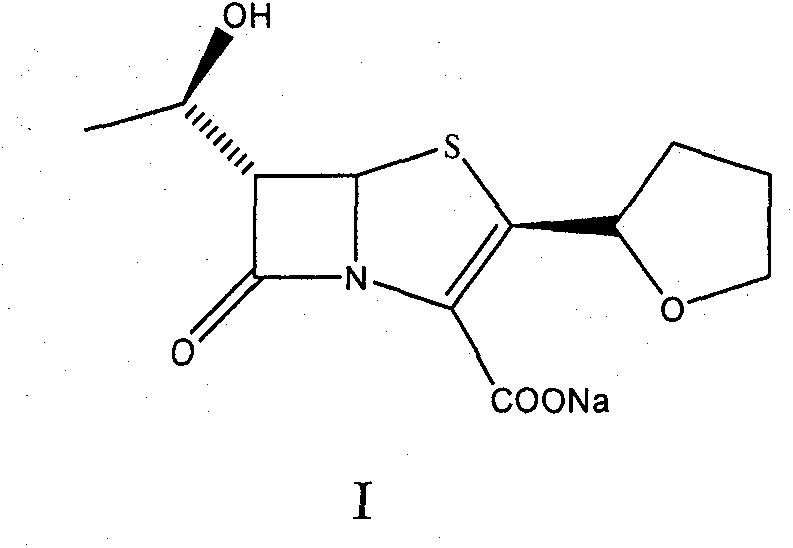
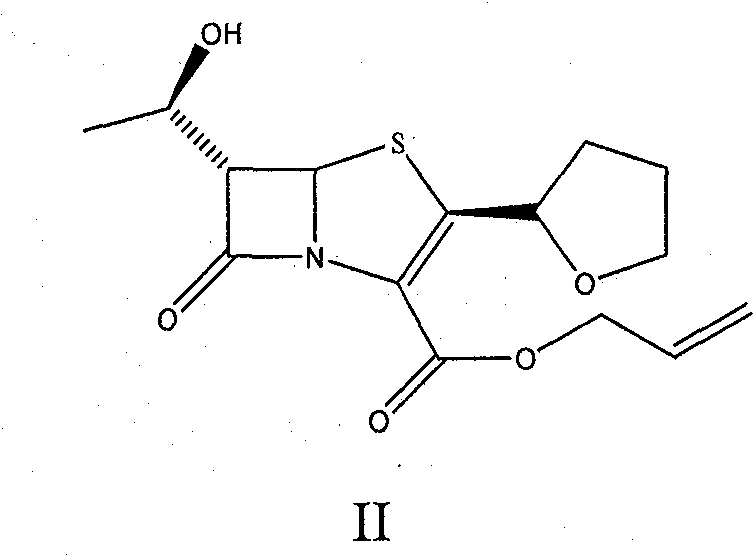
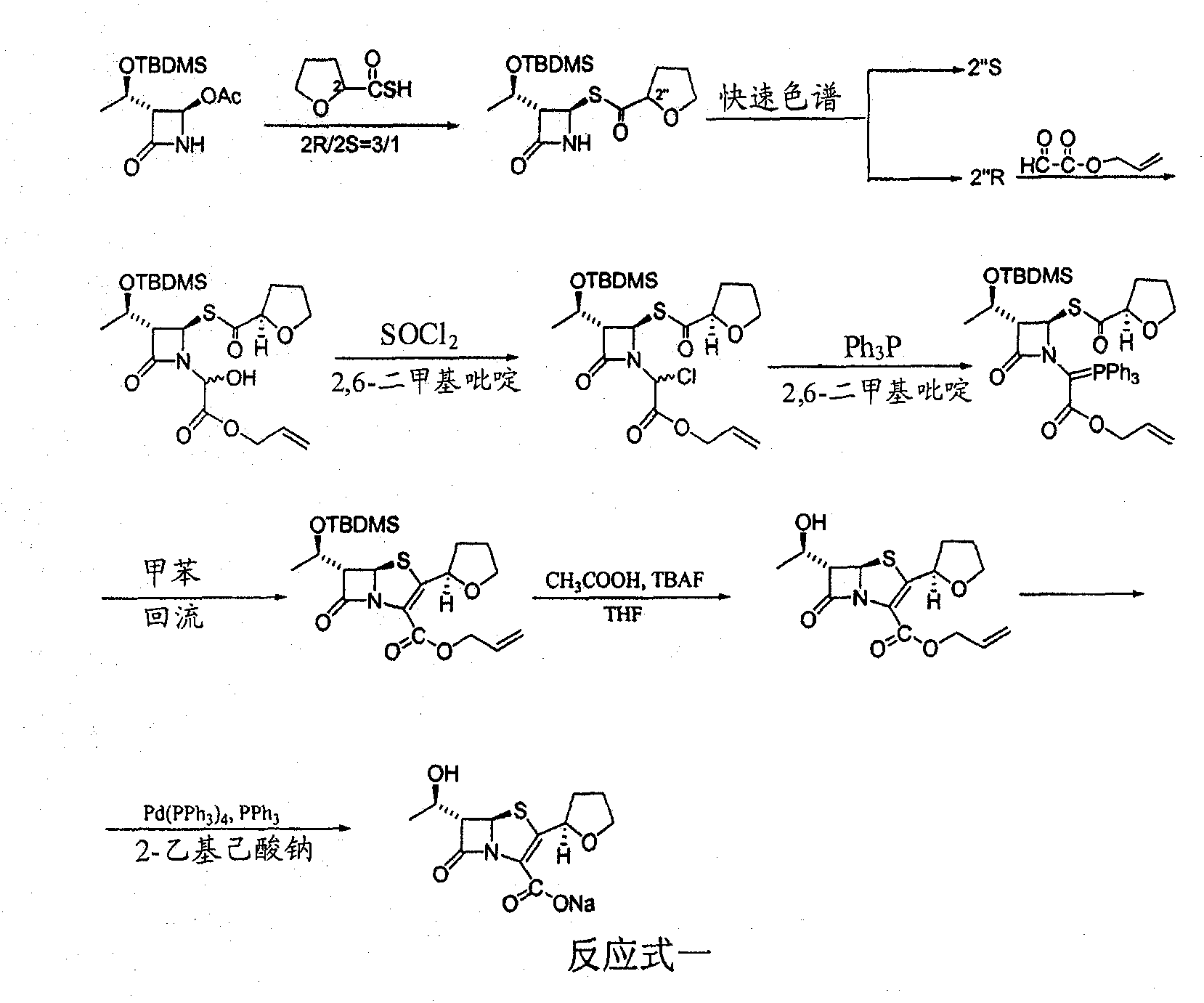
Catalyst composition and method for preparing faropenem sodium
A faropenem sodium and catalyst technology, applied in the field of preparation of faropenem sodium, can solve the problems of excessive heavy metals, deterioration, unstable catalyst properties, etc., achieve high recovery rate, easy separation, and reduce pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 10g of intermediate; 2.0g of triphenylphosphine and 2g of Pd-C (containing 10% palladium) to 60mL of dry methylene chloride in sequence, and add 60mL of ethyl acetate solution prepared by 0.5M sodium 2-ethylhexanoate , stirred at room temperature for 8 hours, stopped the reaction, filtered with suction, recovered Pd-C, added 2ml of water to the reaction solution, stirred until a solid precipitated, and continued to stir for 0.5 hours, filtered with suction to obtain a light yellow solid, which was dissolved in as much Add 0.5 g of activated carbon to a small amount of water, decolorize for 30 minutes, filter, add the filtrate to 5 times the amount of acetone, and place it for crystallization to obtain 7.0 g of faropenem sodium with a yield of 64.5%.
Embodiment 2
