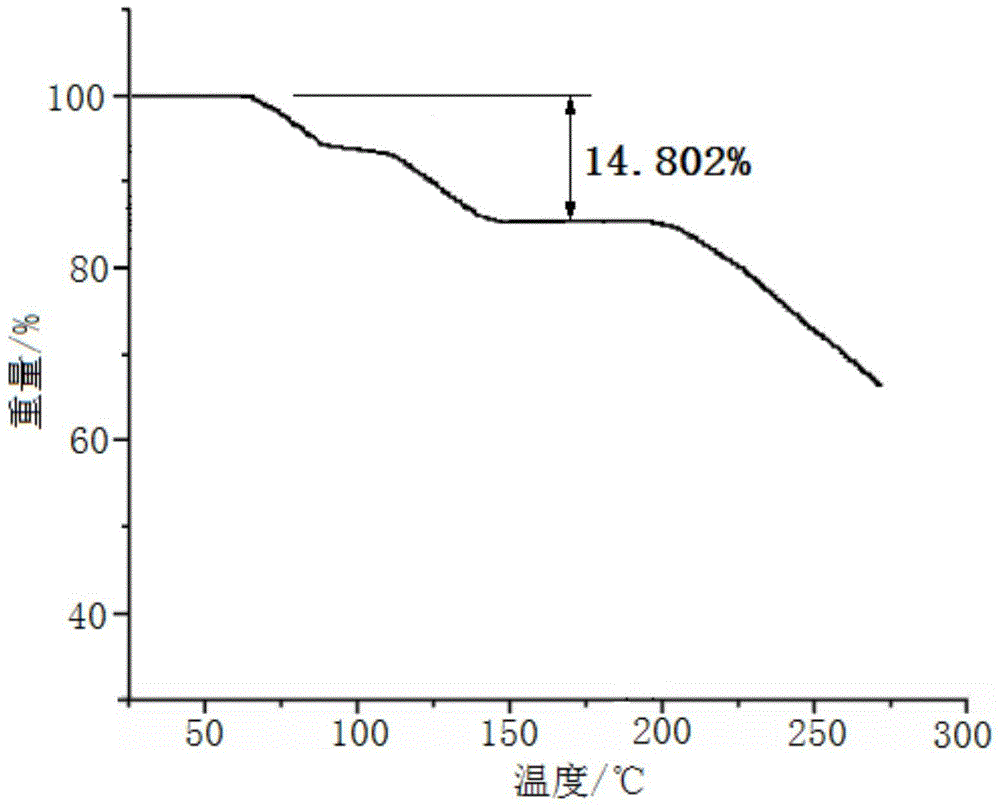
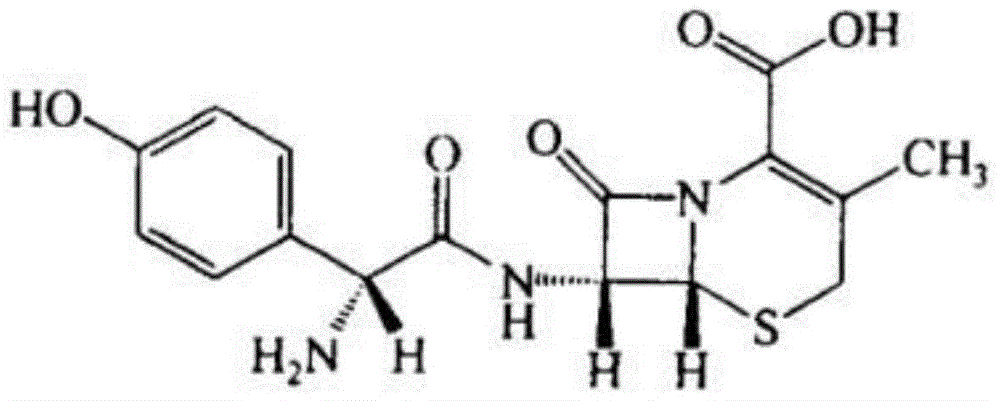
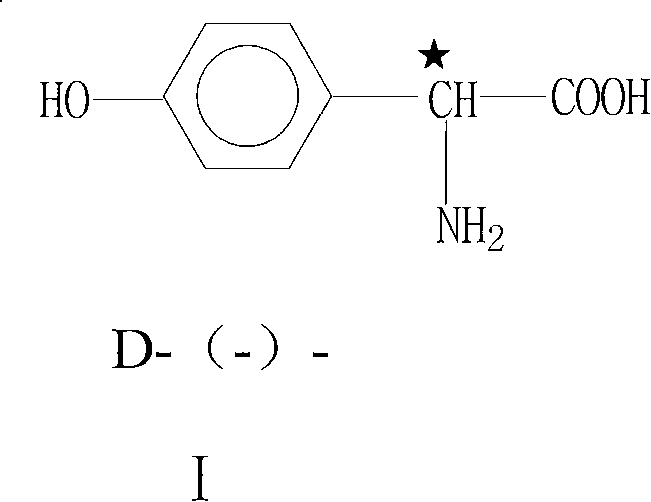
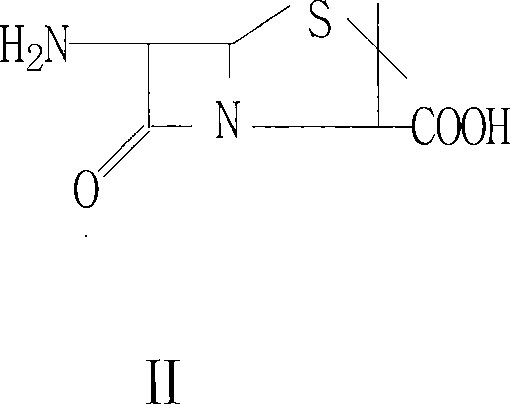
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Cefadroxil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
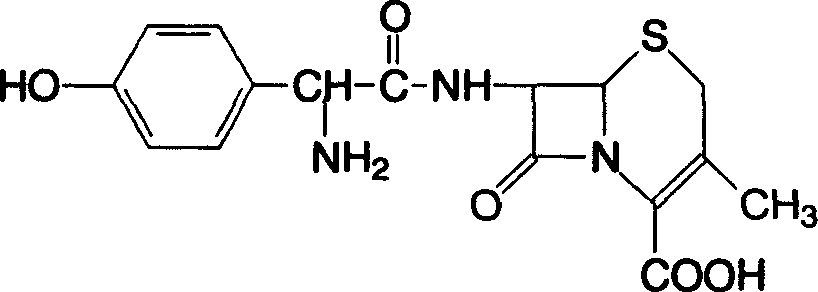
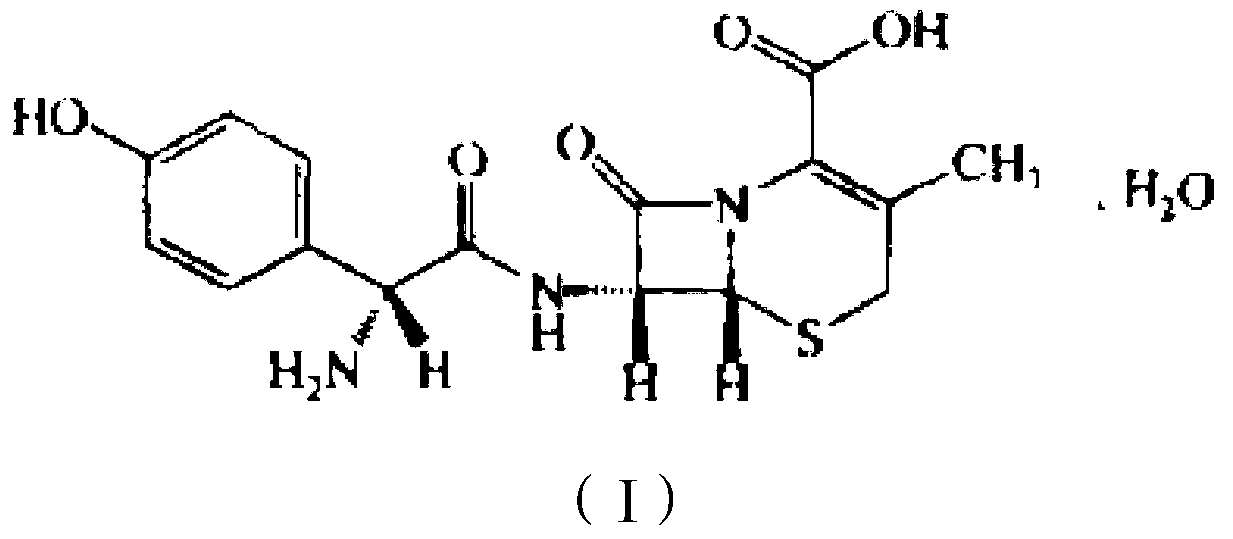
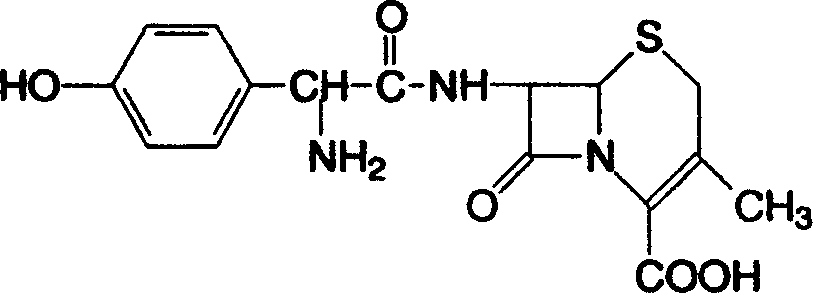
This medication is a cephalosporin-type antibiotic used to treat a wide variety of bacterial infections (e.g., strep throat, skin and urinary tract infections).
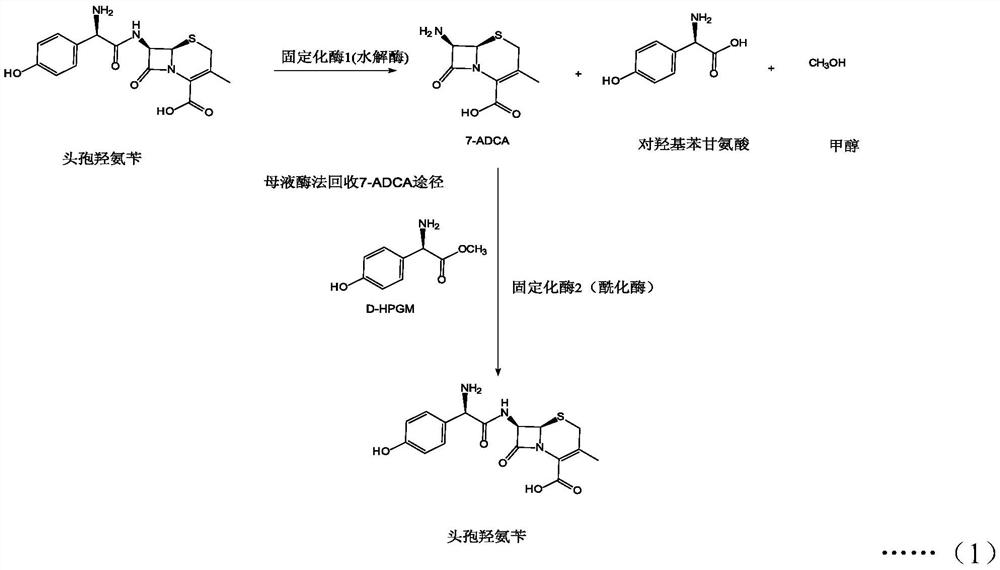
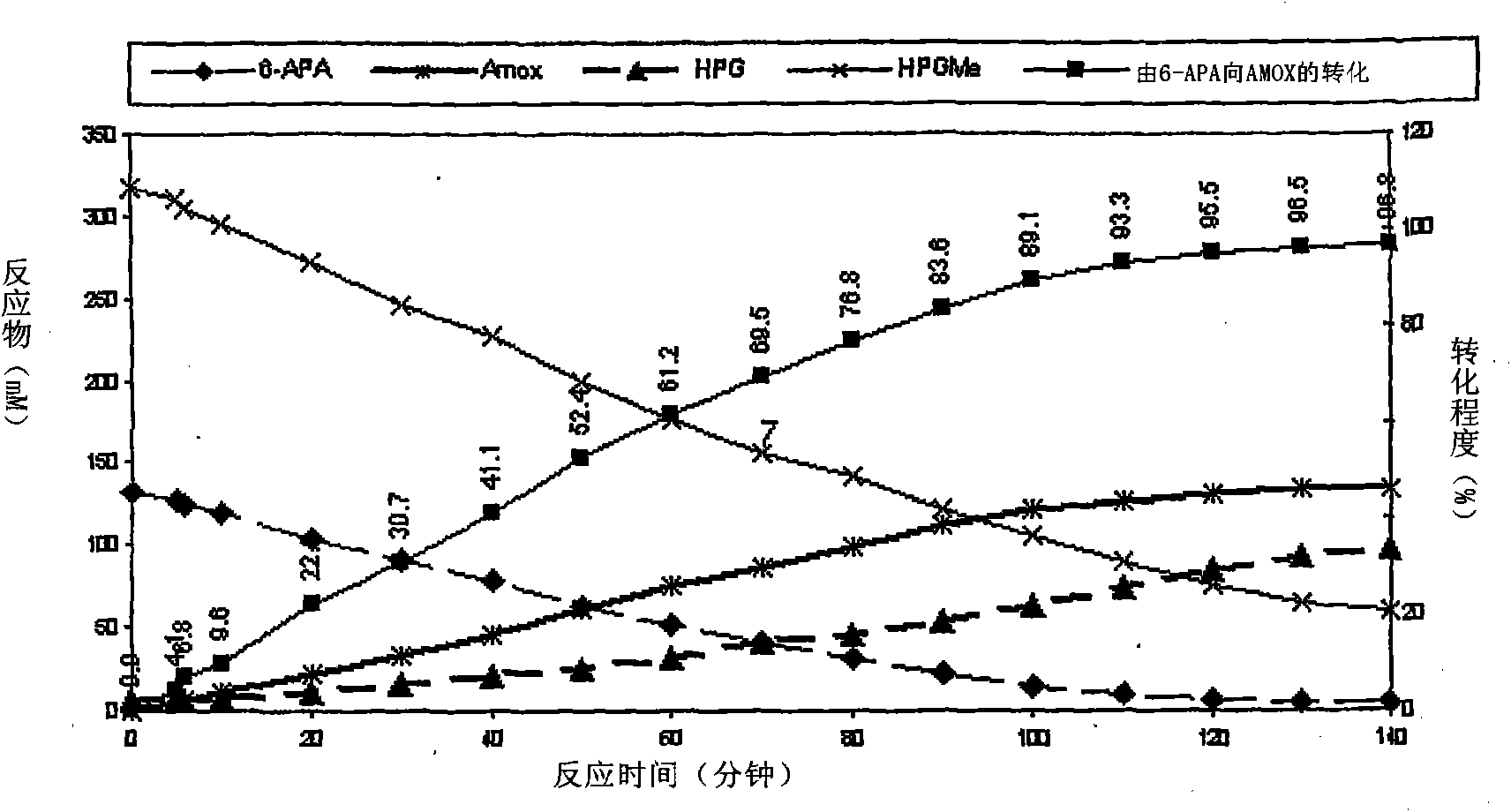
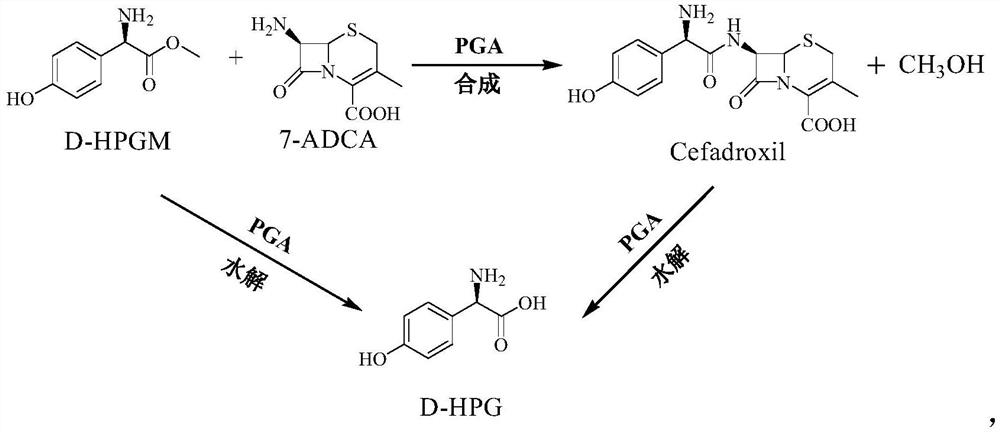
Method of synthesizing cefadroxil by enzyme process
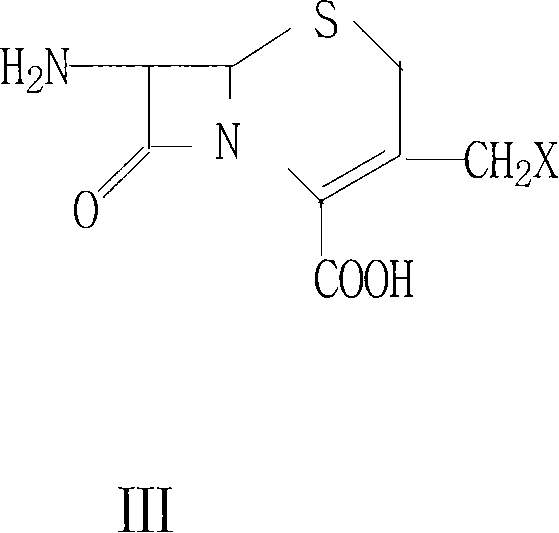
The invention relates to a method of synthesizing cefadroxil by an enzyme process. The method comprises the following steps of: by taking 7-ADCA as an initial raw material, performing a reaction on D-tyrosine methyl ester or D-p-hydroxyl phenylglycine ethyl ester and 7-ADCA in water by directly inputting the solid in the presence of penicillin acylase at 10-25 DEG C; after reaction, separating a cefadroxil coarse product