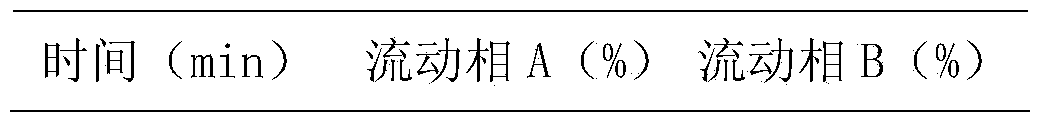
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
78 results about "Cefalexin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat a wide variety of bacterial infections.
Fluorescence immunoassay chromatography test paper for cefalexin residue and preparation of test paper
ActiveCN103901201AEasy to handleThe determination method is simpleMaterial analysisGlass fiberCefalexin
The invention discloses a piece of fluorescence immunoassay chromatography test paper which is rapid, sensitive, simple and convenient to operate to test the residual quantity of cefalexin, and preparation of the test paper. The test paper comprises a sample pad, a binding pad, a nitrocellulose membrane and a water absorbing pad, which are adhered to a substrate in a mutual lap joint manner in sequence, wherein the nitrocellulose membrane is coated with a detection line and a quality control line; the binding pad is coated with a cefalexin monoclonal antibody with a fluorescent mark. The preparation method of the fluorescence immunoassay chromatography test paper for cefalexin residue comprises the following steps: synthesizing cefalexin-ovalbumin coating antigen, preparing a goat anti-mouse immune globulin antibody, coating the cefalexin-ovalbumin coating antigen and the goat anti-mouse immune globulin antibody onthe nitrocellulose membrane to serve as the detection line and the quality control line, preparing a fluorescence nano-particle mark cefalexin monoclonal antibody, then coating glass fiber to serve as the binding pad, sequentially adhering the sample pad, the binding pad, the nitrocellulose membrane and the water absorbing pad to a back plate in the lap joint manner in sequence, cutting the obtained test paper into the width of 4mm, and preserving at normal temperature.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Method for preparing cefalexin
The invention discloses a method for preparing cefalexin. The method comprises the steps of charging a D-2-phenylglycine ester derivative and 7-ADCA (amino desacetoxy cephalosporanic acid) in a molar ratio of (1.15-1.6):1, and adding a catalyst penicillin acylase with amount of 1-2 times of amount of the 7-ADCA for acylation reaction, wherein the coded gene sequence of the penicillin acylase is shown as SEQIDNO:3. The method effectively overcomes reverse reaction during enzyme condensation reaction, so as to greatly reduce the using amount of side chains, avoid the phenomenon that more impurities are generated when high side chains are consumed, and solve the problem that the objective product is difficult to purify; and besides, the 7-ADCA conversion rate can be greatly improved to be up to more than 98%, the product quality is further improved and the production cost is reduced.
Owner:NORTH CHINA PHARMA COMPANY
ELISA detection method for quantitative determination of cephalosporin antibiotic content in animal-derived food
InactiveCN101315372AThe pre-processing process is simpleHigh sensitivityColor/spectral properties measurementsBiotechnologyAntiendomysial antibodies
The invention relates to an enzyme-linked immune detection method for quantitatively detecting the content of spore antibiotic in animal food and belongs to the immune analysis field. The method comprises the steps of adopting the polyclonal antibody of the spore antibiotic with a group-specific antibody as a detection reagent; preparing an enzyme-linked immune detection kit; by using cephalexin as a standard, measurating the concentration of different spore class antibiotics and the relative value of the cephalexin standard through an experiment; and providing the measurated correction factors. The multi-residue detection of the enzyme-linked immune detection kit is expected to be realized. The method has the advantages of simple pretreatment, high sensitivity, high accuracy, etc.
