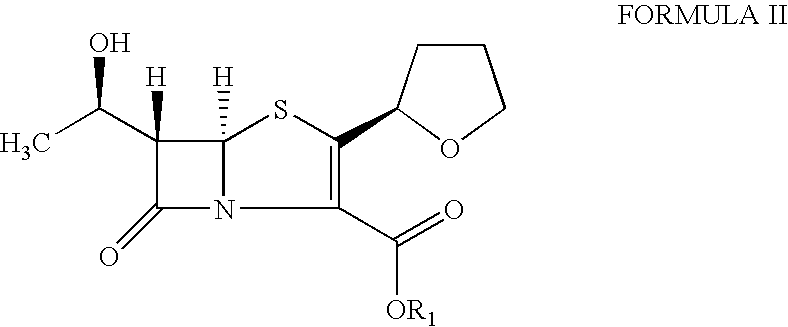
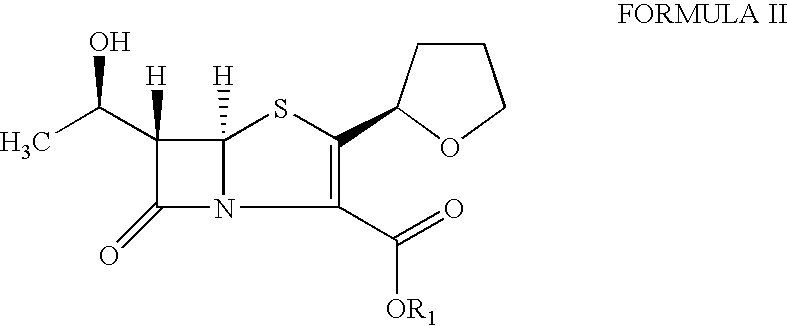
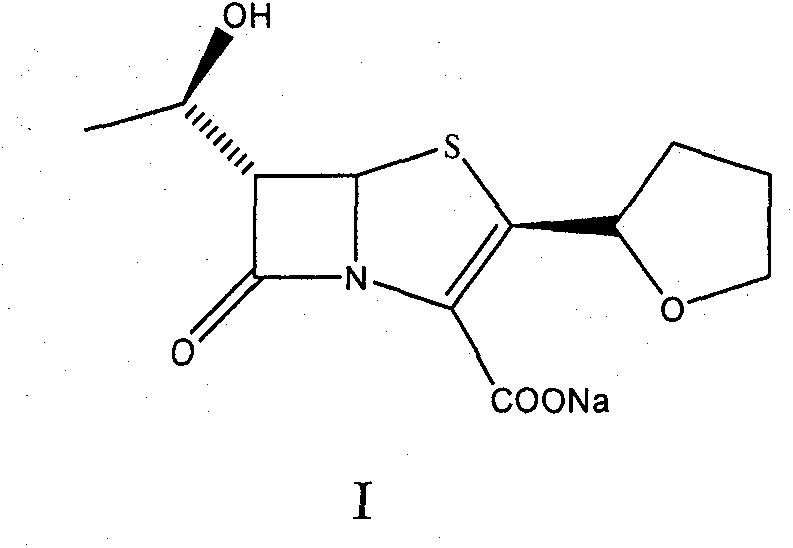
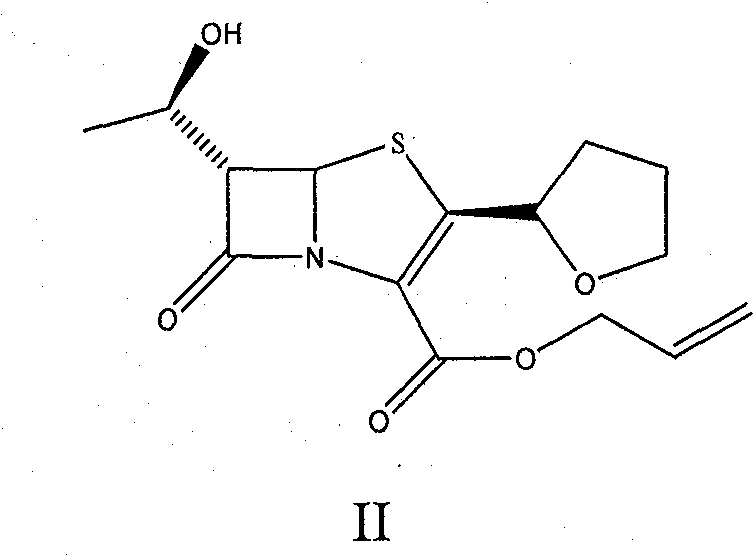
The invention provides a method for preparing penicillenic antibiotics faropenem sodium Iwhich takes natural L-threonine as raw material, and method for preparing important intermediate II. The invention is characterized by high productivity, low cost, simple operation, easy post treatment and suitability for industrialization.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD +1
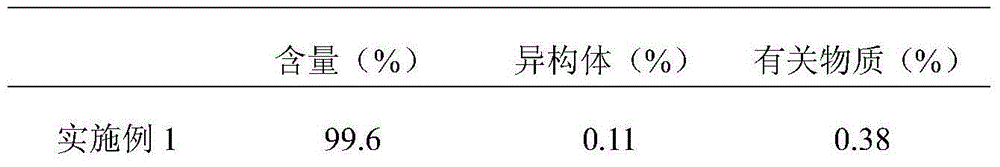
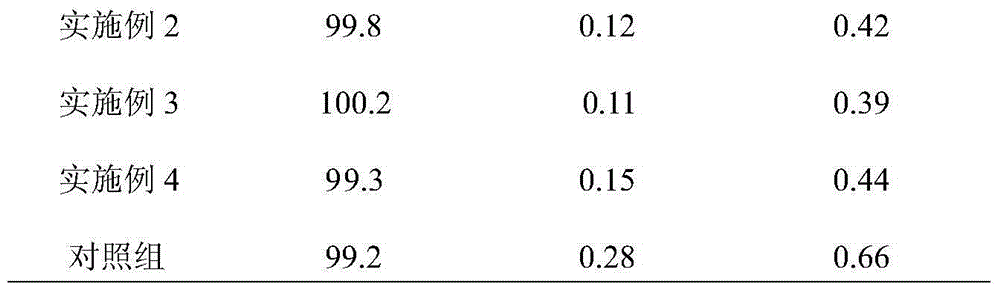
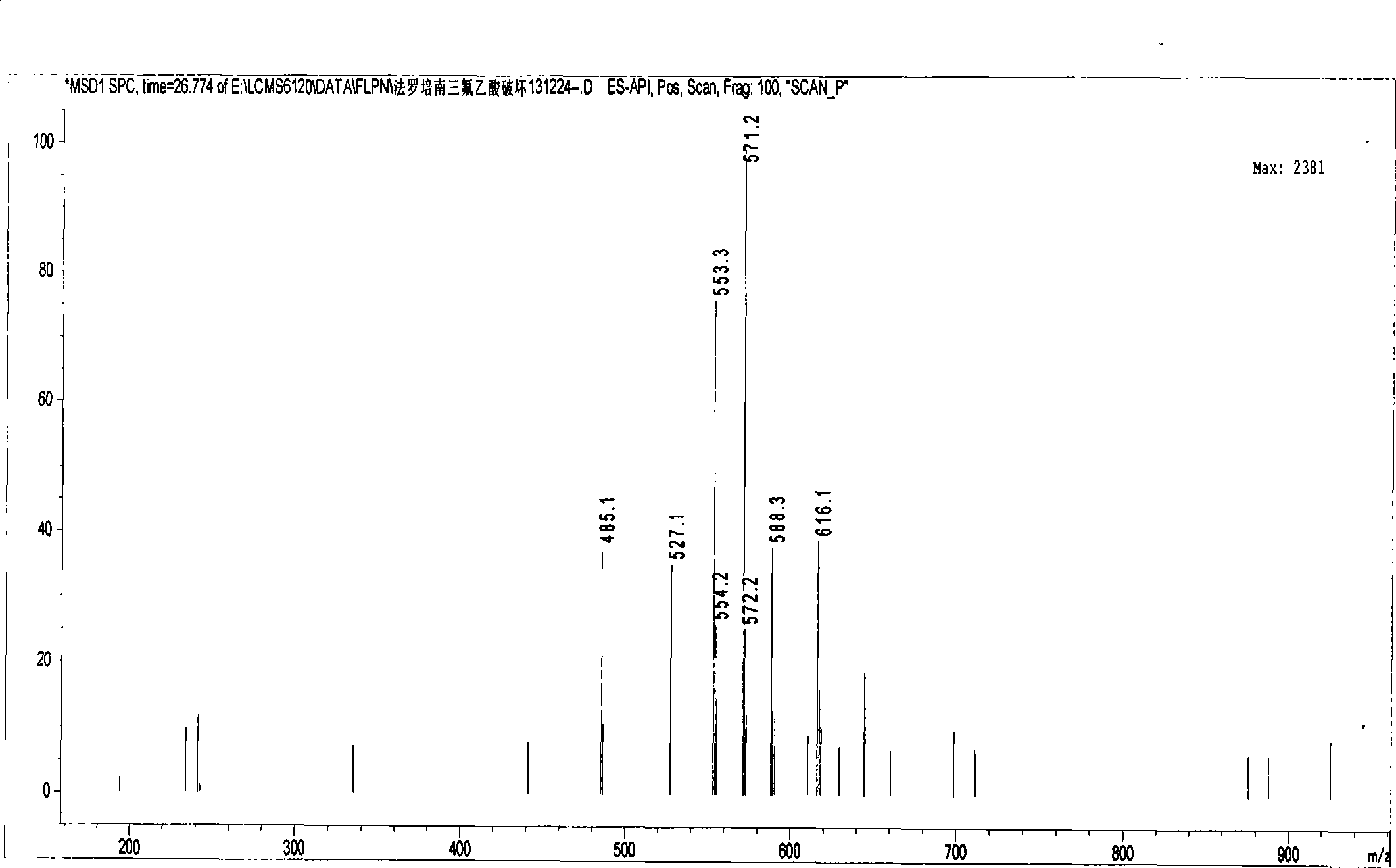
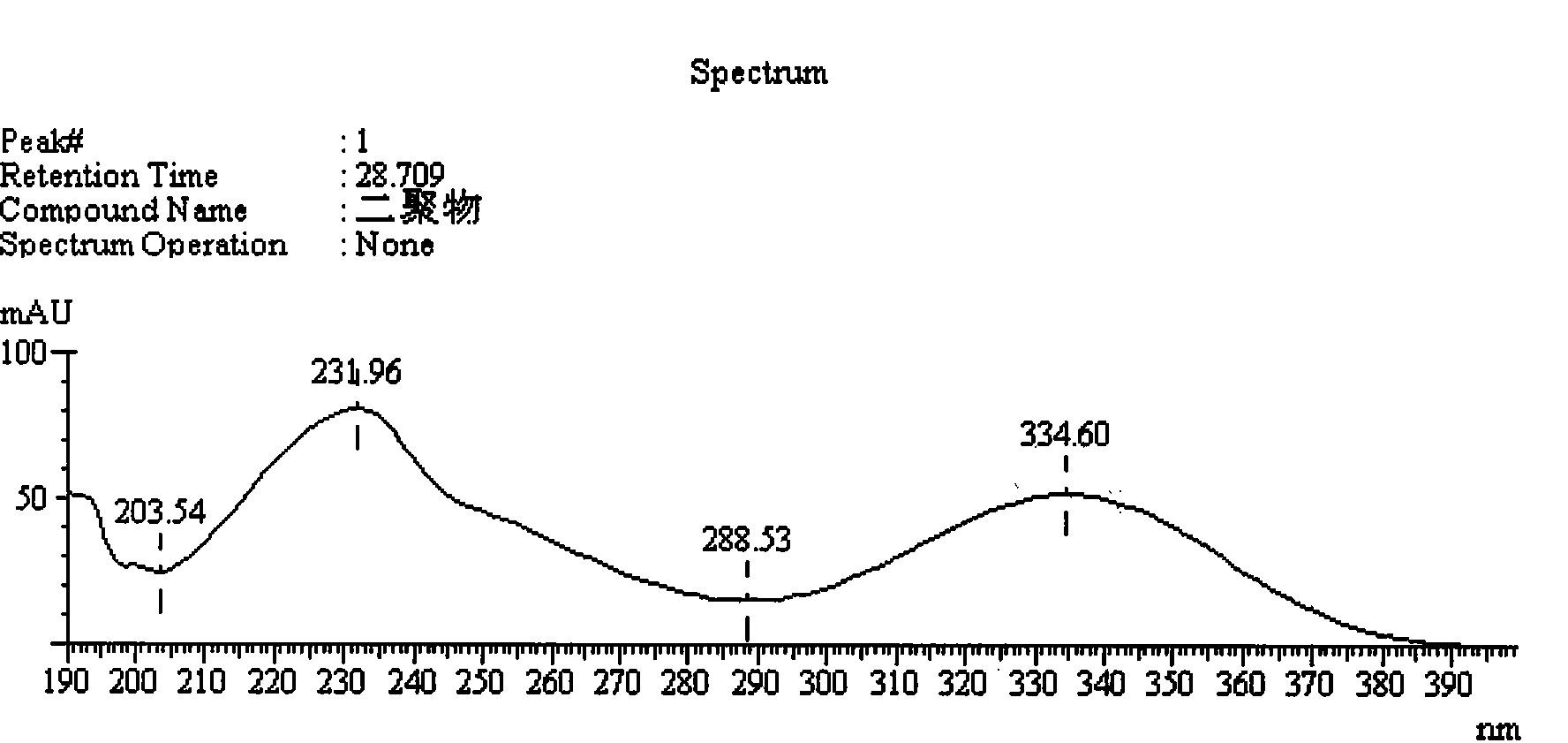
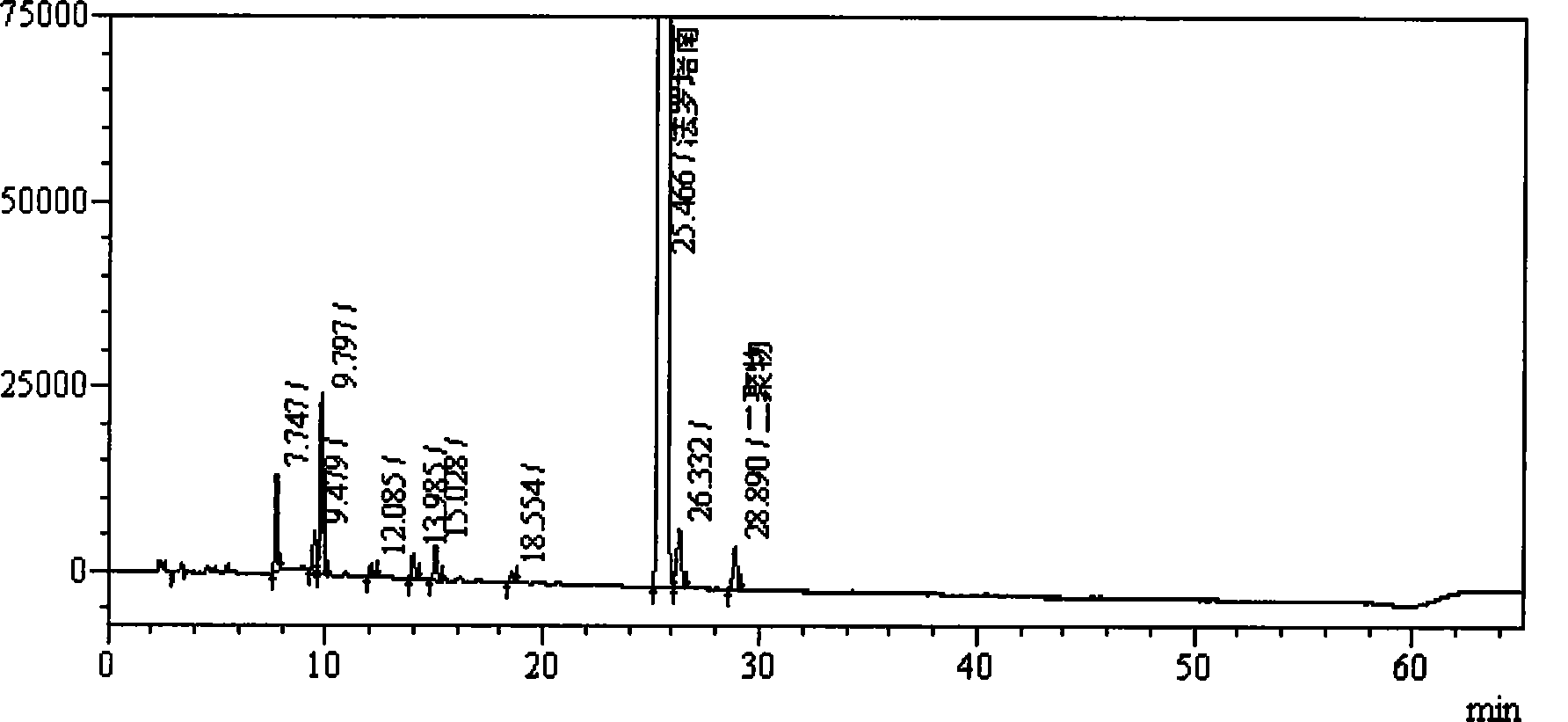
Method for measuring impurity content of faropenem polymers in faropenem sodium raw materials and preparations
ActiveCN101852782ASignificantly progressiveVolume controlOrganic chemistryComponent separationColumn temperatureImpurity
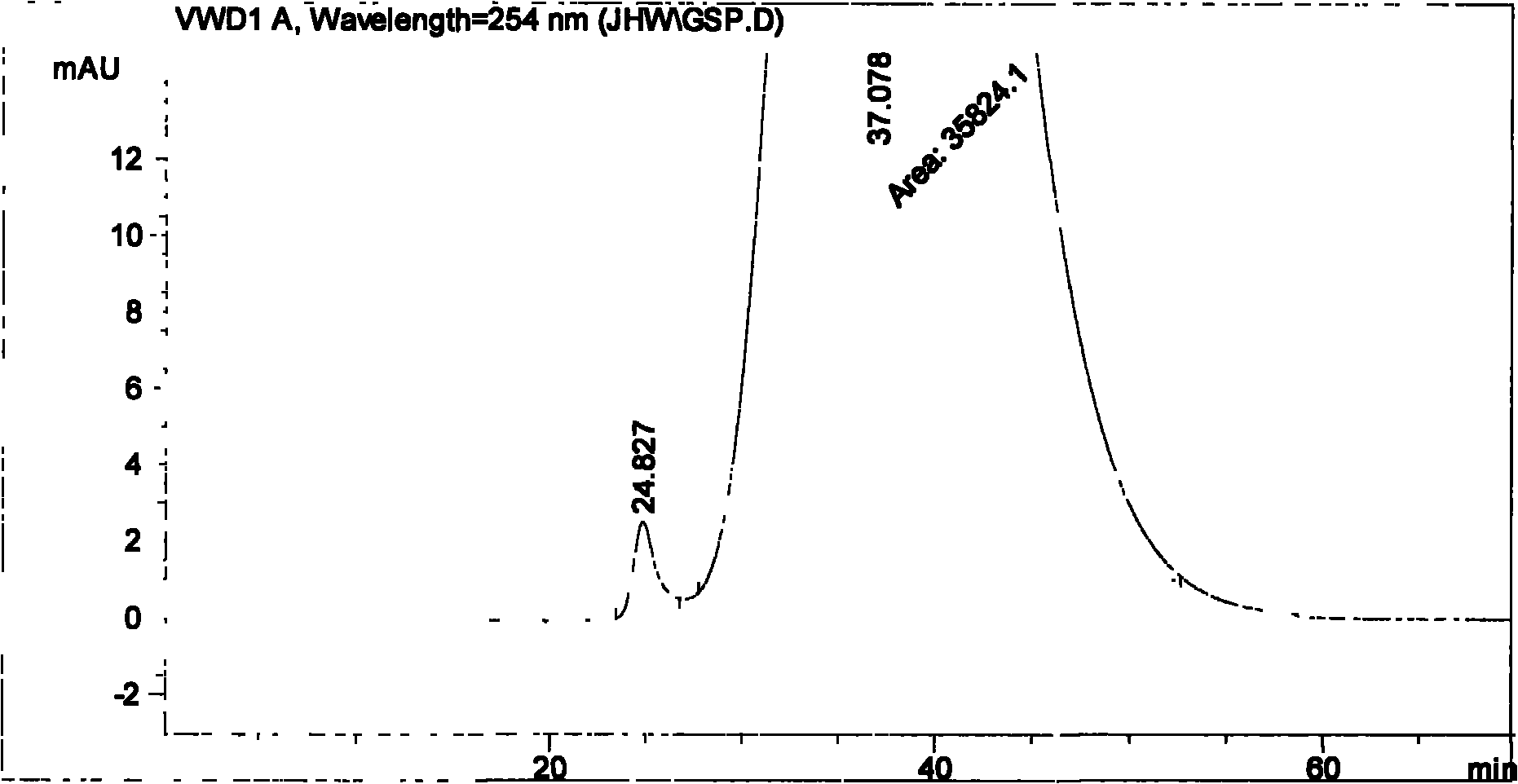
The invention particularly relates to a method for measuring the content of faropenem polymers in faropenem sodium raw materials and preparations by high-efficiency liquid chromatography, which belongs to the field of medicine analysis. The measuring method of the invention is gel chromatography. The high-efficiency liquid chromatogram is used for detection, and the chromatogram conditions are as follows: (1) the gel permeation chromatography has the exclusion molecular weight between 600 and 800 Daltons; (2) the mobile phases as two mobile phases, wherein the mobile phase A is a water phase solution with the pH value between 5.0 and 8.5 and the concentration between 0.01 and 