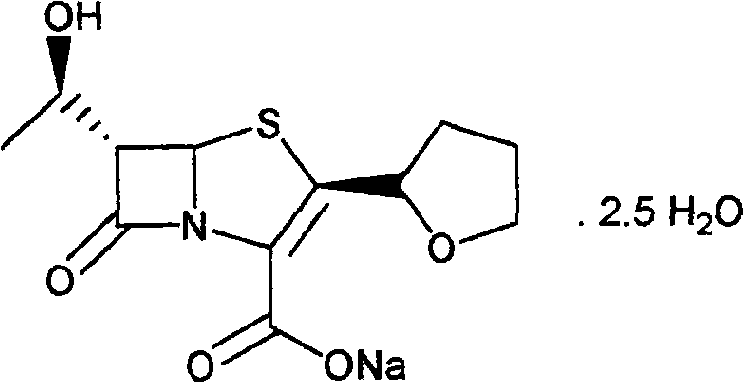
Synthetic method of faropenem sodium
A technology of faropenem sodium and its synthesis method, which is applied in the field of organic chemical industry or pharmaceutical chemical industry, can solve the problems of inconvenient production operation and long reaction steps, and achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
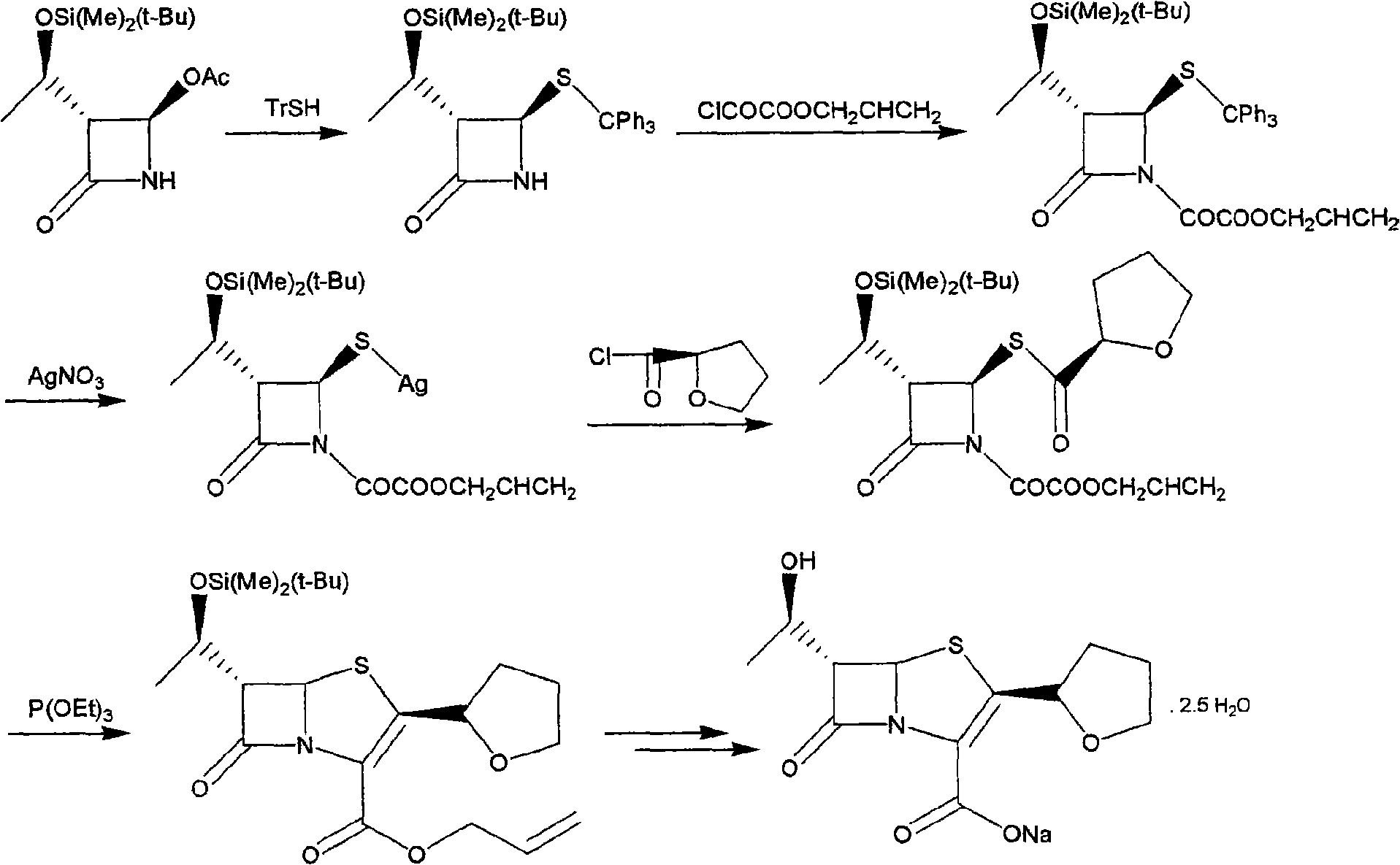
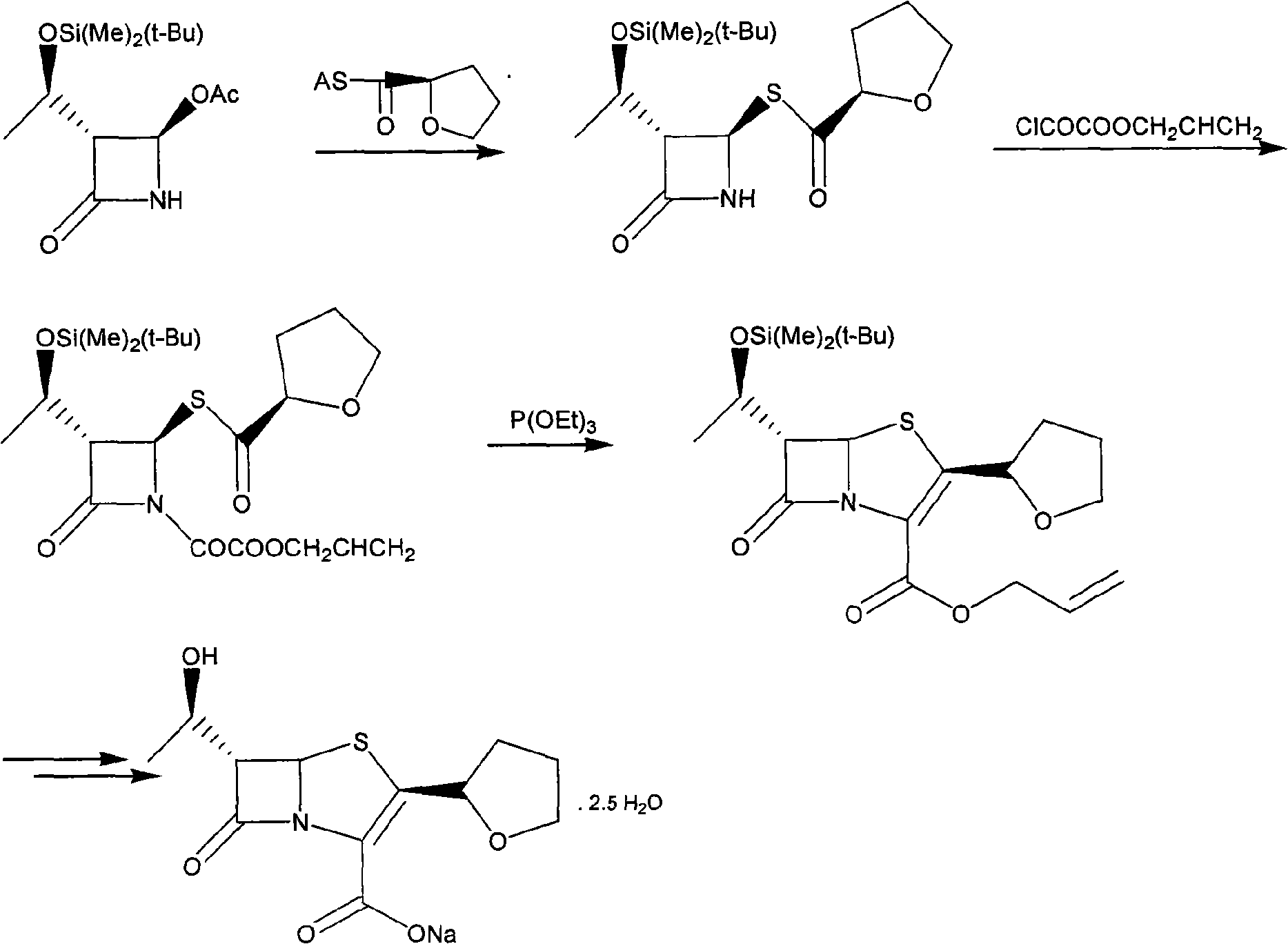
[0024] Example 1: (3R, 4R)-3-[(R)-1-tert-butyldimethylsilyloxyethyl]-4-[(R)-tetrahydrofuran-2-formylthio]nitroheterocycle Synthesis of Butan-2-one (I)
[0025] Dissolve 43.0g (0.120mol) of trityl R-tetrahydrofuran-2-carboxylate and 28.7g (0.10mol) of 4-AA in 100ml of anhydrous tetrahydrofuran, add 7.9g (0.120mol) of sodium sulfide after dissolution, and then add 24g (0.18mol) of anhydrous aluminum trichloride and 10ml of boron trifluoride / diethyl ether solution, stirred and reacted at 40°C