Owner:JIANGNAN UNIV
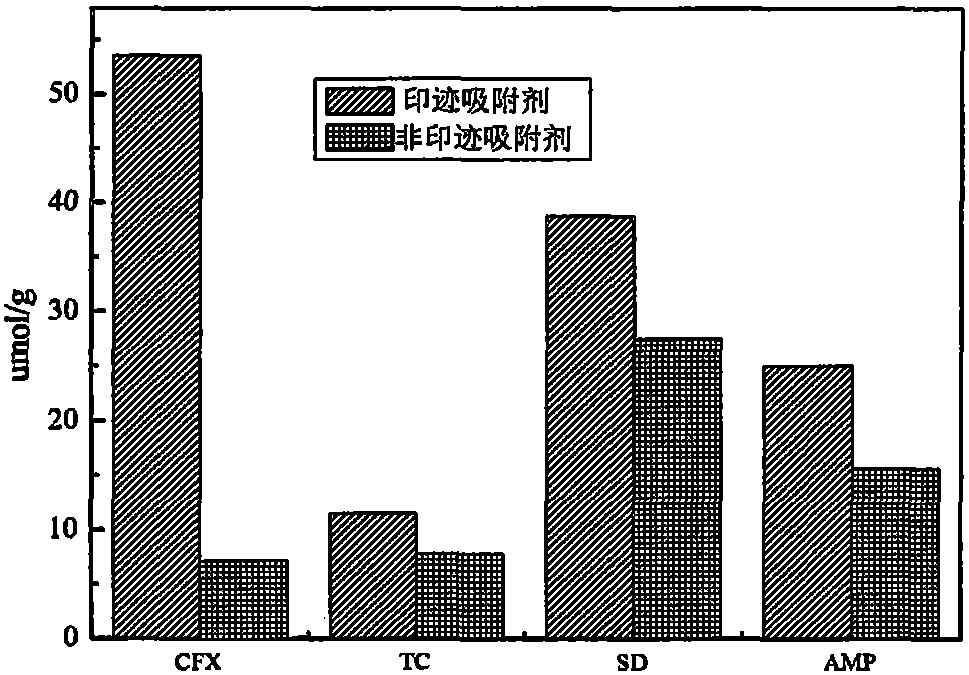
Preparation method for magnetic carbon microsphere surface cephalexin molecular imprinted adsorbent material
ActiveCN105170112AOther chemical processesAlkali metal oxides/hydroxidesHigh absorptionFunctional monomer
The present invention relates to a preparation method for a magnetic carbon microsphere surface cephalexin molecular imprinted adsorbent material. The method comprises: taking a walnut shell as a carbon source to prepare a magnetic carbon microsphere; carrying out ethylene functionalized modification on the surface of the magnetic carbon microsphere by use of 3-(methacryloxy)propyl trimethoxysilane; taking cephalexin (CFX) as a template molecule, methacrylic acid (MAA) as a functional monomer, ethylene alcohol dimethacrylate (EGDMA) and N, N- methylene bisacrylamide (BIS) as a crosslinking agent, and cetane as a porogenic diluent and an organic solvent to prepare a magnetic carbon microsphere surface imprinted adsorbent. The magnetic carbon microsphere surface imprinted adsorbent material prepared by the method disclosed by the invention is low in cost and simple in preparation, and has high recognition, high selectivity, and high separation enrichment capacity on target molecules, and a high absorption speed.
Owner:HENAN UNIV OF URBAN CONSTR
Process for the preparation of dispersible tablets of cephalexin
Owner:RANBAXY LAB LTD
Cefalexin liposome and medicinal composition thereof
InactiveCN101579313AThe encapsulation rate exceedsReduced stabilityAntibacterial agentsOrganic active ingredientsCefalexinMedicine
The invention discloses a cefalexin liposome and a medicinal composition thereof. The cefalexin liposome is mainly prepared from cefalexin, phospholipid, cholesterol and poloxamer 188 in certain proportion.
Owner:HAINAN MEIDA PHARMA
Slow release preparation of cefalexin
InactiveCN101002744AImprove securityImprove effectivenessAntibacterial agentsOrganic active ingredientsCefalexinCurative effect
Owner:刘凤鸣
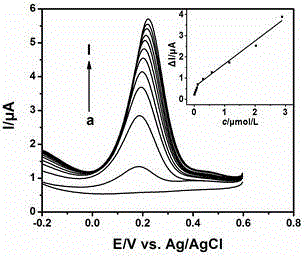
Chemically modified electrode for detecting cefalexin and electrochemical measurement method for cefalexin
ActiveCN104880497AHigh sensitivityLow costMaterial electrochemical variablesCysteine thiolateHydrolysate
The invention relates to the technical field of electrochemical detection and discloses a chemically modified electrode for detecting cefalexin and an electrochemical measurement method for the cefalexin. The chemically modified electrode is prepared through steps that adding colloidal gold solution to a clean gold electrode surface, drying, soaking in hydrochloric acid solution with cysteine, self-assembling at 1 to 6 degrees centigrade, taking out, and using water to wash to clean. The electrochemical detection method includes steps that adding copper sulfate solution to sample solution to be measured, hydrolyzing to obtain hydrolysate, using the chemically modified electrode as a working electrode, and using a square wave voltammetry to measure the concentration of surplus copper ions in the hydrolysate to indirectly measure the content of the cefalexin in the sample. The chemically modified electrode is high in sensitivity, fast, convenient, low in cost and the like for measuring the cefalexin.