and enzyme reaction mother liquor; further purifying the cefadroxil coarse product to obtain a white cefadroxil product; and adding beta-naphthol or 2,7-dioxynaphthalene into the enzyme reaction mother liquor to obtain a cefadroxil compound. Cefadroxil can be further treated and recovered from the cefadroxil compound, so that the recovery rate of cefadroxil is increased. The product obtained by the method is high in yield and purity. The product is white in appearance, multi-step reaction of a chemical process and various solvents and auxiliary materials are not needed, and green synthesis of cefadroxil is realized.
Owner:苏州盛达药业有限公司 +1
Cefadroxil dry suspension and preparing method
ActiveCN1682739AImprove solubilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsSolubilityIrritation
The present invention relates to dry cefadroxil suspension and its preparation process, belongs to the field of medicine technology, and is for raising the water solubility and bioavailability of cefadroxil. The dry cefadroxil suspension consists of cefadroxil and supplementary material comprising including agent, water soluble stuffing, distintegrating agent, suspension assistant and adhesive. The dry cefadroxil suspension has the features of high medicine solubility, high medicine bioavailability, high chemical stability, good taste, convenient taking, high patient's compliance, full absorption in gastrointestinal tract, less irritation to gastrointestinal tract of medicine, etc.
Owner:CSPC OUYI PHARM CO LTD
Cefadroxil compound and pharmaceutical composition comprising same
ActiveCN104447795AUniform particle sizePrevent coalescenceOrganic active ingredientsOrganic chemistrySolubilityWater soluble
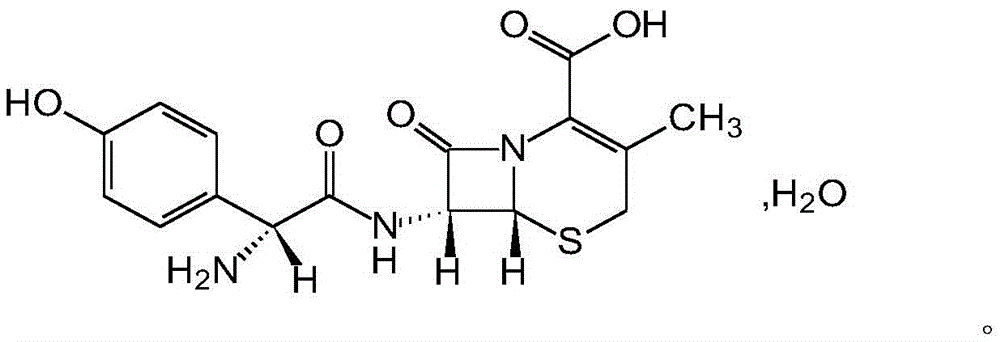
The invention belongs to the technical field of medicines, and particularly relates to a cefadroxil compound and pharmaceutical composition comprising the same. The cefadroxil compound is a cefadroxil hydrate with the molecular formula of C16H17N3O5S*3.5H2O, and the structural formula is shown in the specification. Various pharmaceutically-acceptable dosage forms can be prepared from the pharmaceutical composition comprising the cefadroxil compound, and chewable tablets and capsules are preferred. The cefadroxil compound has the advantage of capability of significantly improving the water solubility and the hygroscopicity of cefadroxil compounds.