0.2 mol / L, and the mobile phase B is water or a sodium dodecyl sulfate water solution or a glycin solution between 0.005 and 0.02 percent; (3) the column temperature is the room temperature; (4) the flowing rate is between 0.5 and 1.0 ml / min; and (5) the detection wavelength is between 210 and 300 nm. The method of the invention has the advantages of strong specificity, good repetitiveness and high automation degree, and the polymers in the faropenem sodium raw materials and the preparations can be accurately qualified.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for removing residual palladium of faropenem sodium
InactiveCN101560215ASimple and fast operationRaw materials are cheap and easy to getOrganic chemistryActivated carbonAlcohol
The invention belongs to the field of medicinal chemistry, and in particular relates to a method for removing residual palladium with high content of a faropenem sodium crude product. The method comprises the following steps: stirring and adsorbing an alcoholic solution or an aqueous solution of the faropenem sodium crude product with the palladium content of 200 to 2,000 ppm and active carbon for 5 minutes to 48 hours at a temperature of between 0 and 45 DEG C; decompressing a filtered filtrate to reclaim the alcoholic solvent; dissolving remnant with deionized water or directly adding an organic solvent dropwise to the filtrate obtaining by filtering the aqueous solution of the faropenem sodium crude product; and precipitating a faropenem sodium hydrate with the palladium residual quantity of below 10 ppm. The method has simple and convenient operation, low cost and good palladium removing effect, and is suitable to be used in the industry.