Owner:ZHONGKAI UNIV OF AGRI & ENG
Preparation method of immune chromatography test paper for detecting cephalosporin antibiotic
InactiveCN101315364ASuitable for on-site testingSimple and fast operationBiological testingCelluloseReagent strip
The invention provides a method for preparing an immunity chromatographic test strip for testing cephalosporins antibiotics, and relates to the immunity testing field. The test strip is composed of a sample pad, a combined pad, a cellulose nitrate membrane, a water absorbing pad and a PVC backing; the sample pad, the combined pad, the cellulose nitrate membrane and the water absorbing pad are sequentially stuck to the PVC backing; the combined pad is coated with a group-specific anti-cephalosporins antibiotic antibody colloidal gold label of anti-cephalosporins antibiotics; and the cellulose nitrate membrane is sequentially coated with a cefalexin-OVA test line and a goat anti-rabbit IgG quality control line. The method prepares the test trip by assembling the test preparation of the combination of the group-specific antibody of the anti-cephalosporins antibiotics and the chromogenic colloidal gold, and carries out a multi-residue screening test of the cephalosporins antibiotics with low cost and rapidness; and the portable test strip which can be used for the cephalosporins antibiotic residue tests of different samples is the application of the immunity chromatographic analysis method.
Owner:JIANGNAN UNIV
Cefalexin tablet and preparation method thereof
ActiveCN105106166AStable in natureEasy molding processAntibacterial agentsOrganic active ingredientsCefalexinReference product
The invention discloses a cefalexin tablet and a preparation method thereof. A dry granulating process is adopted, and not only is the degradation of beta-lactam rings achieved, but also the problem of low dissolution of products produced through conventional wet granulation is solved. The product is high in stability, a dissolution curve is similar to that of a reference product, and a production process is simple.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Compound cefalexin injection
The invention discloses a method for preparing compound cefalexin injection. The composition comprises the following components in parts by weight: 2-5 parts of cefalexin, 1-2 parts of protostemonine, 1-5 parts of PVC (Polyvinyl Chloride), 1-2 parts of ethyecellulose, 1-3 parts of lecithin, 0.5-1 part of sorbitan fatty acid, 0.5-1 part of polysorbate and 1-3 parts of PEG. The composition disclosed by the invention is high in drug stability, high in solubility and simple in preparation process.
Owner:CHONGQING TAITONG ANIMAL PHARMA
Antibody chip kit and method for detecting residual cephalosporin antibiotics in food
The invention discloses an antibody chip kit for detecting residual cephalosporin antibiotics in food, and belongs to the technical field of the drug residue detection. The kit comprises a chip, an antibody, a second antibody marked by Cy3, and an extraction reagent, the antibody is composed of a cefalexin monoclonal antibody and a ceftiofur monoclonal antibody, and the cefalexin monoclonal antibody is secreted by hybridoma 3A6 with a preservation number of CCTCC NO: C201340; the ceftiofur monoclonal antibody is secreted by hybridoma 4D5 with a preservation number of CCTCC NO: C201341, and the chip fixes cefalexin coating antigen and cefalexin coating antigen. The invention further discloses an antibody chip method for detecting residual cephalosporin antibiotics in food, the method can be used for simultaneously detecting 8 kinds of cephalosporins, and has the advantages of high accuracy, high precision, high efficiency, etc.
Owner:HUAZHONG AGRI UNIV
Method for improving antibacterial activity of cephalexin through synergy of nanometer copper oxide
InactiveCN103933066ALower doseGood curative effectAntibacterial agentsOrganic active ingredientsEscherichia coliCefalexin
The invention specifically relates to a method for improving antibacterial activity of cephalexin through synergy of nanometer copper oxide, which belongs to the technical field of biological medicine. According to the method, a nanometer copper oxide inorganic nano-material is added into cephalexin so as to improve antibacterial activity on Escherichia coli. According to results of antibacterial experiments, it is found that nanometer copper oxide has a good synergistic effect on antibacterial activity of cephalexin; drug effects produced in combined usage of nanometer copper oxide and cephalexin are far better than drug effects produced in separate usage of nanometer copper oxide and cephalexin. The method has the advantages of easy availability of raw materials, low cost and simple operation.