for 24 hours, after the reaction was detected by TLC, the reaction solution was cooled to 0°C, and 100ml of saturated sodium bicarbonate was slowly added dropwise Solution, keep the temperature not exceeding 10°C. After the dropwise addition, control the temperature of the water bath to not exceed 40°C to remove tetrahydrofuran by distillation under reduced pressure, add 200ml ethyl acetate to the residue, filter out the solid, separate the organic phase from the filtrate, and then use 100ml...
Embodiment 2
[0026] Example 2: (3R, 4R)-1-allyloxyoxalyl-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4-[(R)-tetrahydrofuran- Synthesis of 2-formylthio]azetidin-2-one (II)
[0027] Dissolve 22.0g (0.061mol) of intermediate I in 100ml of dry dichloromethane, cool to 0°C and dropwise add 14.6g (0.098mol) of allyloxy oxalyl chloride. After the addition is complete, add 14.5 ml of triethylamine, after the addition, keep at 0-5°C for 2 hours. After the reaction is detected by TLC, add 100ml of water to the reaction solution and stir, then remove the water phase, and then use 50ml of saturated sodium bicarbonate solution, 50ml of water, and 50ml of saturated Washed with brine, dried over anhydrous magnesium sulfate and evaporated to dryness to obtain Compound II as a colorless oil, weighing 25.6 g.
Embodiment 3
[0028] Example 3: (5R, 6S)-6-[(R)-1-tert-butyldimethylsiloxyethyl]-2-[(R)-tetrahydrofuran-2-formylthio]penem -Synthesis of propenyl 3-carboxylate (III)
[0029] Dissolve 13g (0.0276mol) of intermediate II in 20ml of xylene, add 11.5g (0.069mol) of triethyl phosphite and 50mg of hydroquinone, reflux for 2 hours, wash the reaction solution with water, dry and evaporate to dryness to obtain Yellow oil, the yellow oil was recrystallized from ethyl acetate / petroleum ether to obtain compound III as a yellow solid with a weight of 7.6 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com