Owner:FUDAN UNIV
Faropenem sodium-containing granules and preparation method thereof
InactiveCN104208028ASignificant progressGreat tastePharmaceutical non-active ingredientsGranular deliveryLactoseElderly patient
The invention relates to faropenem sodium-containing granules, belonging to the technical field of pharmaceutic preparation. The faropenem sodium-containing granules comprise 10-20% of faropenem sodium, 78-89.5% of lactose and 0.5-2.0% of steviosin. The faropenem sodium-containing granules adopt dry granulation, and the technology is simple, feasible and beneficial to operation, so that the faropenem sodium-containing granules are suitable for industrial mass production; the product is stable in properties and good in mouth feel, thus being very suitable for children and elderly patients.
Owner:SHANDONG NEWTIME PHARMA
Catalyst composition and method for preparing faropenem sodium
ActiveCN101941981AReduce pollutionEasy to separateAntibacterial agentsOrganic chemistryTriphenylphosphinePalladium
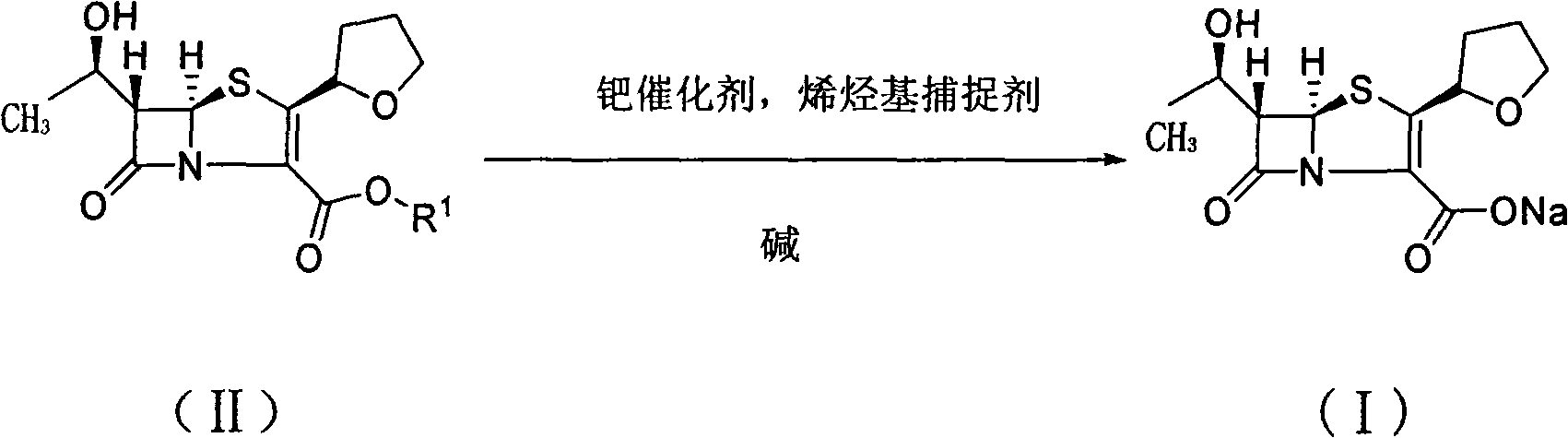
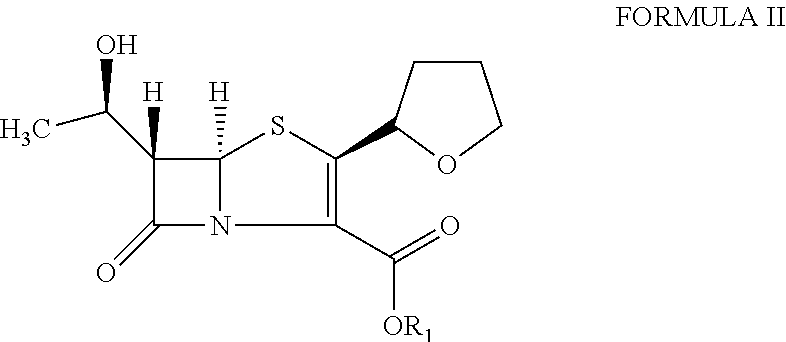
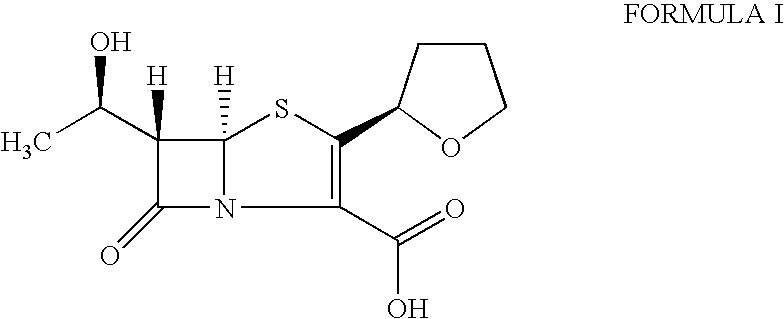
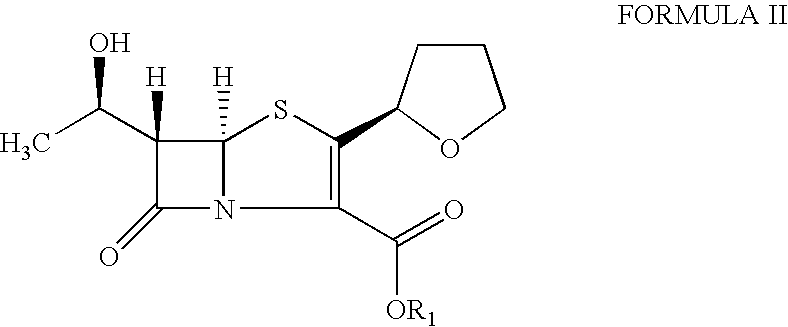
The invention provides a catalyst composition, mainly containing palladium carbon (Pd / C) and triphenylphosphine, wherein the molar ratio of palladium to the triphenylphosphine is 1:2-1:6. The invention also provides a method for preparing faropenem sodium of a formula I, comprising the following step of: performing a reaction of a compound as an intermediate in a formula II and an allyl receptor under the action of a catalyst for removing allyl to produce the faropenem sodium of the formula I. The adopted catalyst for removing the allyl is the catalyst composition containing the palladium carbon (Pd / C) and the triphenylphosphine. The catalyst composition is easy to recover, thereby greatly reducing pollution of the palladium in a product.
Owner:HUNAN WARRANT PHARMA +1
Industrial production of Fallopeinan sodium
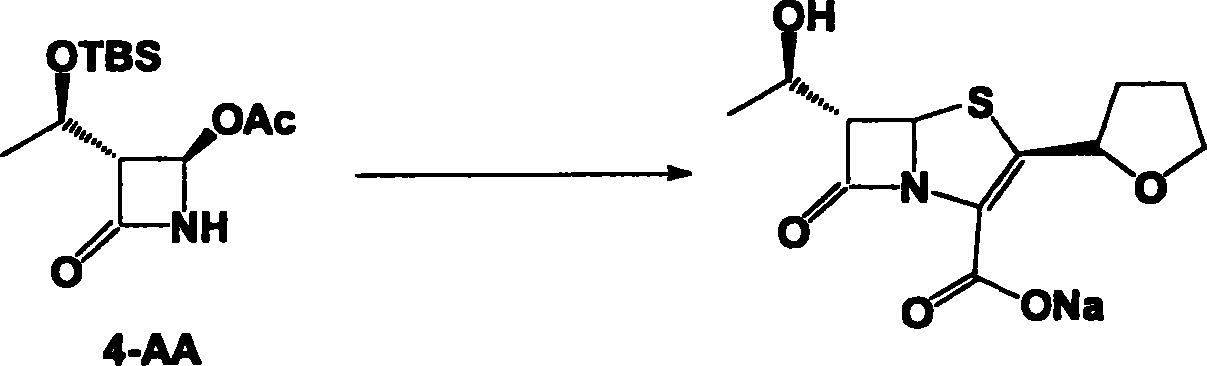
Industrial production of Faropena sodium is carried out by taking 4-AA as raw material and substituting allyloxy-oxalyl chloride with low-grade alkoxy-oxalyl chloride to obtain the final product. It's cheap, convenient and efficient, adopts 'one-pan' method and avoid producing double-bond polymer and has no need for purification.