Owner:金鸿药业股份有限公司
Cefadroxil oral disintegrant tablet, and its prepn. method
ActiveCN1618428APromote dissolutionQuickly exert the therapeutic effect of the whole bodyAntibacterial agentsOrganic active ingredientsWater solubleExcipient
An oral disintegrating tablet of dracefal contains dracefal and the medicinal excipient (water-soluble filler, disintegrant, lubricant, wetting agent, or adhesive). Its preparing process is also disclosed. Its advantages are high disintegrating speed, quickly taking its effect, less residue and low by-effect.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Cefadroxil tablet and preparation method thereof
ActiveCN105534937AExcellent pharmaceutical propertiesImprove stabilityAntibacterial agentsOrganic active ingredientsCarboxymethyl starchHigh humidity
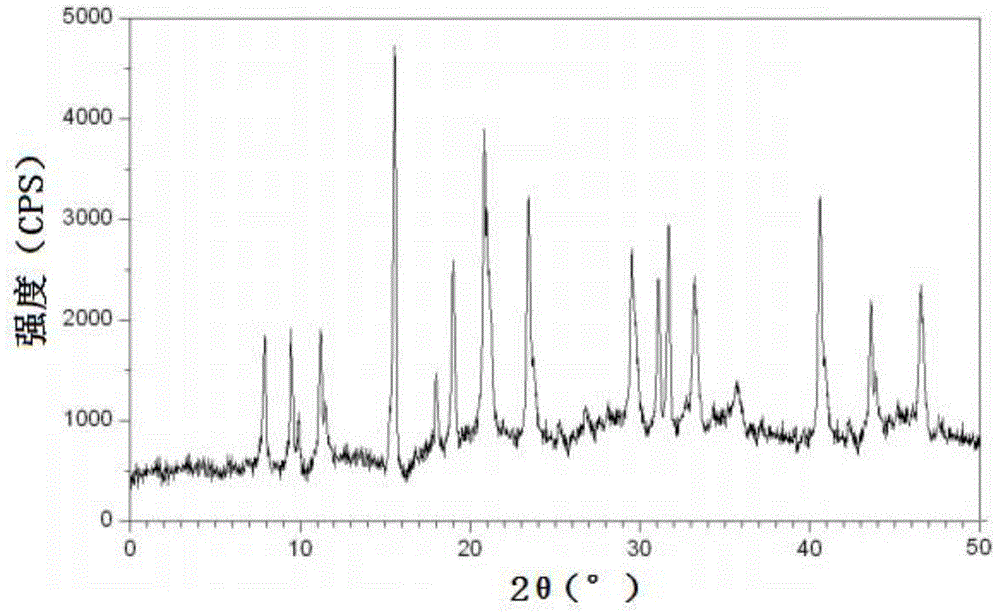
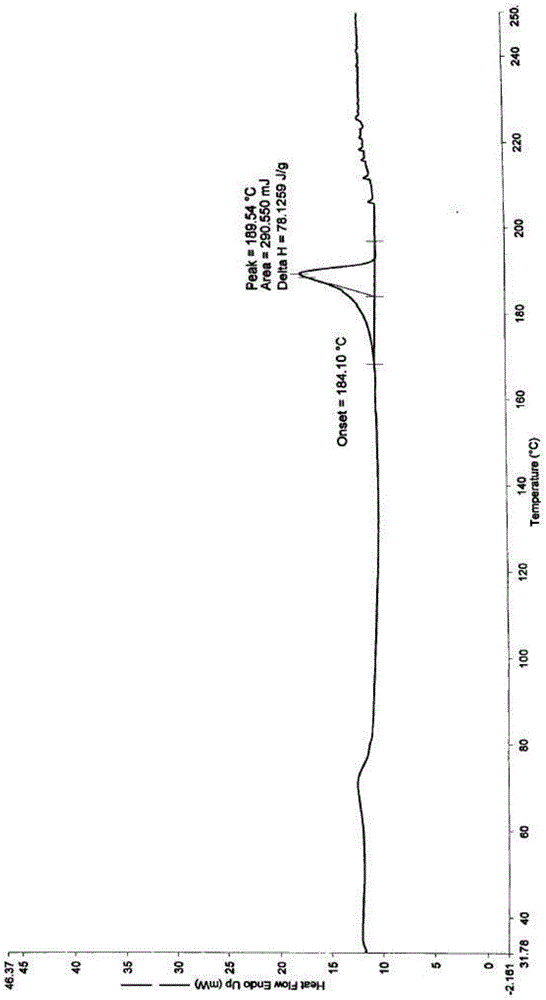
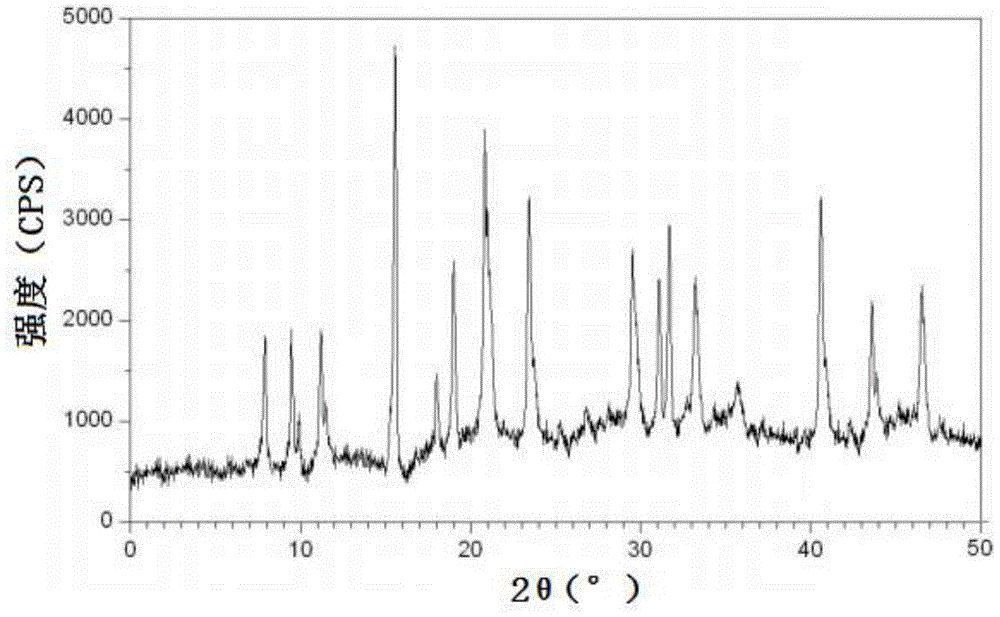
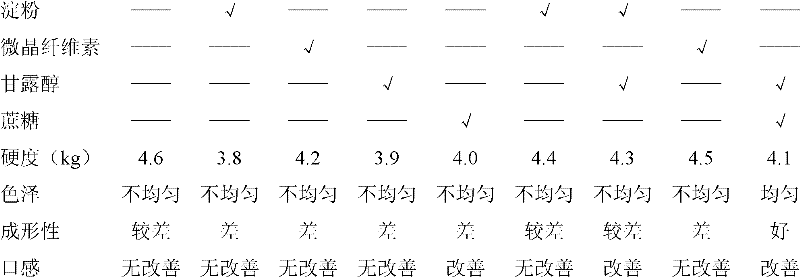
The invention relates to a cefadroxil tablet and a preparation method thereof, and belongs to the technical field of medicines. The cefadroxil tablet comprises the following components in parts by weight: 125-500 parts of cefadroxil, 35-60 parts of starch, 40-120 parts of 10% starch paste, 2-6 parts of magnesium stearate, and 3-12 parts of sodium carboxymethyl starch, wherein X-ray powder diffraction pattern of cefadroxil has characteristic peaks at 2theta of 4.27+ / -0.2 degree, 8.52+ / -0.2 degree and the like; and the differential scanning calorimetry pattern of cefadroxil has characteristic peaks at 189-190 DEG C. Compared with the prior art, the impurity content is not significantly changed under the conditions of high temperature, high humidity and strong light, and the stability is greatly improved; pharmacokinetic experiment results show that the bioavailability of the cefadroxil tablet is significantly improved compared with that of commercially available cefadroxil tablets.