Owner:TONGJI UNIV
Resistant starch content measuring method for rice
InactiveCN102533938AInhibition of fermentationDoes not affect activityMicrobiological testing/measurementCefalexinPenicillin
The invention provides an effective method for accurately and objectively measuring resistant starch content in rice by adding different types of antibiotics into starch hydrolyzate to discuss the effect of the antibiotics on starch hydrolysis and influence on a measuring result of the resistant starch content. By comparing the influences of the antibiotics on the starch hydrolysis effect and the resistant starch content of a detected sample, a result shows that the fermentation effect of miscellaneous bacteria in the starch hydrolysis process can be effectively suppressed by the antibiotics without affecting the activity of amylase; the most effective antibiotic is mixed antibiotic (four antibiotics, namely 0.40-0.52 mg / mL of penicillin, 15,000-17,000 UI / mL of gentamicin sulphate, 0.8-1.2 mg / mL of cefalexin and 0.85-1.30 mg / mL of erythromycin lactobionate, are mixed according to a proportion of 1.2:1:1.2:1 ); and the best dose is 35 microlitre.
Owner:SHANGHAI ACAD OF AGRI SCI
Method for concentrating cefalexin through membrane method and enzyme concentration method
ActiveCN103788115AReduce energy consumptionEasy to automateOrganic chemistryCefalexinMembrane method
The invention relates to a method for concentrating cefalexin, in particular to the method for concentrating cefalexin through a membrane method and an enzyme concentration method. The method mainly solves the problem that the environment is polluted and energy dissipation is high due to the fact that the existing method for concentrating the cefalexin needs to use the extracting solvent. The method comprises the steps that 1, cefalexin mother solution prepared by the enzyme concentration method is stored into a mother solution storing tank; 2, the cefalexin mother solution passes through a refined filtration unit; 3, the cefalexin mother solution passes through an ultrafiltration membrane unit; 4, the cefalexin mother solution passes through a nanofiltration membrane unit unit; 5, the cefalexin mother solution is conveyed to a reverse osmosis membrane unit, and then the concentrated cefalexin is obtained. By means of the method, the original cefalexin solution can be concentrated more than 10 times, energy dissipation is low, alternative use of the extracting solvent is greatly reduced, harm to human bodies is reduced, and pollution to the environment is avoided. The method is used for concentrating the cefalexin.
Owner:HARBIN INST OF TECH
Cefalexin capsules and preparation method
InactiveCN110613697AMeet the needs of fillingImprove liquidityAntibacterial agentsOrganic active ingredientsCefalexinCarboxymethyl cellulose
The invention discloses cefalexin capsules and a preparation method. The capsules comprise the following components in percentage: 90.6% of cefalexin, 6.4-8.3% of microcrystalline cellulose, 0.6-1.6%of carboxymethyl cellulose sodium and 0.5-1.4% of magnesium stearate, wherein the sum of weight percentages of the components is 100%. The preparation process of the capsules comprises the following steps: performing pre-mixing for 16 minutes at a frequency of 10Hz, performing pelletizing, performing batch mixing for 10 minutes at a frequency of 10Hz, and performing capsule filling, wherein pelletizing conditions are that the stirring speed is 25rpm, the spiral rotation speed is 55-85rpm, the rotation speed of rollers is 16rpm, the gap of the rollers is 0.9-1.3mm, the rotation speed of granuleshaping is 80rpm, the pressure of the rollers is 100-130bar, the aperture of a granule shaping mesh size is 1.0mm, and the vacuum degree is 0.1bar. The flowability and the dissolution stability of the capsules disclosed by the invention can be improved, and the method for preparing the capsules is low in energy consumption and green and environment-friendly.