Owner:LUNAN PHARMA GROUP CORPORATION
Faropenem sodium freeze-dried powder injection
ActiveCN102133201AImprove securityQuality improvementAntibacterial agentsPowder deliveryPhysical chemistryPowder injection
The invention relates to a Faropenem sodium freeze-dried powder injection, which comprises only one composition: Faropenem sodium. Preferably, injection water is added into Faropenem sodium powder with the particle size of 75 to 100mum to be prepared into Faropenem sodium solution with concentration of 5-20 percent for drying, thus obtaining a finished product. The Faropenem sodium freeze-dried powder injection is simple and feasible in preparation process, beneficial to operation, and suitable for needs for large-scale industrial production.
Owner:鲁南新时代生物技术有限公司
Detection of bacteria having a resistance to carbapenems
Disclosed is a process for detecting and / or identifying, in a biological sample, bacteria exhibiting a resistance to carbapenems, including: a) contacting said sample with a reaction medium including at least one chromogenic agent and faropenem and / or doripenem; b) incubating the whole so as to allow the bacteria to grow; and c) detecting the strains exhibiting a resistance to carbapenems. The medium employed in step a) also contains cloxacillin and / or a combination of cloxacillin and PAbetaN.
Owner:BIOMERIEUX SA
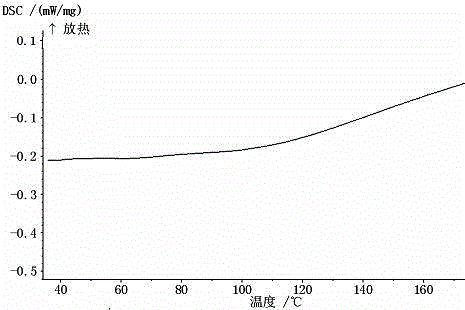
Faropenem sodium crystallization and preparation method thereof
ActiveCN101033233AImprove stacking effectImprove liquidityAntibacterial agentsOrganic chemistryCrystallographyBeta lactam antibiotic
This invention relates to the crystallization of compounds, specifically involving beta-lactam antibiotics faropenem Na crystallization and its preparation method.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Faropenem sodiumcomposition for direct tabletcompression and preparation method of faropenem sodiumcomposition
ActiveCN104473892AAvoid exposure to heat and humidityImprove stabilityAntibacterial agentsPill deliveryInorganic saltsLubricant
The invention belongs to the technical field of medicinepreparation, and particularly relates to faropenem sodiumcomposition for direct tabletcompression and a preparation method of the faropenem sodiumcomposition. The composition comprises, in percentage by weight, 40%-70% of faropenem sodium and 30%-60% of an auxiliary material, wherein the faropenem sodium is fine powder with theaverageparticle size ranging from 150 mum to 250 mu m; the auxiliary material comprises a disintegrating agent, a binding agent, 0%-5% of a lubricating agent, 0%-5% of a flow aid and one or more of microcrystalline cellulose, cellulose, starch, saccharides and inorganic salts. Compared with the prior art, thefaropenem sodiumcomposition has the advantages that tablets can be prepared from the faropenem sodiumcomposition with a direct tabletcompression method, processes that medicines are contacted with humid and heat are omitted, the product stability is improved, technological steps are reduced, work hours are saved, the production cost is reduced, and the production efficiency is improved.
Owner:CISEN PHARMA
Faropenem sodium powder injection
ActiveCN102133193AImprove stabilityOvercome the problem of low stabilityAntibacterial agentsPowder deliveryPhysical chemistryPowder injection
The invention relates to a Faropenem sodium powder injection, which comprises only one composition: raw Faropenem sodium powder without any additive. As raw Faropenem sodium is solid powder and in isolating state, the oxidization and hydrolyzation are hard to carry out; in addition, as a current filling machine can be forcibly filled, the filling amount is stable. Particularly, when the humidity of a split charging workshop is controlled under 30 percent, the stability of the Faropenem sodium powder is better.
Owner:鲁南新时代生物技术有限公司
Faropenem sodium granules and preparing method thereof
InactiveCN104027310AImprove stabilityAvoid tabooAntibacterial agentsGranular deliveryPhysical chemistryCyclodextrin
Faropenem sodium granules and a preparing method thereof are disclosed. The faropenem sodium granules can be prepared by: uniformly mixing faropenem sodium and hydroxypropyl-beta-cyclodextrin, adding an aqueous ethanol solution, granulating, extruding, rolling, drying, uniformly mixing with pharmaceutically accepted auxiliary materials and packaging.
Owner:QINGDAO UNIV
Tablet containing faropenem sodium
ActiveCN101744782ASignificant progressDissolution constantAntibacterial agentsDigestive systemHigh humidityOlder people
The invention relates to a tablet containing faropenem sodium, belonging to the field of pharmaceutical preparation. The faropenem sodium tablet of the invention comprises 20-50 parts of faropenem sodium, 20-25 parts of disintegrant, 2-10 parts of 95% ethanol solution containing 5% of polyvinylpyrrolidone, 20-30 parts of filler and 0.5-2 parts of lubricant. The faropenem sodium tablet of the invention has the advantages of fast disintegration speed and stable properties in high-temperature and high-humidity environment, can effectively improve the effect-taking concentration and bioavailability of faropenem, and is very suitable for old people and children with dysphagia to take.
Owner:LUNAN BETTER PHARMA
Orally disintegrating tablet containing faropenem sodium and preparation method of orally disintegrating tablet
ActiveCN104257618ASignificant progressReduce usagePharmaceutical non-active ingredientsPill deliveryMANNITOL/SORBITOLOrally disintegrating tablet
The invention belongs to the field of medicines and provides an orally disintegrating tablet containing faropenem sodium. The orally disintegrating tablet also contains mannitol and a lubricating agent. According to the orally disintegrating tablet, less auxiliary materials are used; the composition formula is simple; the orally disintegrating tablet which is prepared from powder through tabletting still has good fluidity; the obtained tablet is reliable in hardness and high in disintegrating speed; and the dissolution behavior of the main drug is excellent.
Owner:SHANDONG NEWTIME PHARMA
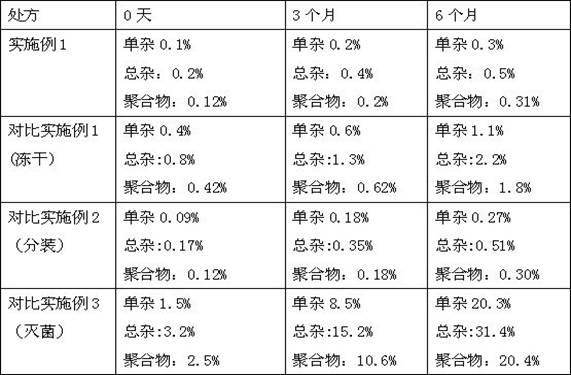
Method for determining content of bipolymer impurities in faropenem sodium
The invention relates to the field of pharmacy and provides a method for determining the content of bipolymer impurities in faropenem sodium. The method comprises the following steps: detecting with high performance liquid chromatography under chromatographic conditions that a reversed-phase chromatographic column is an octadecyl silane bonded silica gel column, a mobile phase A is acetonitrile and a mobile phase B is a 0.05% trifluoroacetic acid aqueous solution; and carrying out linear gradient elution. The method is good in correlation, high in sensitivity and good in repeatability, and can be used for quickly judging the content of faropenem bipolymers in faropenem sodium, and therefore, the quality of faropenem sodium can be guaranteed.
Owner:ZHUHAI UNITED LAB
Faropenem sodium granule and preparation method thereof
ActiveCN104224729AReduce contentImprove stabilityPharmaceutical non-active ingredientsGranular deliveryPolyvinyl alcoholDextran
The invention provides a faropenem sodium granule and a preparation method thereof. The faropenem sodium granule contains faropenem sodium, polyvinyl alcohol, dextran and a sweetening agent. The faropenem sodium granule has the advantages of low polymer content, good stability and simple preparation technology.
Owner:SHANDONG NEWTIME PHARMA
Combination comprising zidovudine and an antimicrobial compound
PendingCN112218633AAntibacterial agentsHeterocyclic compound active ingredientsCefalexinPharmaceutical medicine
The invention provides a combination comprising zidovudine or a pharmaceutically acceptable derivative thereof and an antimicrobial compound selected from nitrofurantoin, mecillinam, fosfomycin, cephalexin and faropenem, or a pharmaceutically acceptable derivative or prodrug thereof. These combinations are particularly useful for the treatment of microbial infections.