Owner:CSPC OUYI PHARM CO LTD
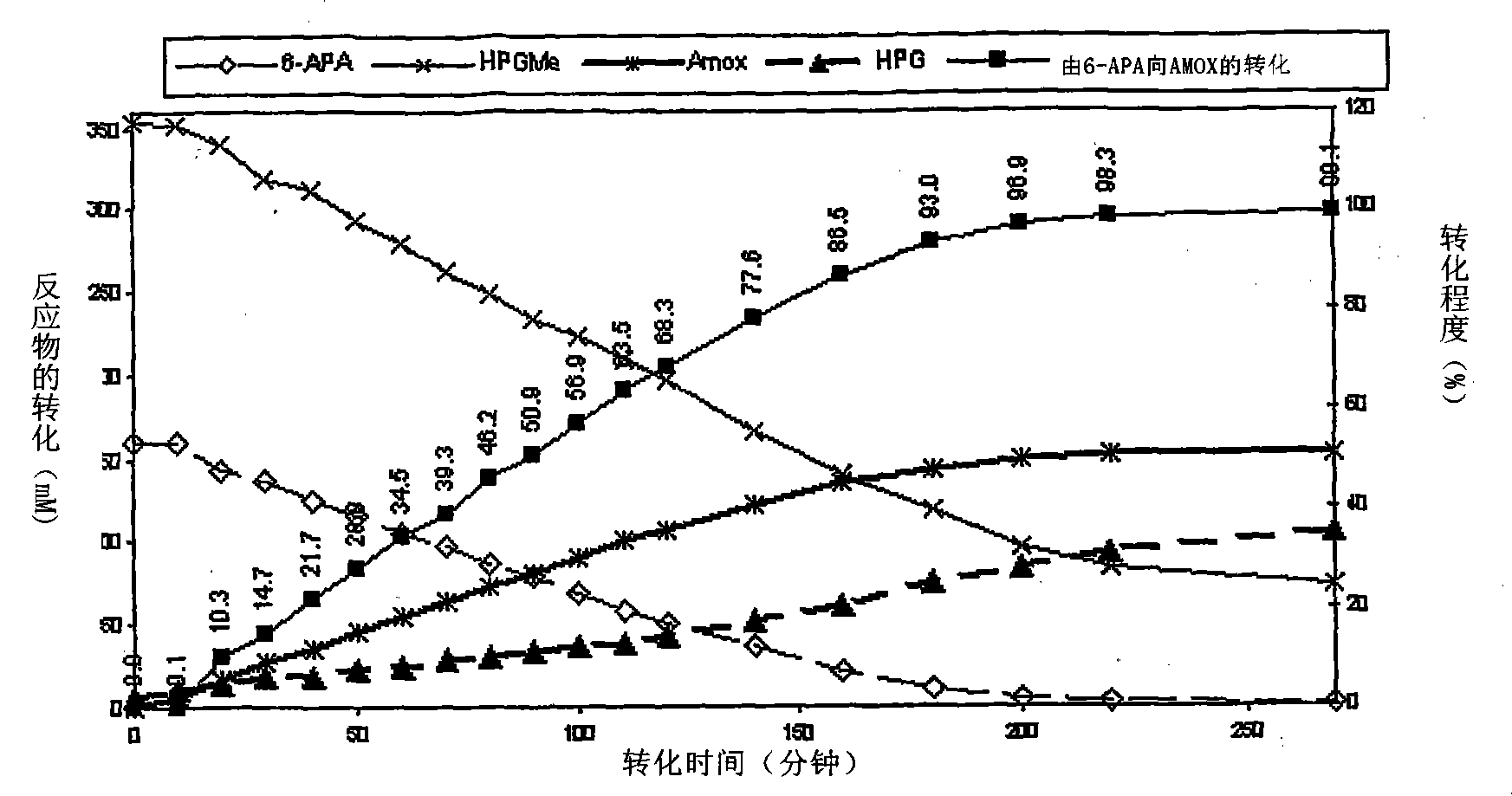
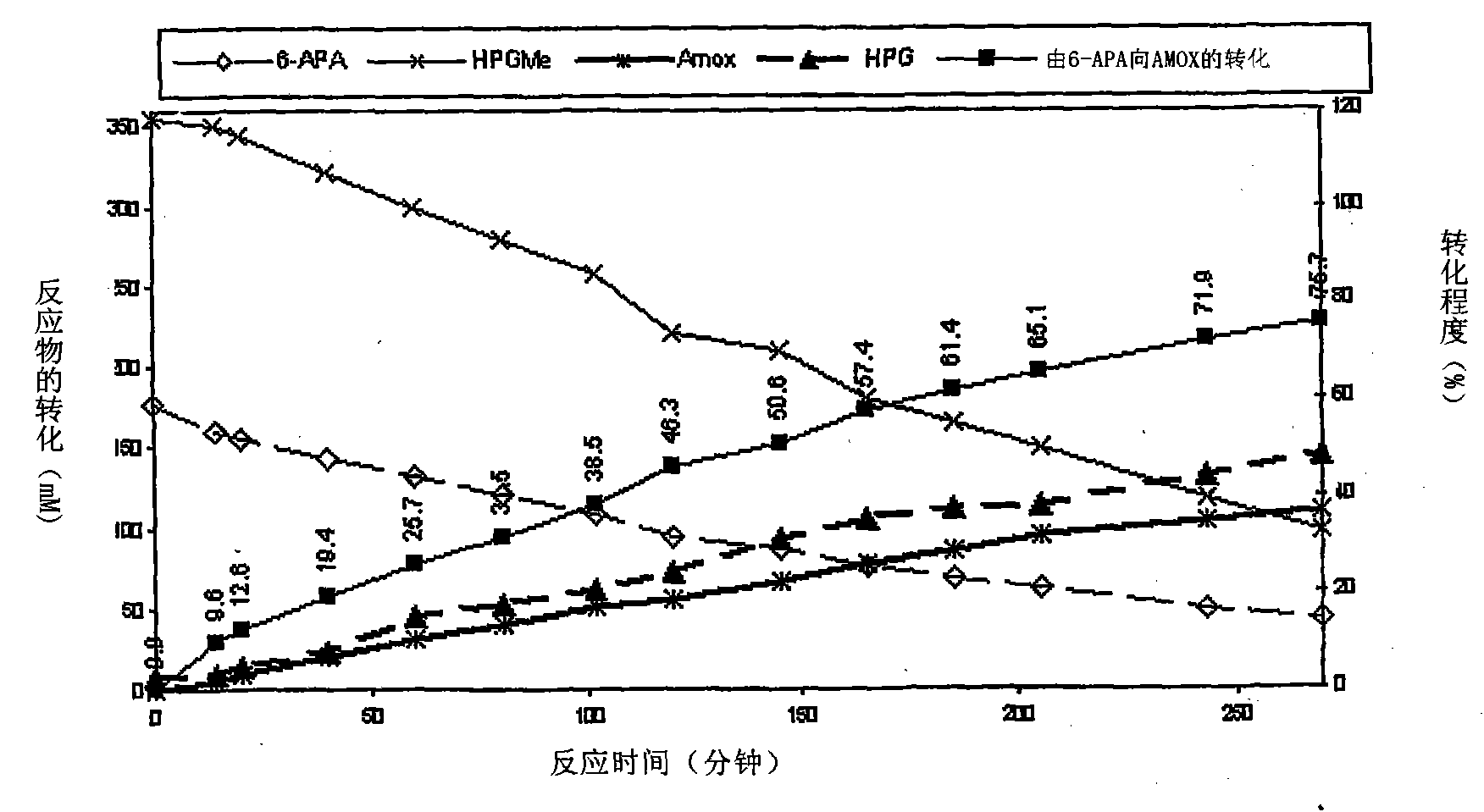
Process for the preparation of immobilized recombinant penicillin acylase catalyst from Achromobacter sp. CCM 4824 expressed in E. coli BL 21 CCM 7394 and its use for the synthesis of beta-lactam antibiotics
ActiveCN101802212AImprove synthesis abilityExtended active timeFermentationAmpicillinBeta lactam antibiotic
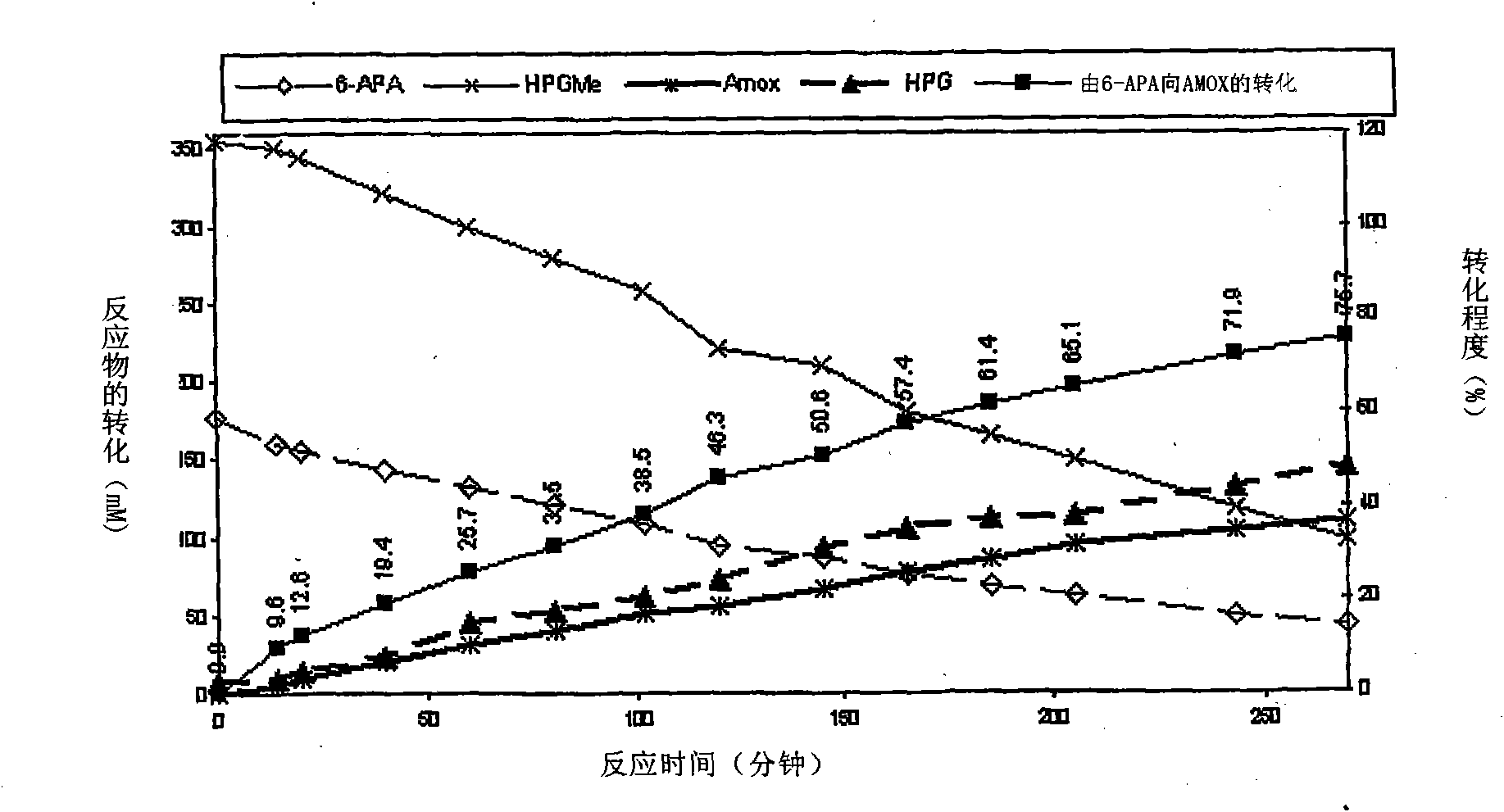
The present invention discloses isolation of Penicillin Acylase (PA) from Achromobacter sp CCM 4824 expressed in recombinant strain E. coli BL21 CCM 7394 bearing the recombinant plasmid pKXIP1 and processing of PA into biocatalyst useful for the industrial synthesis of antibiotics. More particularly the invention discloses a synthesis of semi-synthetic [beta]-lactam antibiotics in the reaction mixture consisting of activated acyl-donor (D-p-hydroxyphenylglycine methyl ester or amide for Amoxicillin and Cefadroxil; D-phenylglycine methyl ester or amide for Ampicillin and Cephalexin) and nucleophile (6-APA or 7-ADCA) catalyzed by PA obtained from recombinant E. coli BL21 CCM 7394 as the biocatalyst.