Owner:长春迪瑞制药有限公司
Method for recycling cefalexin from enzyme synthesis type cefalexin mother liquor
The invention relates to the field of recycling of cefalexin, in particular to a method for recycling cefalexin from an enzyme synthesis type cefalexin mother liquor. The method comprises the following steps of (1) separating the acidified cefalexin mother liquor by a film, so as to obtain a concentrated solution of the cefalexin; (2) enabling the obtained concentrated solution of the cefalexin instep (1) to be in contact with an alkaline regulator, and crystallizing, so as to obtain a crystal slurry of the cefalexin. The cefalexin obtained by the method has the characteristics that the purity is high, and the recycling rate is high; the preparation technology is simple, the cost is lower, and more environment-friendly effect is realized; under more preferable conditions, such as the stirring condition, the crystal is separated out, and the stirring phase is set to the phase 2 or phase 3, so that the granularity of the obtained cefalexin crystal is more uniform.
Owner:SHANXI WEIQIDA PHARMA IND
High-throughput screening method of beta-lactam antibiotic synthetase
ActiveCN103667418AJudging the size of the transformation abilityEasy to operateMicrobiological testing/measurementEnzymatic synthesisHigh-Throughput Screening Methods
The invention discloses a high-throughput screening method of beta-lactam antibiotic synthetase. The method comprises the steps as follows: a synthetic liquid is added in plate holes of an ELISA (enzyme linked immunosorbent assay) plate, then a to-be-tested enzyme sample is added, the mixture reacts in a microplate oscillation reactor at the temperature of 10-30 DEG C for 0.1-3 hours, in the reaction process, the ELISA plate is taken out every 1-30 minutes, a PDAB (paradimethylaminobenzaldehyde) color development solution is added for color development, and a light absorption value in the position of 415 nm is measured through ELISA; according to variation trend of the light absorption values and a minimum value of the light absorption values, enzyme with high activity and good synthesis capacity is screened out; the synthetic liquid is prepared by dissolving mother nucleuses and side chains of beta-lactam antibiotics into a KPB solution; and the to-be-tested enzyme sample is beta-lactam antibiotic synthetase. The high-throughput screening method of beta-lactam antibiotic synthetase can be used for screening enzymatic synthesis enzyme of lactam antibiotics such as amoxicillin, cefalexin, cefaclor, cephadroxil, ampicillin and the like, has the characteristics of simplicity in operation, low cost, accurate detection and the like, and is suitable for large-scale high-throughput screening of synthesis enzyme.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Cefalexin capsule
InactiveCN104644610AImprove stabilityPromote dissolutionAntibacterial agentsOrganic active ingredientsCefalexinPolyethylene glycol
The invention relates to a cefalexin capsule which comprises cefalexin and auxiliary materials. The auxiliary materials comprise filler, a disintegrating agent, a surfactant, a stabilizer, a lubricant and a glidant, wherein the surfactant is one or more of lauryl sodium sulfate, polysorbate-80, poloxamer, polyethylene glycol tricaprylin and polyethylene glycol stearin. The cefalexin capsule disclosed by the invention is short in disintegration time, and drugs dissolve out rapidly.
Owner:南京多宝生物科技有限公司
A kind of method for preparing cephalexin
The invention discloses a method for preparing cefalexin. The method comprises the steps of charging a D-2-phenylglycine ester derivative and 7-ADCA (amino desacetoxy cephalosporanic acid) in a molar ratio of (1.15-1.6):1, and adding a catalyst penicillin acylase with amount of 1-2 times of amount of the 7-ADCA for acylation reaction, wherein the coded gene sequence of the penicillin acylase is shown as SEQIDNO:3. The method effectively overcomes reverse reaction during enzyme condensation reaction, so as to greatly reduce the using amount of side chains, avoid the phenomenon that more impurities are generated when high side chains are consumed, and solve the problem that the objective product is difficult to purify; and besides, the 7-ADCA conversion rate can be greatly improved to be up to more than 98%, the product quality is further improved and the production cost is reduced.
Owner:NORTH CHINA PHARMA COMPANY
Monoclonal antibody, enzyme-linked immunosorbent assay method and kit for detecting cefalexin, cefadroxil and cefradine
ActiveCN104558189AHigh recognition sensitivityExcellent recognition sensitivityMicroorganism based processesTissue cultureCefalexinCefradine
The invention discloses a specific monoclonal antibody capable of distinguishing cefalexin, cefadroxil and cefradine, and an enzyme-linked immunosorbent assay method and kit for detecting the cefalexin, the cefadroxil and the cefradine. According to the invention, the monoclonal antibody is secreted by a hybridoma cell strain 3A6 of which the preservation number is CCTCC No.C201340. Compared with the prior art, the monoclonal antibody, prepared by the invention, can be used for distinguishing the cefalexin, the cefadroxil and the cefradine at the same time. The enzyme-linked immunosorbent assay method and kit disclosed by the invention have the advantages of high detection efficiency, high sensitivity, high precision, high accuracy and the like.