Owner:HELPERBY THERAPEUTICS LTD
Process for the Preparation of Faropenem
Owner:RANBAXY LAB LTD
Method for preparing faropenem daloxate
ActiveCN101235044AEasy to operateLow costOrganic chemistryAntiinfectivesCyclopenteneChemical synthesis
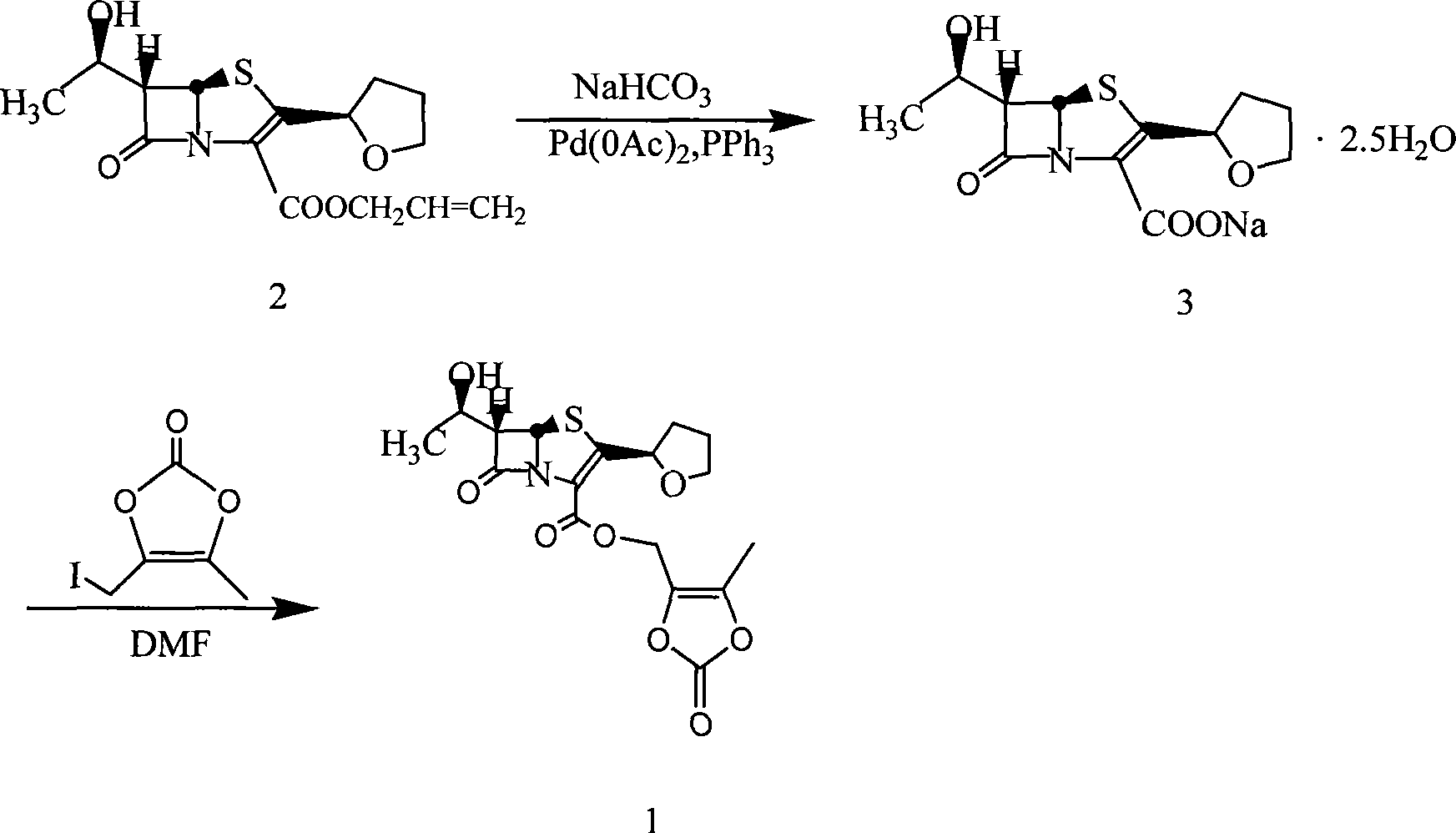
The invention relates to a preparation method of faropenem medoxomil, belonging to chemical synthesis technical field, which is characterized in that faropenem and 4-halogenated methyl radical-5-methyl-1, 3-dioxane cyclopentene-2-ketone, in alkali condition and solvent A, via phase transfer catalyst and reaction promoter containing iodine are synthesized to obtain faropenem medoxomil, or the inorganic salt of faropenem medoxomil and 4-halogenated methyl radical-5-methyl-1, 3-dioxane cyclopentene-2-ketone, in solvent B, via phase transfer catalyst and reaction promoter containing iodine are synthesized to obtain faropenem medoxomil. The method has simple operation, mild reaction and saved cost, while the synthesized faropenem medoxomil has high yield and purity.
Owner:NANJING HUAWE MEDICINE TECH DEV +1
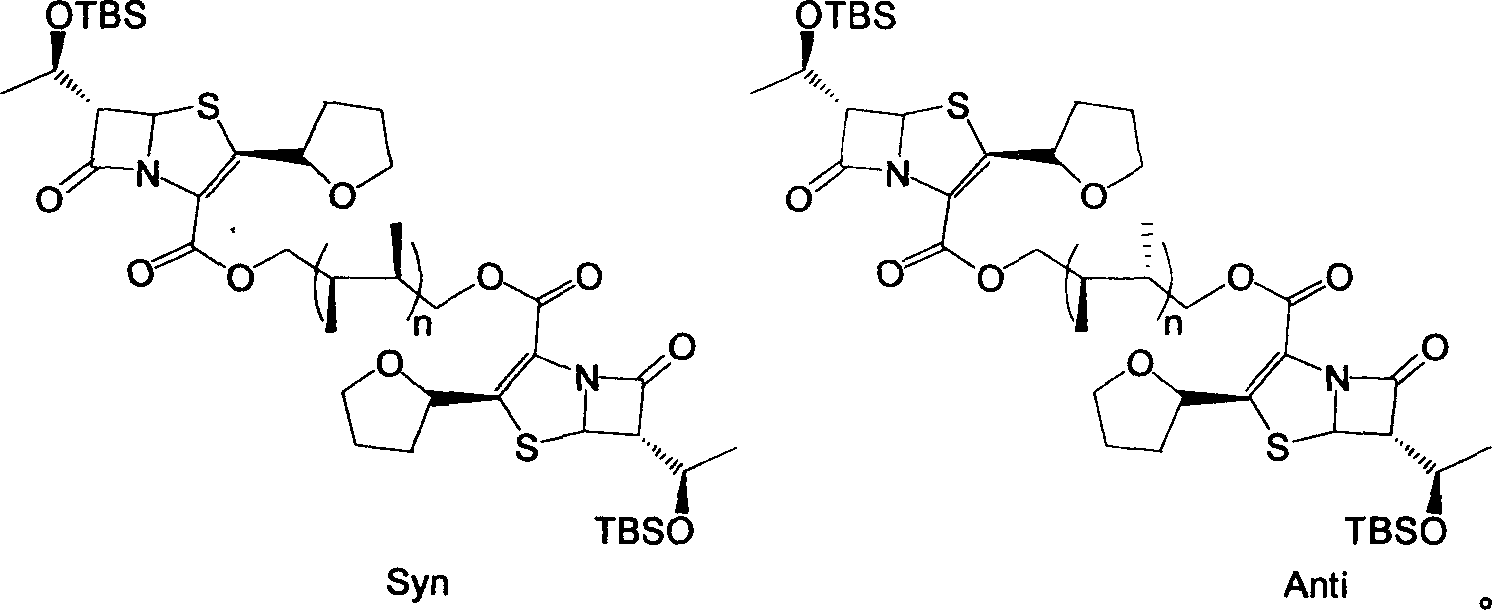
Faropenem sodium synthesis method from reaction by-product
InactiveCN1733771AIncrease profitImprove the overall yield of preparationOrganic chemistrySynthesis methodsPhotochemistry
