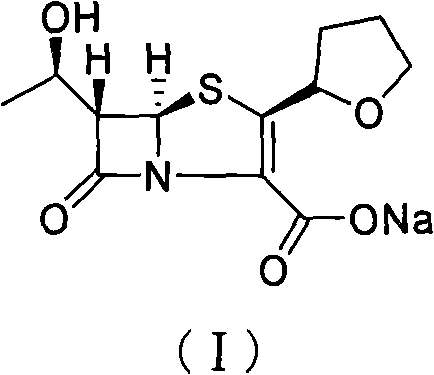
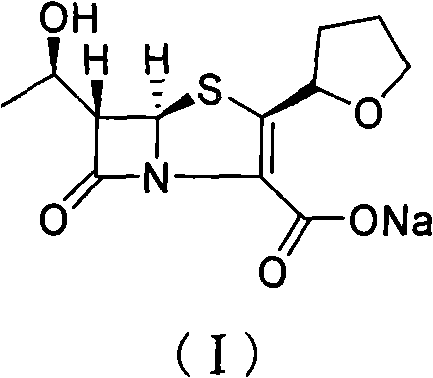
Method for removing residual palladium of faropenem sodium
A technology of faropenem sodium and removal method, which is applied in the field of medicinal chemistry, can solve problems such as palladium residues and the like, and achieves the effects of simple operation, easy availability of raw materials and good adsorption effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Sodium isooctanoate (4.33g, 0.026mol) and deionized water (3.4mL) were placed in a reaction flask and stirred until dissolved, then (1′R, 2″R, 5R, 6S)-6-[(1 '-Hydroxyethyl)-2"-tetrahydrofuryl] penem-3-carboxylic acid allyl ester (8.5g, 0.026mol) and ethyl acetate (34mL) solution, and after replacing the air in the reaction flask with nitrogen, Tetrakis(triphenylphosphine)palladium (0.3 g, 0.26 mmol) and triphenylphosphine (0.34 g, 1.3 mmol) were added rapidly. After stirring at 25°C for 5 hours, cool to 0°C and continue to stir for 0.5 hours, filter, and wash the filter cake with ethyl acetate to obtain the crude product of faropenem sodium (I). The crude product is detected by an ICP emission spectrometer, and the palladium content is 1068ppm. Then dissolve the crude product of faropenem sodium (I) in 85 mL of methanol, add 767 injections of activated carbon (0.1 g), stir at 25 °C for 12 h, filter with Buchner funnel, spin dry the alcohol solvent in the filtrate at 25...
Embodiment 2
[0021] After sodium bicarbonate (5.2g, 61.4mmol) and deionized water (8mL) were placed in the reaction flask and stirred and dissolved, 5,5-dimethylcyclohexanedione (5.2g, 37.2mmol) was added in batches and waited Continue to stir until the reaction solution is clear after the addition; Allyl carboxylate (20g, 61.4mmol) and ethyl acetate (80mL) solution, and after replacing the air in the reaction flask with nitrogen, quickly add two (triphenylphosphine) palladium dichloride (1.0g, 1.43mmol) . Stir at 25°C for 5 hours, then cool to 0°C and continue stirring for 0.5 hours, filter, and wash the filter cake with ethyl acetate to obtain the crude product of faropenem sodium (I). The crude product is detected by ICP emission spectrometer, and the palladium content It is 1004ppm. Then dissolve the crude product of faropenem sodium (I) in 200 mL of methanol, add 767 injections of activated carbon (0.24 g), stir at 25 ° C for 12 h, filter it with a Buchner funnel, and spin dry the a...
Embodiment 3
[0023] After sodium bicarbonate (5.2g, 61.4mmol) and deionized water (8mL) were placed in the reaction flask and stirred and dissolved, 5,5-dimethylcyclohexanedione (5.2g, 37.2mmol) was added in batches and waited Continue to stir until the reaction solution is clear after the addition; Allyl carboxylate (20g, 61.4mmol) and acetone (80mL) solution, and after the air in the reaction flask was replaced with nitrogen, palladium dichloride (0.25g, 1.43mmol) and triphenylphosphine (0.674g, 2.57 mmol). Stir at 25°C for 5 hours, then cool to 0°C and continue stirring for 0.5 hours, filter, and wash the filter cake with ethyl acetate to obtain the crude product of faropenem sodium (I). The crude product is detected by ICP emission spectrometer, and the palladium content 2000ppm. Then the crude product was dissolved with 200mL of methanol, 767 injection of activated carbon (0.24g) was added, and after stirring for 12h at 25°C, it was suction-filtered with a Buchner funnel, and the al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com