Owner:HUAZHONG AGRI UNIV
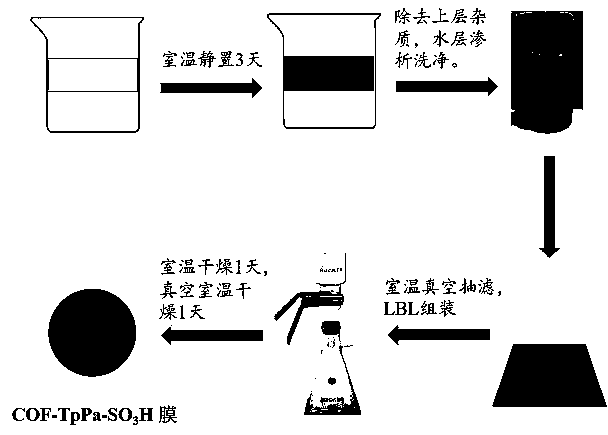
Research on pure two-dimensional covalent organic framework material membrane for removing liquid-phase antibiotics
The invention discloses a COF-TpPa-SO3H membrane with efficient nanofiltration separation effect on the antibiotic cefalexin, belonging to the field of novel membrane materials. A preparation method for the membrane comprises the following steps: firstly, acquiring a great number of COF nanosheets with a high area-thickness ratio by using the hydrophilicity of sulfonate radicals in a molecular structure through a simple interfacial polymerization method, and then carrying out vacuum filtration in a layer-by-layer stacking manner to obtain the COF-TpPa-SO3H membrane with high mechanical strength and high chemical stability. The removal rate of the pure COF-TpPa-SO3H membrane prepared by vacuum filtration at normal temperature on the antibiotic cefalexin is as high as 95%, with an error range being + / - 0.6%; and a water flux is up to 800 L.m<-2>.H<-1>.MPa<-1>, and the highest water flux of the obtained pure COF membrane is 2500 L.m<-2>.H<-1>.MPa<-1> by changing the deposition mass of COF. The water flux of the COF membrane prepared by the method is higher than the water flux of a currently researched GO nanofiltration membrane and other ultrathin two-dimensional material membranes, and the COF membrane has good application potential in antibiotic removal.
Owner:BEIJING UNIV OF CHEM TECH
Compound cefalexin capsule and preparation method thereof
InactiveCN104606166ASmall molecular weightGood water solubilityAntibacterial agentsOrganic active ingredientsCefalexinPOLACRILIN POTASSIUM
The invention relates to a compound cefalexin capsule and a preparation method thereof. The compound cefalexin capsule is prepared from the following raw materials in parts by weight: 220-270 parts of cefalexin, 2-20 parts of ambroxol, 25-125 parts of polacrilin potassium, 50-100 parts of chito-oligosaccharide, 5-50 parts of talc and 10-75 parts of starch. The compound cefalexin capsule is reasonable in formula, high in drug dissolution, simple in preparation method without pollution and remarkable in efficacy without adverse reaction.
Owner:SHANGHAI HUAYUAN ANHUI RENJI PHARMA
Beta-lactam antibiotic multi-template molecularly imprinted magnetic composite material, preparation method and application thereof
ActiveCN112552547AHigh adsorption selectivityImprove adsorption capacityOther chemical processesCefazolin SodiumAdsorption selectivity
The invention relates to the technical field of adsorption materials, in particular to a beta-lactam antibiotic multi-template molecularly imprinted magnetic composite material, a preparation method and application thereof. The invention discloses a beta-lactam antibiotic multi-template molecularly imprinted magnetic composite material, which has strong adsorption selectivity, can specifically andquickly adsorb cefalexin, cefazolin sodium, penicillin G sodium, oxacillin sodium and amoxicillin, and has large adsorption capacity. The composite material also shows superparamagnetism, solid-liquid separation can be rapidly and effectively realized, and the working efficiency is improved. The composite material can be combined with high performance liquid chromatography to be used for detecting the beta-lactam antibiotics in a complex environment water sample, and the beta-lactam antibiotics in the complex environment water sample can be effectively detected and separated.