The invention provides a process for synthesizing Faropenem from reaction by-products, which has the advantages of low cost, high yield, and easy realization of reaction conditions.
Owner:LUNAN PHARMA GROUP CORPORATION
Synthetic method of faropenem sodium
InactiveCN101585847ASimple and fast operationSuitable for industrial productionAntibacterial agentsOrganic chemistryFormic acidTetrahydrofuran
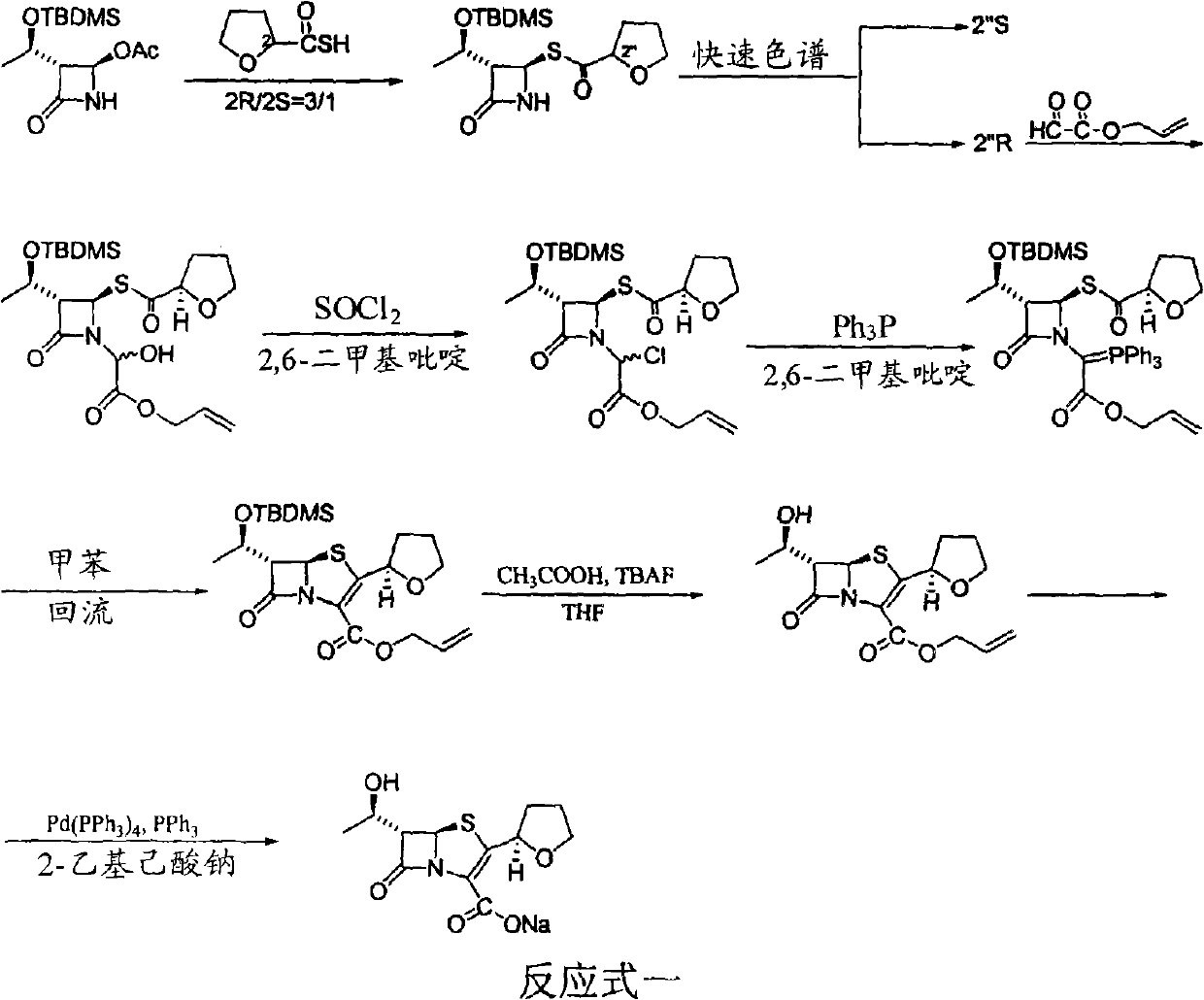
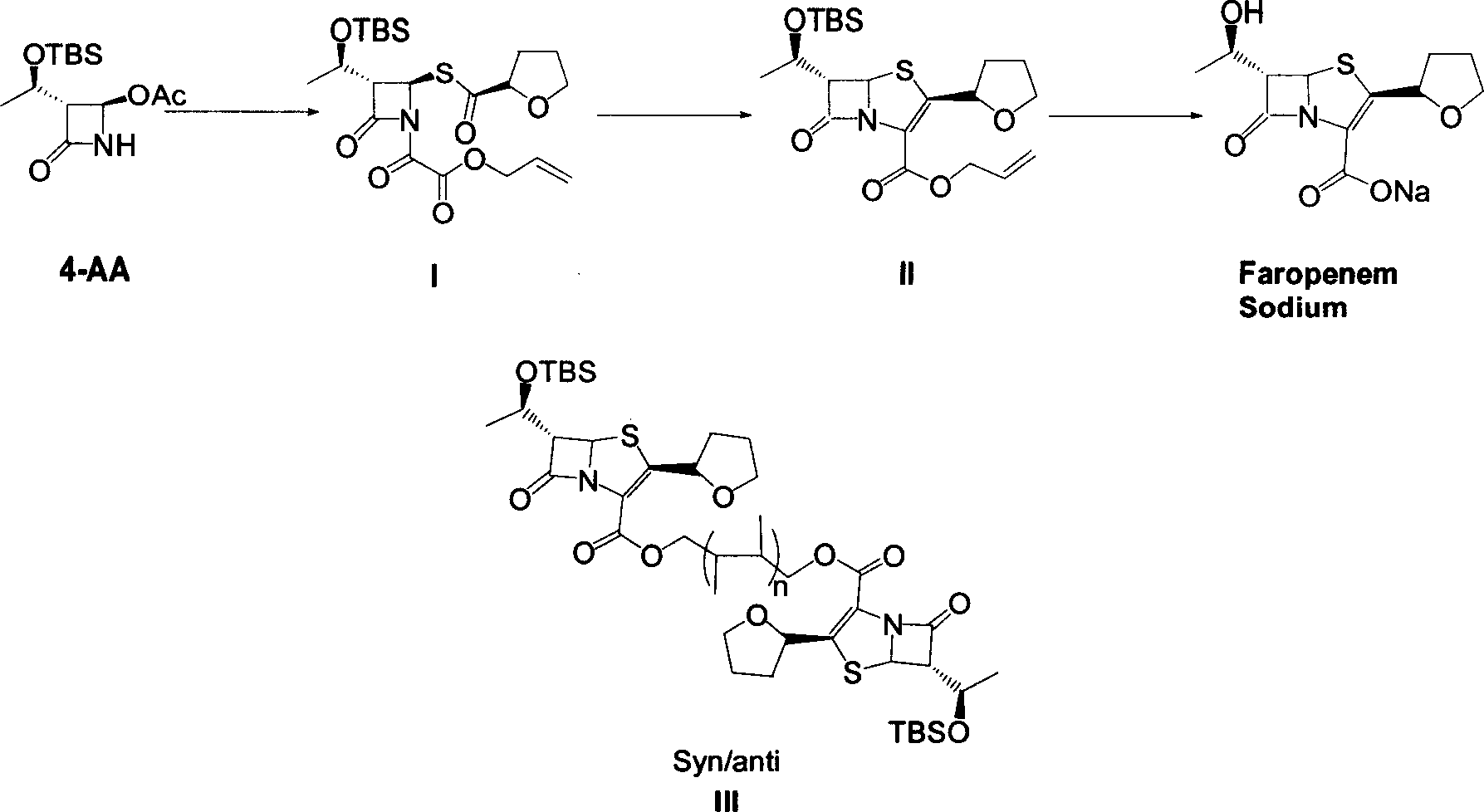
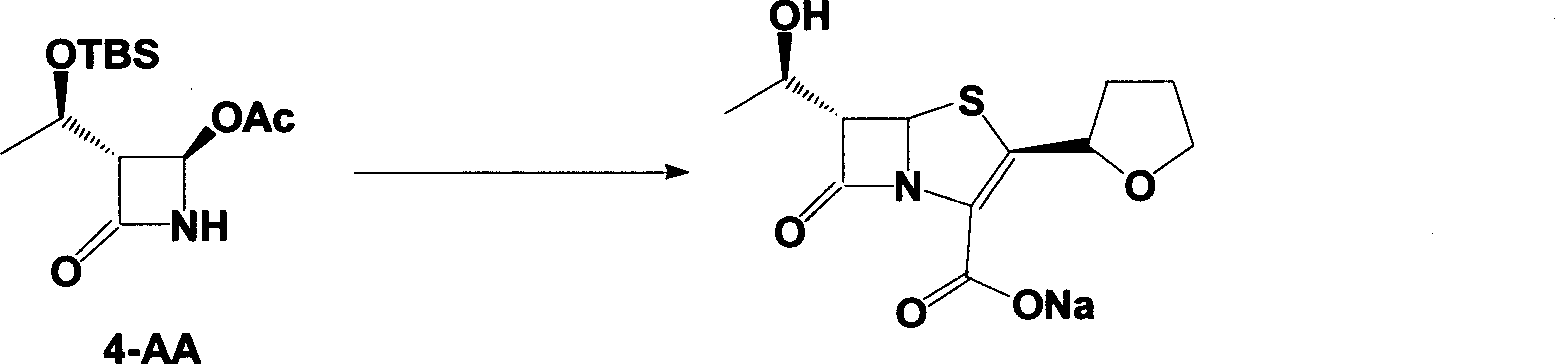
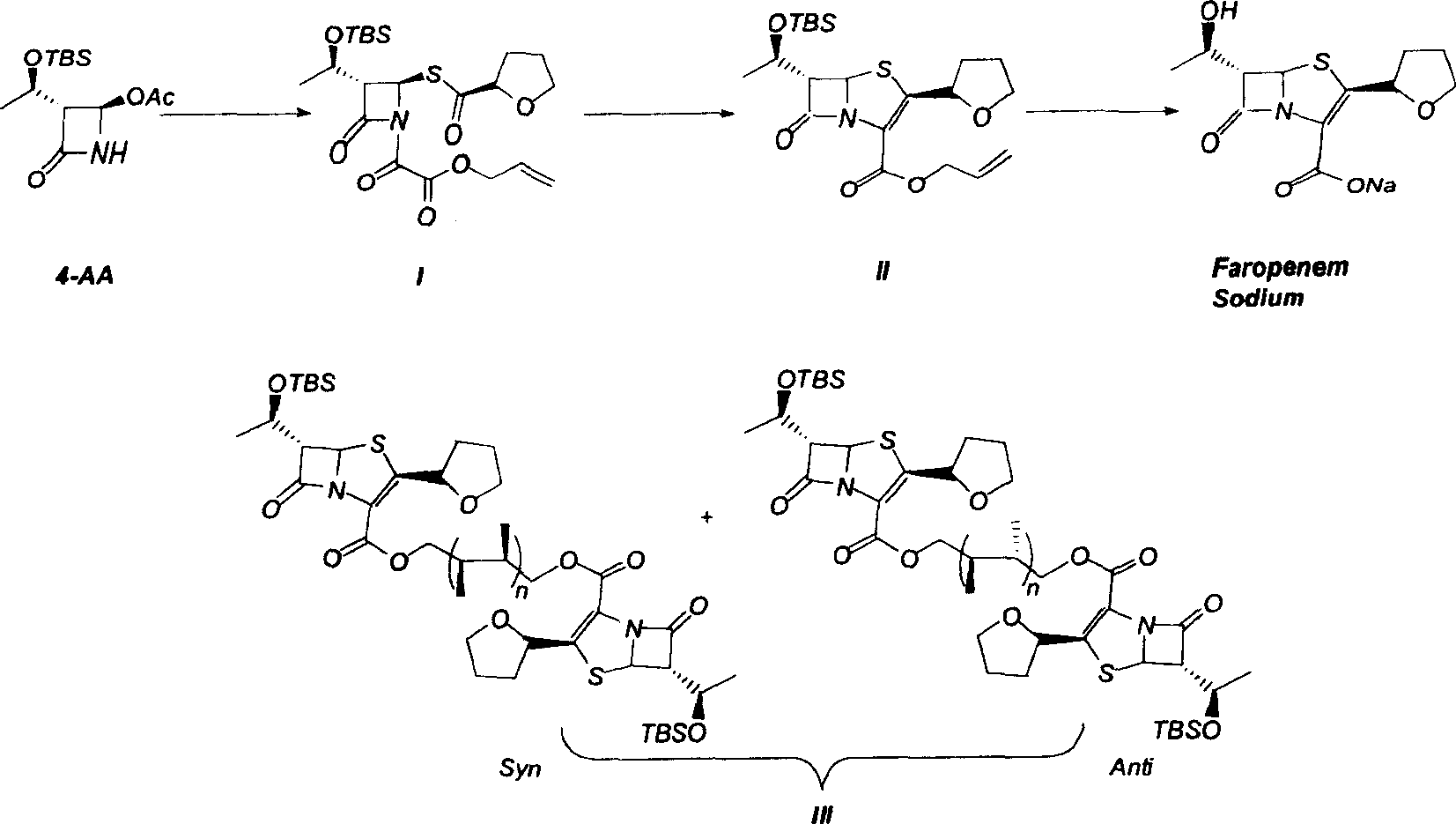
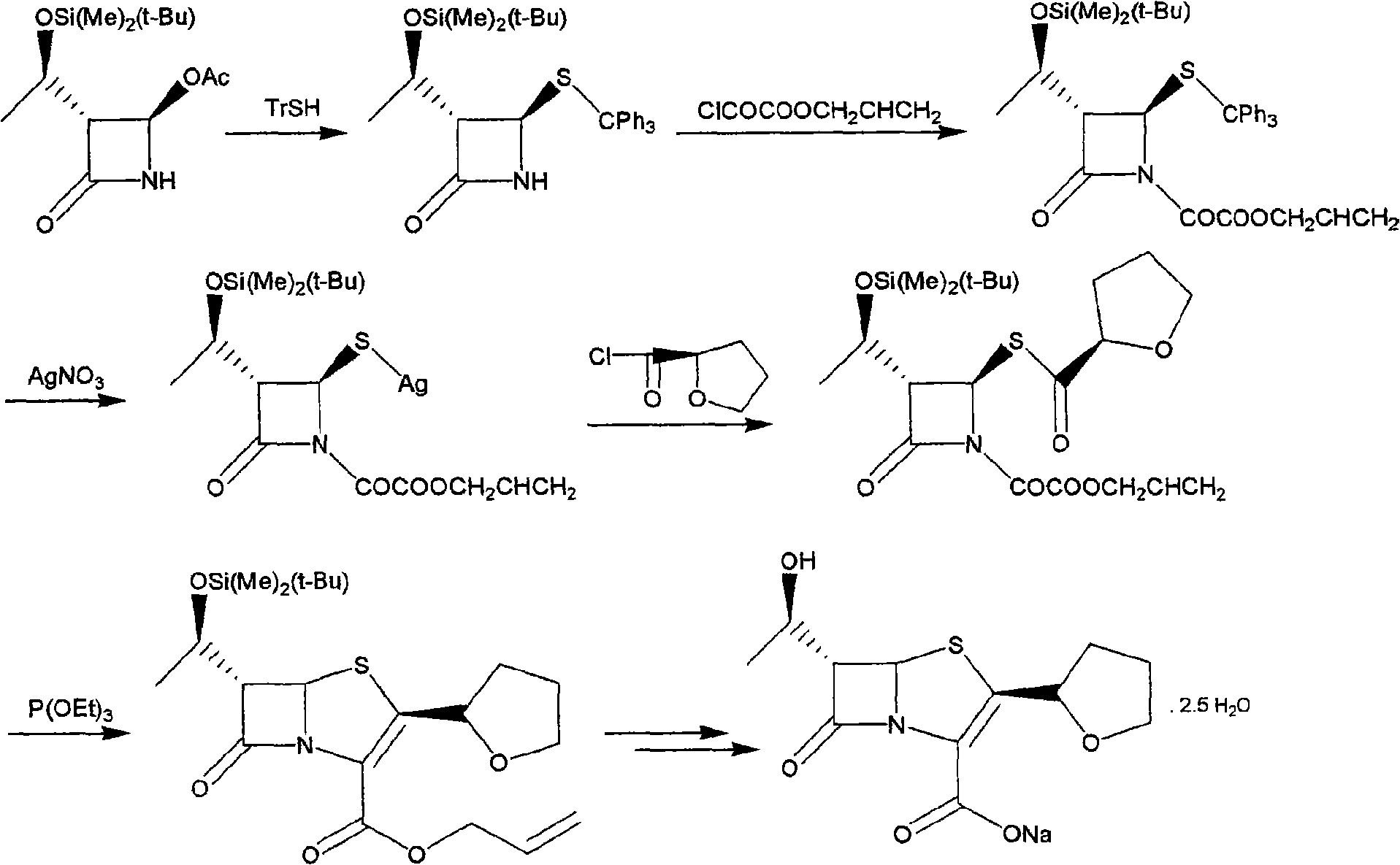
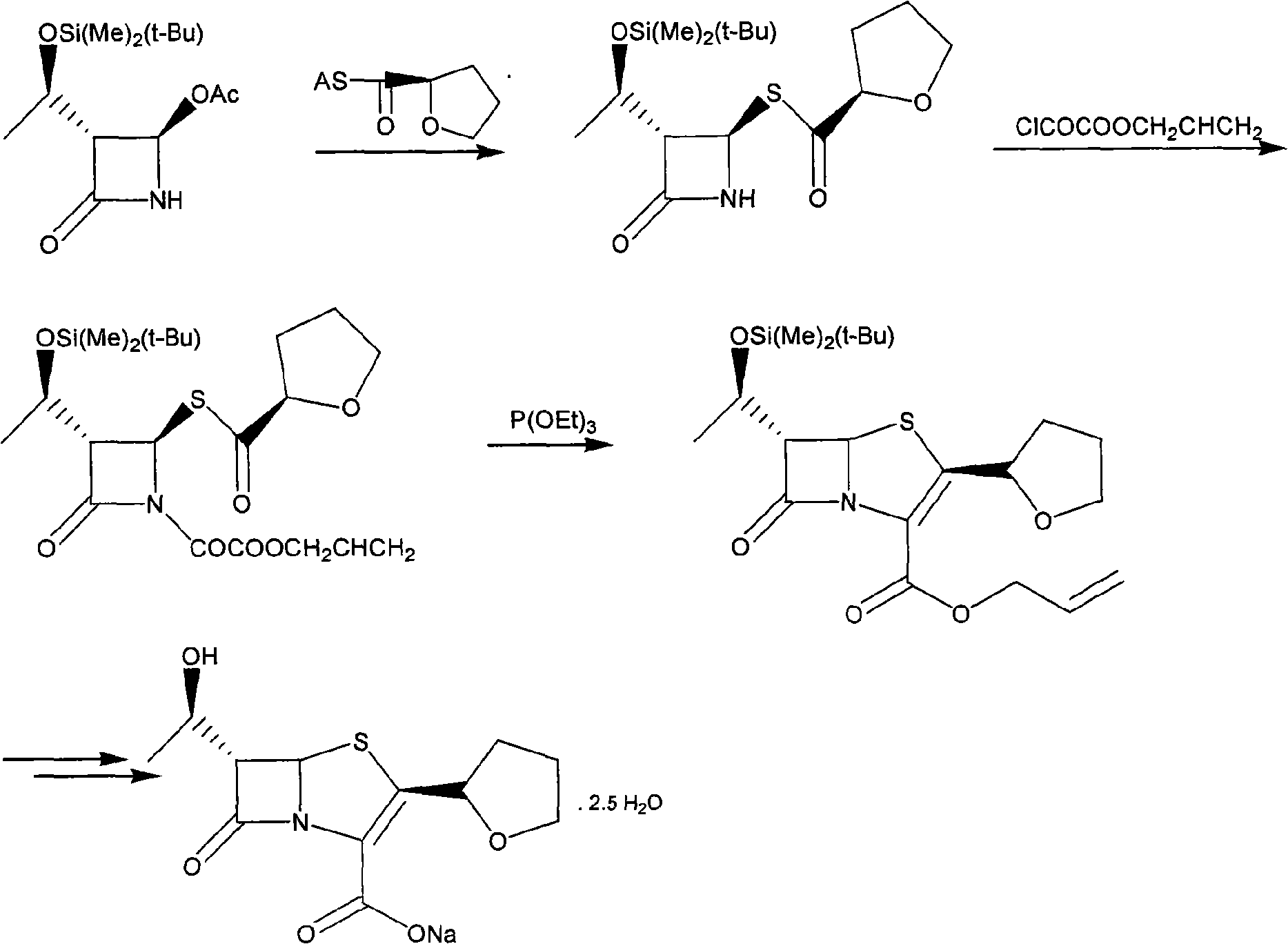
The invention discloses a synthetic method of faropenem sodium. The method uses (3R,4R)-3-[(R)-1-tert-butyldiemthylsilyloxyethyl]-4-[(R)-acetoxyl]azetidin-2-one (4-AA) as raw material and prepares the faropenem sodium through a five-step reaction. The method does not use a silver salt as a condensing agent, does not use 4-tetrahydrofuran-2-formic acid which is previously made into mercaptan acid, has simple and convenient operation, and is suitable for industrial production. The product purity and the total yield all exceed the prior art.
Owner:CHENGDU QIAOFENG TECH DEV
Preparation method of Faropenem sodium
