Faropenem sodium powder injection
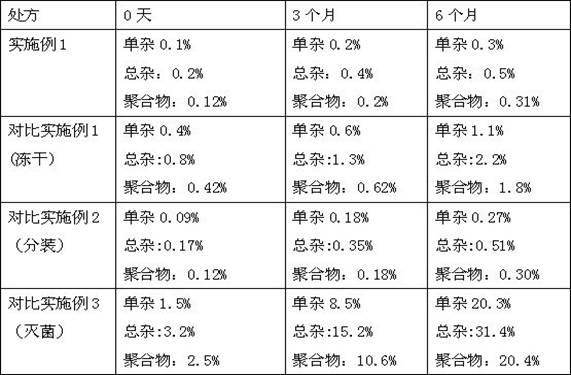
A technology of faropenem sodium powder and faropenem sodium, which is applied in the fields of powder transportation, antibacterial drugs, and active ingredients of heterocyclic compounds, etc., which can solve the problems of difficult control of loading volume, poor solution stability, and long production cycle, etc., and achieve loading volume Stable, good stability, high preparation stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] Preparation of control solution Take an appropriate amount of faropenem sodium reference substance (approximately equivalent to 20mg of faropenem), weigh it accurately, put it in a 100ml measuring bottle, add water to dissolve and dilute to the mark, and shake well.
[0020] Assay Take an appropriate amount of this product (approximately equivalent to 50mg of faropenem), weigh it accurately, put it in a 100ml measuring bottle, add water to dissolve and dilute to the mark, shake well, immediately accurately measure 100μl, inject it into a liquid chromatograph, and use mobile phase A The mobile phase was used for determination, and the chromatogram was recorded; in addition, 100 μl of the control solution was accurately measured, injected into the liquid chromatograph, and the mobile phase B was used as the mobile phase, and the same method was used for determination. According to the peak area calculation by the external standard method, the faropenem-containing po...
Embodiment 1
[0022] Faropenem sodium (calculated as faropenem) 100g
[0023] --------------------------------------
[0024] Make 1000 pieces
[0025] Preparation Process:
[0026] 1. Bottle washing, sterilizing and drying: the vials are first cleaned by an ultrasonic bottle washing machine, then sterilized and dried by a sterilizing dryer at 320°C, and then sent to the filling room for later use.
[0027] 2. Butyl rubber stopper treatment: After the butyl rubber stopper is cleaned by a rubber stopper cleaning machine, it is steam-pressurized at 121°C for 20 minutes, and dried for later use.
[0028] 3. Control the humidity of the packaging environment below 50%, fill each vial with powder containing faropenem sodium, add a rubber stopper, and crimp the cap.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com