Owner:GUANGDONG UNIV OF TECH
Application of V2O5/CeO2 composite nano-material in degradation of cefalexin-containing wastewater
InactiveCN106315818AImprove efficiencyObvious oxidation catalysisWater treatment compoundsNature of treatment waterWastewaterAntibiotic Y
The invention belongs to the technical field of treatment of antibiotics and particularly discloses an application of a V2O5 / CeO2 composite nano-material in degradation of cefalexin-containing wastewater. The application comprises the steps of adjusting the initial concentration of cefalexin in the wastewater to 20mg / L to 120mg / L, adjusting the pH value of the wastewater to 2 to 7, then, adding 5mg to 30mg of the V2O5 / CeO2 composite nano-material into per 20mL of wastewater, and finally, carrying out isothermal oscillation for 1 to 6 hours at the temperature of 25 DEG C to 70 DEG C. The V2O5 / CeO2 composite nano-material has an obvious oxidative catalysis action on cefalexin antibiotic, the degradation effect is optimal under the conditions that the concentration of the wastewater is 40mg / L, the pH of the wastewater is 3, the dosage is 20mg, the temperature is 50 DEG C, and the oscillation time is 2 hours, and the removal efficiency can reach 64.54% to 69.13%. Under the optimal degradation conditions of the V2O5 / CeO2 composite nano-material, the V2O5 / CeO2 composite nano-material has better effect and higher efficiency to the degradation of the cefalexin antibiotic compared with pure nano-CeO2.
Owner:ZHENGZHOU UNIVERSITY OF AERONAUTICS
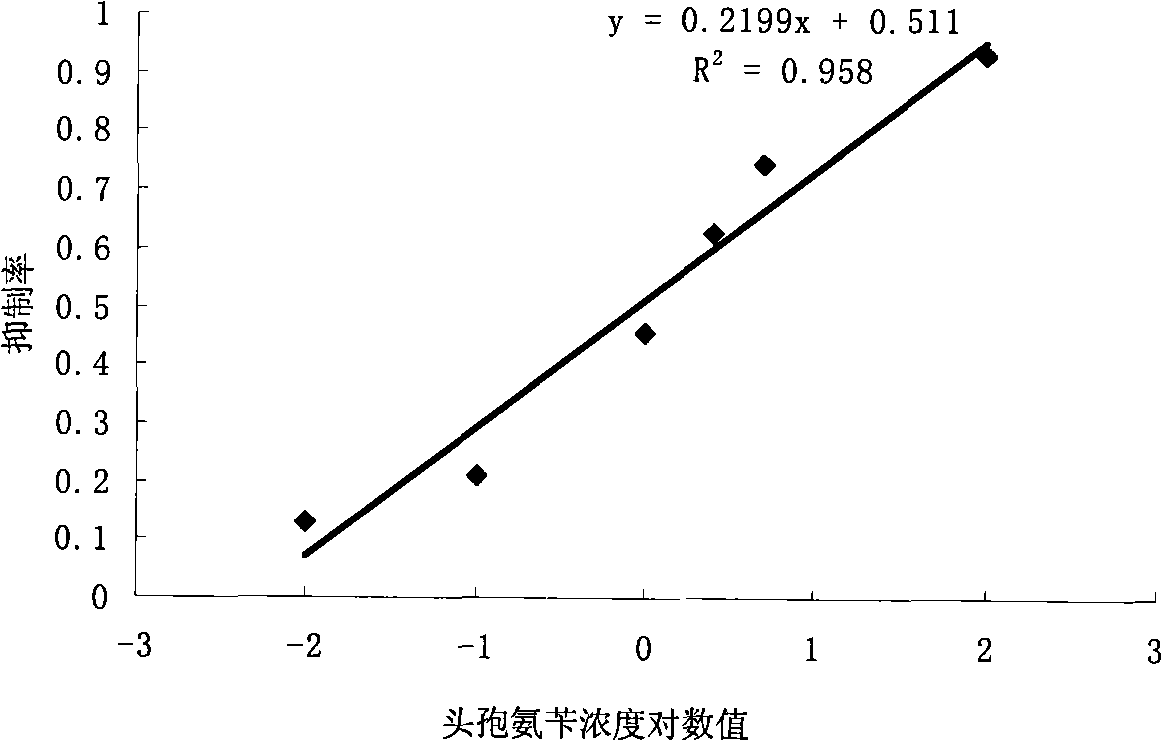
Preparation method of ratiometric fluorescent probe for detecting cefalexin residues and fluorescent probe prepared by preparation method and application thereof
ActiveCN111647407ASimple preparation processRaw materials are easy to getFluorescence/phosphorescenceLuminescent compositionsFluoProbesCefalexin
The invention discloses a preparation method of a ratiometric fluorescent probe for detecting cefalexin residues, the fluorescent probe prepared by the preparation method and application. The preparation method comprises the following steps of: preparing carbon quantum dots, preparing cadmium telluride quantum dots, preparing silicon-coated carbon dots and synthesizing the fluorescent probe. The preparation process is simple, raw materials are easily available, the cost is low, and large-scale production is easy. The ratiometric fluorescent probe for detecting the residual cefalexin is high insensitivity, has high selectivity and specificity, only has fluorescence response to the cefalexin, and has no response to other antibiotics. The ratiometric fluorescent probe for detecting the cefalexin residues, prepared by the method, can be used for effectively and quantitatively detecting the cefalexin residues, is more efficient, cost-saving and more accurate, and has important significancein controlling food safety and protecting human health.
Owner:NANJING NORMAL UNIVERSITY
Child cefalexin composition