InactiveCN101585846AMild reaction conditionsSimple and fast operationAntibacterial agentsOrganic chemistryCarboxylic acidTert butyl
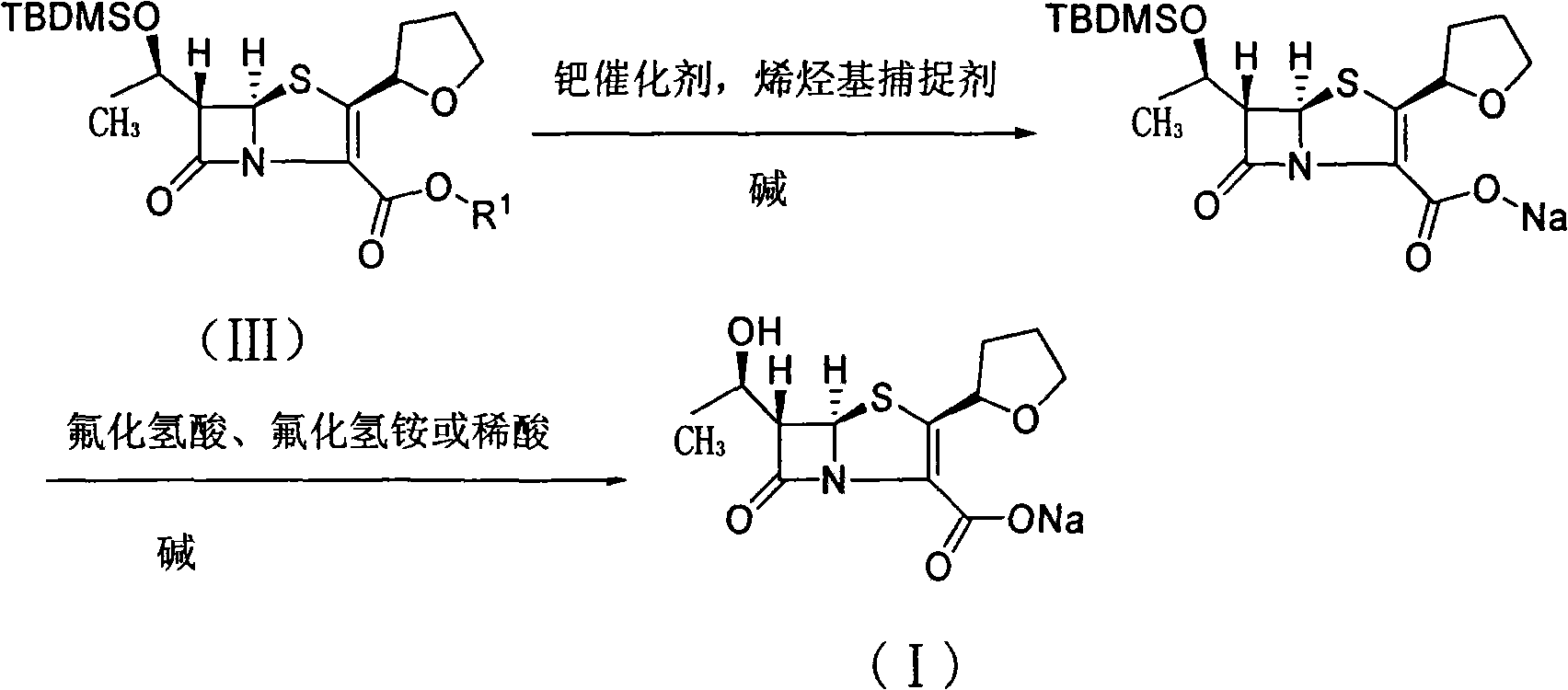
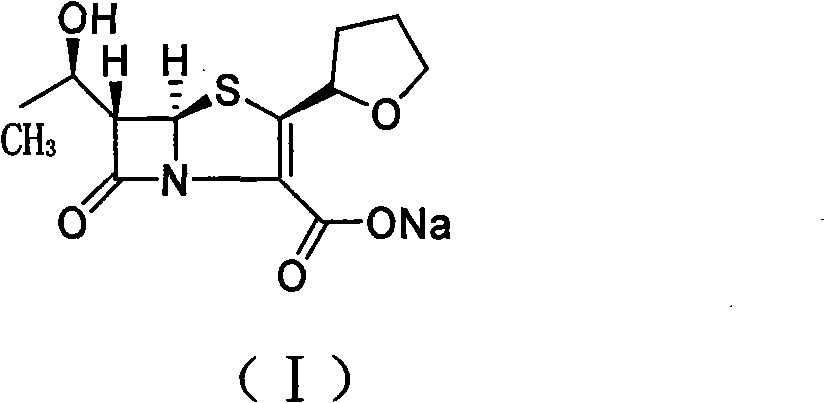
The invention relates to a preparation method of Faropenem sodium (I). The preparation method of Faropenem sodium includes: using (1'R, 2''R, 5R, 65)-6-[(1'-R)-2''-tetrahydrofuran base]penem-3-carboxylic ether as raw material, R is hydroxyethyl, or tert-butyl dimethyl silica ethyl, in the presence of palladium catalyst and alkylene capturing agent, removing allyl group of carboxyl acid or cinnamon base protecting group, in the presence of alkaline producing Faropenem sodium; Or, B: when the R is tert-butyl dimethyl silica ethyl, in the presence of palladium catalyst, triphenylphosphine and alkylene capturing agent, after removing allyl group of carboxyl acid or cinnamon base protecting group, under the actions of fluorine hydride acid, ammonium acid fluoride or dilute acid, removing silicone base protecting group of hydroxyl group, under the action of alkali, producing Faropenem sodium, the alkylene capturing agent is 5, 5-dimethyl cyclohexanedione; the palladium catalyst contains palladiurn bichloride, mixture of palladiurn bichloride and triphenylphosphine, and di (triethoxy phosphine) palladiurn bichloride, etc.