Owner:FERMENTA BIOTECH
Cefadroxil tablet and preparation method thereof
ActiveCN104800177ADisintegrates quicklyEvenly dispersedAntibacterial agentsOrganic active ingredientsCarboxymethyl starchCellulose
The invention provides a cefadroxil tablet. The cefadroxil tablet is prepared by mixing and tabletting cefadroxil particles, micro-crystal cellulose and a lubricating agent. The cefadroxil particle comprises the following ingredients: 250 parts by weight of cefadroxil, 10 to 20 parts by weight of micro-crystal cellulose, 2 to 4 parts by weight of starch, and 4 to 6 parts by weight of carboxymethyl starch sodium. Compared with the prior art, the cefadroxil tablet adopts the carboxymethyl starch sodium as a disintegrating agent which is unique in swelling effect; moreover, the micro-crystal cellulose is respectively added in the granulating and tabletting process, so that the cefadroxil is more uniformly dispersed, a product tablet core has good disintegrating effect, the particles can be rapidly disintegrated, the dissolution rate of the product is high, and the disintegrating time is short. The experiment result shows that the dissolution rate of the cefadroxil tablet is 87 to 103 percent, and the disintegrating time is 4 to 6 minutes.
Owner:HUNAN KELUN PHARMA
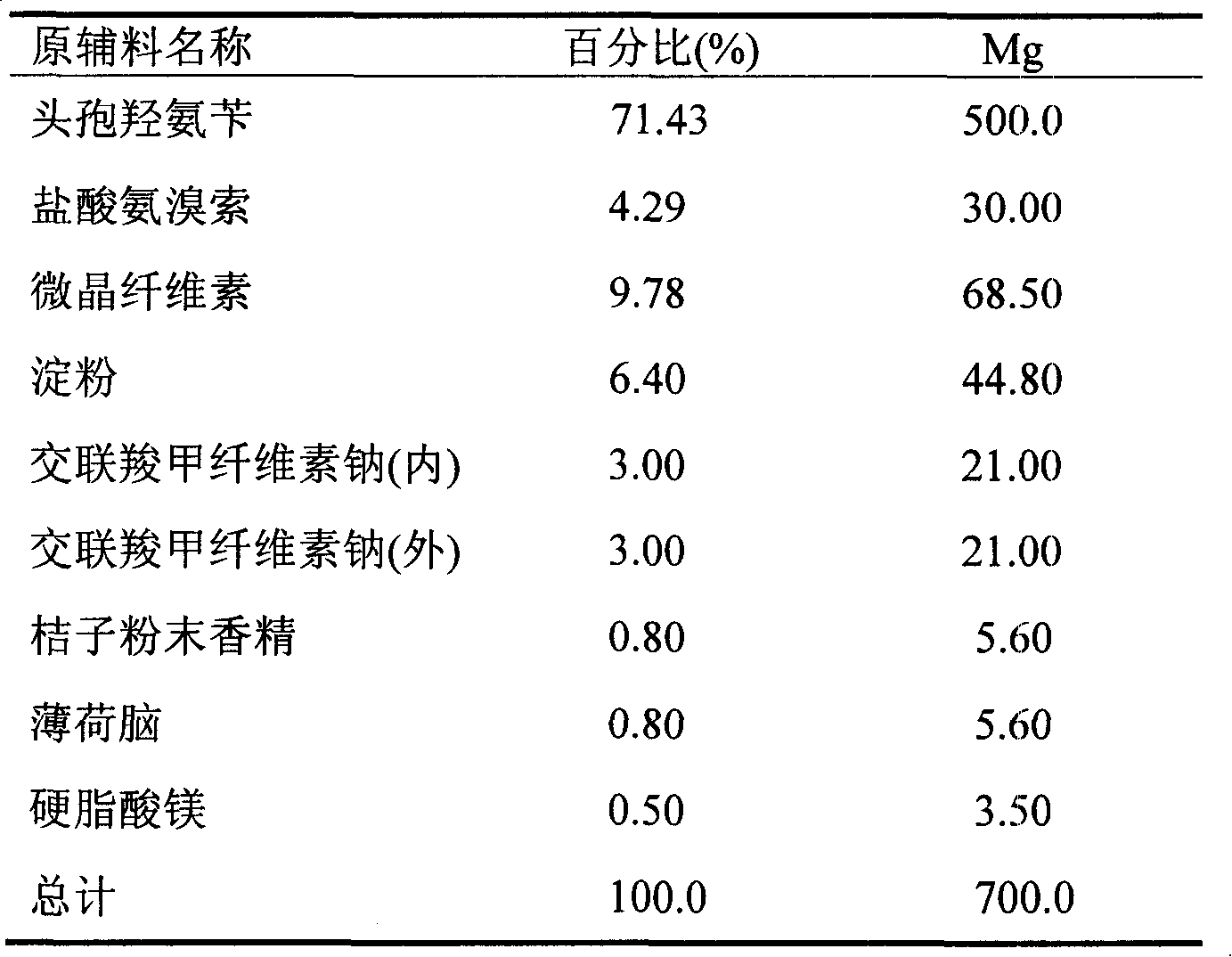
Cefadroxil chewable tablets used for pets and preparation method thereof
InactiveCN109512791AImprove complianceImprove taste effectAntibacterial agentsOrganic active ingredientsFlavorHigh humidity
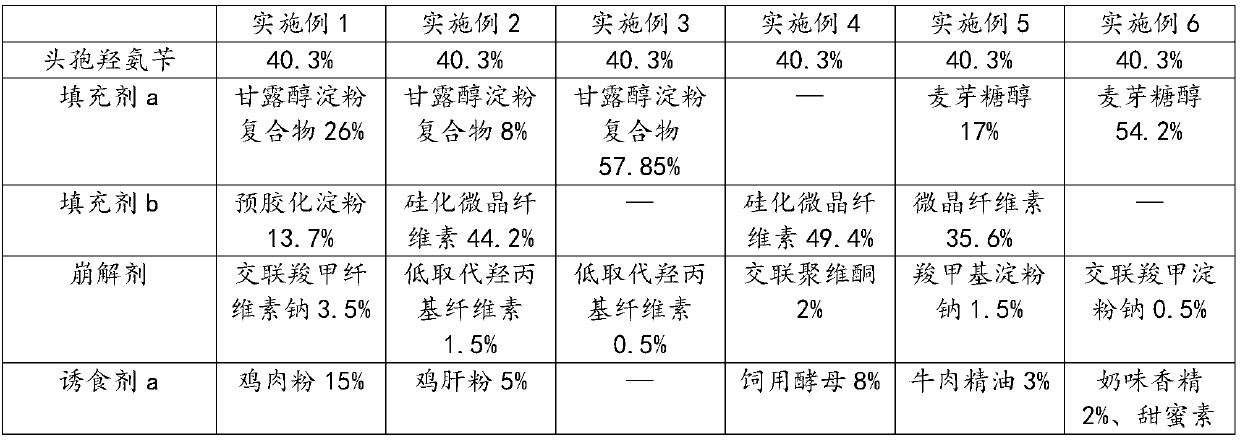
The invention provides cefadroxil chewable tablets used for pets and a preparation method thereof. The cefadroxil chewable tablets used for the pets are mainly prepared from the following raw materials: cefadroxil, filler, a disintegrating agent, phagostimulant and a lubricant; the phagostimulant comprise one or more of phagostimulant a, phagostimulant b and phagostimulant c. After being subjectedto taste masking treatment, the chewable tablets have cool and refreshing taste and a flavor which dogs and cats like, have a good taste modifying effect, the taking compliance of the dogs and the cats is good, and medication is convenient. According to a preparation technology of the cefadroxil chewable tablets used for the pets, production conditions of high temperature and high humidity are avoided, an obtained chewable tablet product is stable in various indexes, storage is facilitated, and the safety and the effectiveness of a medicine are guaranteed.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
A kind of synthetic method of cefadroxil
The invention discloses a synthetic method of cefadroxil. The synthetic method of cefadroxil comprises the following steps of: dissolving 7-aminodesacetoxycephalosporanic acid by adopting organic base at the temperature ranging from minus 10 DEG C to minus 50 DEG C to obtain 7-aminodesacetoxycephalosporanate; carrying out reaction on HPCDane salt and pivaloyl chloride at the temperature ranging from minus 20 DEG C to minus 70 DEG C to generate mixed anhydride and carrying out condensation reaction on the mixed anhydride and 7-aminodesacetoxycephalosporanate, extracting and regulating pH value of a water layer, then separating salt and regulating pH value, and crystallizing to obtain cefadroxil. By adopting the manner, the synthetic method of cefadroxil has the advantages that a process is simple, solvate does not need to be formed in a synthetic process, crystallization can be directly carried out in water, consumption of an organic solvent is reduced, production cost is reduced, yield of cefadroxil is high, various quality indexes are qualified, pharmacopeia standards are met, and implementation of industrial production can be facilitated.
Owner:苏州盛达药业有限公司
A kind of cefadroxil compound and its pharmaceutical composition
ActiveCN104447795BNot easy to absorb moistureImprove solubilityOrganic active ingredientsOrganic chemistrySolubilityWater soluble