The invention discloses a child cefalexin composition, which relates to the technical field of medicinal preparations. A prescription of the composition consists of the following components in percentage by weight: 25 percent of cephalexin, 25-75 percent of mannitol, 2-4 percent of gelatin and 0.1-0.15 percent of sucralose. The invention further provides a child cefalexin composition freeze-driven oral disintegrated tablet containing the child cefalexin composition. The freeze-driven oral disintegrated tablet has the advantages of simple composition, no need of water for taking, no need of chewing, disintegration time of not more than 2 seconds in the oral cavity of a human being, quick response, small residues in intestinal canals, full absorption, small side effects, good mouthfeel and particular suitability for infant patients.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Method for producing cephalexin
InactiveUS20050084925A1Raise the ratioBetter technical application propertyOrganic chemistryFermentationOrganic chemistryCopolymer
The invention relates to a process for the preparation of cephalexin with the aid of a penicillin amidase immobilized on a crosslinked hydrophilic copolymer which has binding activity for ligands having nucleophilic groups and is in bead form.
Owner:ROEHM GMBH & CO KG
Composite medicine for treating toothache quickly and effectively
InactiveCN101185758AGood effectSafe and reliable medicineAntisepticsDigestive systemDiseasePhenylbutazone
The invention particularly relates to a drug for rapid-acting treatment of toothache. The drug integrates various causes of toothache disease and carries out the compounding of antibiotic anti-inflammatory drug, anti-spirit sensitivity drug, anti-anaerobic infection drug, anti-inflammatory analgesic drug and other various western patent drugs, and the compound drug mainly consists of drug cefalexin or other antibiotic anti-inflammatory drug, tiapride hydrochloride, metronidazole or other anti-anaerobic infection drug, indometacin enteric-coated tablet, phenylbutazone and prednisone hydrochloride sequentially according to the mixing ratio by weight parts. The drug of the invention is rapid in acting for the toothache disease which is formed by various causes, and generally the drug can act in 1 to 1.5 hours after the drug administration, the pain can be stopped after one week of the drug administration, and the effect is significant; the effective rate of the rapid pain stopping is 98 percent and the cure rate is 96.5 percent according to the statistics; the used drugs of the invention are the patent drugs which are proved by the state and the efficacies of the drugs are safe and reliable; the administration is convenient, which is easy to be accepted by patients.
Owner:严晓丽
Combination comprising zidovudine and an antimicrobial compound
PendingCN112218633AAntibacterial agentsHeterocyclic compound active ingredientsCefalexinPharmaceutical medicine
The invention provides a combination comprising zidovudine or a pharmaceutically acceptable derivative thereof and an antimicrobial compound selected from nitrofurantoin, mecillinam, fosfomycin, cephalexin and faropenem, or a pharmaceutically acceptable derivative or prodrug thereof. These combinations are particularly useful for the treatment of microbial infections.
Owner:HELPERBY THERAPEUTICS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com