Owner:FUDAN UNIV
Purification method of faropenem sodium hydrate
InactiveCN103588786AReduce residualImprove crystallization yieldOrganic chemistryPurification methodsAlcohol
The invention discloses a purification method of a faropenem sodium hydrate, which comprises the following steps: 1) dissolving a faropenem sodium hydrate crude product in a lower alcohol solvent at -20-40 DEG C, adding active carbon for decoloration, filtering, 2) adding water into filtrate, performing decompression concentration and evaporation at 0-40 DEG C to remove the lower alcohol solvent, and 3) adding residual into a ketone insoluble solvent, stirring, crystallizing and filtering to form the pure faropenem sodium hydrate, wherein for the faropenem sodium hydrate crude product, the water content is 1g:(0.5-2mL). According to the method, the faropenem sodium hydrate crude product is dissolved in the lower alcohol solvent at the low temperature, a little water is added, and the lower alcohol solvent is evaporated off at the low temperature; the whole purification process is performed at the low temperature all the time, so that the crystallization yield is increased obviously and reaches above 90%; and residual products in a mother solution are fewer, so that the product cost is lowered.
Owner:HUANGGANG LUBAN PHARM
Faropenem sodium preparation method
The invention discloses a process for preparing Faropenem by using 4-AA as the raw material through a 'one-pot' operation, wherein the intermediates can be used directly for the next step reaction without the need for purification. The method has the advantages of low cost, high yield, and easy reaction conditions.
Owner:LUNAN PHARMA GROUP CORPORATION
Faropenem sodium crystallization and preparation method thereof
ActiveCN100484942CImprove stacking effectImprove liquidityAntibacterial agentsOrganic chemistryCrystallographyBeta lactam antibiotic
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Faropenem sodium particles and preparation method thereof
InactiveCN111202714ASimple processSolve the problem of growthAntibacterial agentsInorganic non-active ingredientsDesiccantCurative effect
The invention belongs to the field of pharmaceutical preparations, and relates to faropenem sodium particles and a preparation method thereof. The invention provides the faropenem sodium particles with a simple production process, stable and controllable quality and a safe and reliable curative effect. The particles contain the following components in percentages by weight: 5-20% of faropenem sodium, 75-95% of an excipient, and 0.3-1% of a desiccant. The stability of the faropenem sodium particles prepared by the method is greatly improved, the quality indicators of the substances, related tothe main components, such as related substances, polymers and moisture are well controlled, and the safety and shelf life standards of medication are improved.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
Application of faropenem in preparation of medicine for initial treatment of pathogen-positive pulmonary tuberculosis
InactiveCN111184718AQuick effectSignificant effectAntibacterial agentsHeterocyclic compound active ingredientsInitial treatmentSide effect
The invention belongs to the technical field of medicines, and relates to a new application of faropenem, in particular to the new application of the faropenem in preparation of a medicine for initialtreatment of pathogen-positive pulmonary tuberculosis. Compared with an existing standard scheme, when being used for initial treatment of the pathogen-positive pulmonary tuberculosis, a pharmaceutical composition containing the faropenem is remarkable in effect, rapid in effect taking, small in side effect and high in safety, and effectively overcomes the defects that the tuberculosis treatmentis long in treatment course and high in toxic and side effects.
Owner:LUNAN PHARMA GROUP CORPORATION
Process for the preparation of faropenem
The present invention is related to processes for the preparation of faropenem, which comprises treating the compound of Formula II,with an alkali metal salt of a substituted or unsubstituted C5-10 carboxylic acid and a catalytic amount of a palladium complex in the presence of an organic solvent, followed by the treatment of the reaction mixture of with water and a water miscible solvent, and isolating a hydrate of an alkali metal salt of faropenem from the reaction mass, wherein water is not removed from the reaction mixture in water treatment or isolation steps.
Owner:RANBAXY LAB LTD
Preparation method of faropenem sodium
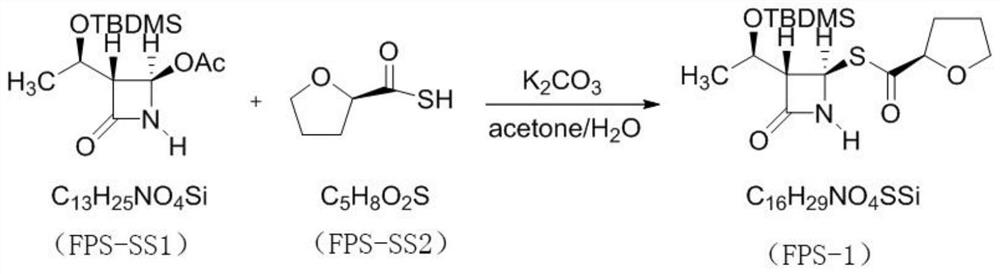
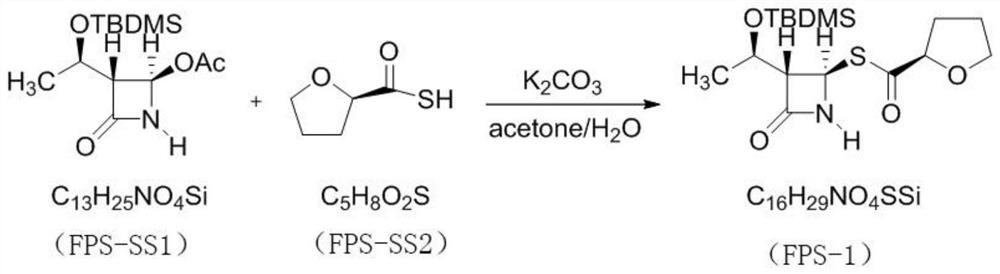
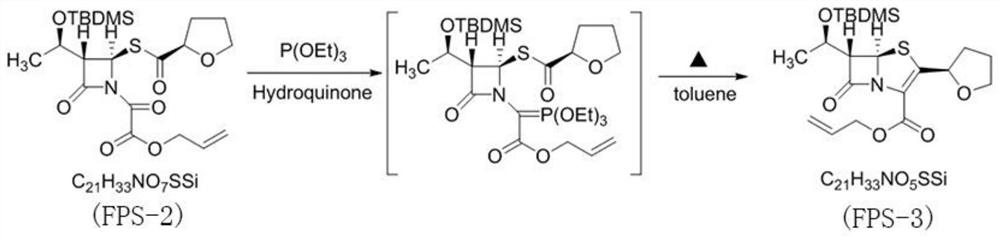
PendingCN114315863AHigh yieldHigh purityOrganic chemistryAntiinfectivesPhosphorous acidTriethylphosphite
The invention discloses a preparation method of faropenem sodium, which comprises the following steps: mixing potassium carbonate, R-(+)-thiotetrahydrofuran-2-formic acid and (3R, 4R)-4-acetoxyl-3-[(R)-(tert-butyldimethylsiloxy) ethyl] azetidin-2-ketone, and reacting to obtain FPS-1; mixing the FPS-1, triethylamine and oxalyl chloride monoallyl ester, and carrying out a reaction so as to obtain FPS-2; mixing the FPS-2 with triethyl phosphite, and carrying out a reaction so as to obtain FPS-3; mixing the FPS-3 with tetrabutylammonium fluoride, and carrying out a reaction so as to obtain FPS-4; and mixing the FPS-4, triphenylphosphine, sodium isooctoate and tetrakis (triphenylphosphine) palladium, and reacting to obtain the faropenem sodium. According to the preparation method disclosed by the invention, high-purity and high-yield faropenem sodium can be obtained.
Owner:赤峰万泽药业股份有限公司
Catalyst composition and method for preparing faropenem sodium
ActiveCN101941981BReduce pollutionEasy to separateAntibacterial agentsOrganic chemistryTriphenylphosphinePalladium
The invention provides a catalyst composition, mainly containing palladium carbon (Pd / C) and triphenylphosphine, wherein the molar ratio of palladium to the triphenylphosphine is 1:2-1:6. The invention also provides a method for preparing faropenem sodium of a formula I, comprising the following step of: performing a reaction of a compound as an intermediate in a formula II and an allyl receptor under the action of a catalyst for removing allyl to produce the faropenem sodium of the formula I. The adopted catalyst for removing the allyl is the catalyst composition containing the palladium carbon (Pd / C) and the triphenylphosphine. The catalyst composition is easy to recover, thereby greatly reducing pollution of the palladium in a product.
Owner:HUNAN WARRANT PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com