[0035] Add 10g of intermediate; 2.0g of triphenylphosphine and 2g of Pd-C (containing 10% palladium) to 60mL of dry dichloromethane successively, add 60mL of ethyl acetate solution prepared by 0.5M sodium p-benzenesulfinate, Stir at 40°C for 3 hours, stop the reaction, filter with suction, recover Pd-C, add 2ml of water to the reaction solution, stir until a solid precipitates, and continue stirring for 0.5 hours, filter with suction to obtain a light yellow solid, dissolve the solid in as much Add 0.5 g of activated carbon to a small amount of water, decolorize for 30 minutes, filter, add the filtrate to 5 times the amount of acetone, and place it for crystallization to obtain 6.5 g of faropenem sodium, with a yield of 60.2%.
Embodiment 3
[0037]Add 10g of the intermediate; 2.0g of triphenylphosphine and 4.2g of Pd-C (containing palladium 5%) to 60mL of dry methylene chloride in turn, and add 0.5M ethyl acetate solution prepared by sodium 2-ethylhexanoate 60mL, stirred at 25°C for 7 hours, stopped the reaction, filtered with suction, recovered Pd-C, added 2ml of water to the reaction solution, stirred until a solid precipitated, and continued to stir for 0.5 hours, filtered with suction to obtain a light yellow solid, which was dissolved in Add 0.5 g of activated carbon to as little water as possible, decolorize for 30 minutes, filter, add the filtrate to 5 times the amount of acetone, and place it for crystallization to obtain 7.0 g of faropenem sodium, with a yield of 64.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com