The invention belongs to the technical field of medicines, and particularly relates to a cefadroxil compound and pharmaceutical composition comprising the same. The cefadroxil compound is a cefadroxil hydrate with the molecular formula of C16H17N3O5S*3.5H2O, and the structural formula is shown in the specification. Various pharmaceutically-acceptable dosage forms can be prepared from the pharmaceutical composition comprising the cefadroxil compound, and chewable tablets and capsules are preferred. The cefadroxil compound has the advantage of capability of significantly improving the water solubility and the hygroscopicity of cefadroxil compounds.
Owner:金鸿药业股份有限公司
Technique for producing D-(-)-p-hydroxyphenylglycine by aqueous-phase resolution method
InactiveCN101613298AOrganic compound preparationAmino-carboxyl compound preparationSolubilitySide chain
The invention provides a technique for producing D-(-)-p-hydroxyphenylglycine by aqueous-phase resolution method, which belongs to the field of pharmaceutical intermediate production. With the wide application of amoxicillin, cefadroxil and the like, the market of a side chain D-HPG thereof continually expands, and people pay more and more attention to synthesis and resolution methods thereof. By chemical methods, the technique uses resolution agent and racemic DL-HPG to form salts and utilizes different solubility thereof in particular solvents to separate two optical isomers so as to achieve the aim of resolution. The technique for producing D-(-)-p-hydroxyphenylglycine by aqueous-phase resolution method uses non-chiral resolution agents which are cheap and readily available and selects water as a solvent, thereby obtaining the D-HPG with low cost and high optical purity.
Owner:HENAN NEWLAND PHARMA
Cefadroxil compound and preparation method thereof
Owner:HAINAN MEIDA PHARMA
Monoclonal antibody, enzyme-linked immunosorbent assay method and kit for detecting cefalexin, cefadroxil and cefradine
ActiveCN104558189AHigh recognition sensitivityExcellent recognition sensitivityMicroorganism based processesTissue cultureCefalexinCefradine
The invention discloses a specific monoclonal antibody capable of distinguishing cefalexin, cefadroxil and cefradine, and an enzyme-linked immunosorbent assay method and kit for detecting the cefalexin, the cefadroxil and the cefradine. According to the invention, the monoclonal antibody is secreted by a hybridoma cell strain 3A6 of which the preservation number is CCTCC No.C201340. Compared with the prior art, the monoclonal antibody, prepared by the invention, can be used for distinguishing the cefalexin, the cefadroxil and the cefradine at the same time. The enzyme-linked immunosorbent assay method and kit disclosed by the invention have the advantages of high detection efficiency, high sensitivity, high precision, high accuracy and the like.
Owner:HUAZHONG AGRI UNIV
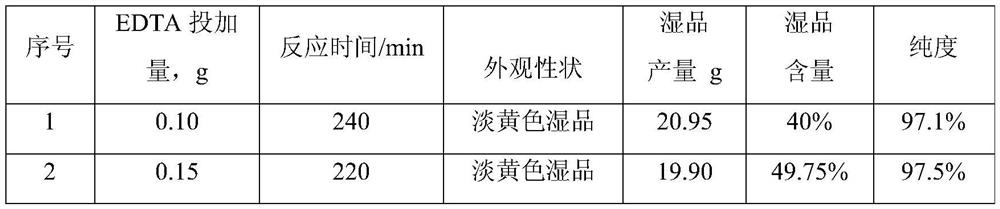
7-ADCA recovery method
ActiveCN113699209AImprove product qualityObvious safety and environmental benefitsProcess efficiency improvementFermentationChemical compoundPhysical chemistry
The invention provides a 7-ADCA recovery method, and belongs to the technical field of heterocyclic compounds. The 7-ADCA recovery method comprises the following steps that mother liquor after crystallization of cefadroxil is taken and heated to 10-30 DEG C; the pH value of the mother liquor is adjusted to 7.0-8.0, EDTA is added, and stirring is carried out; an immobilized enzyme is added, the pH value is maintained at 7.0-8.0 and the temperature at 10-30 DEG C, and stirring is carried out until the concentration of the cefadroxil does not exceed 0.5 mg / ml; separating is carried out to remove the immobilized enzyme, the solution obtained by separation is decolored and filtered; the pH value of the filtrate is adjusted to 5.0-6.0, the temperature is controlled to be 10-30 DEG C, and crystals are grown; and filtering and separating are carried out, and a wet product is rinsed to obtain 7-ACDA with the purity of more than 98.5%. The7-ADCA recovery method is green and environment-friendly in technical route, simple to operate, high in yield, stable and reliable, and obvious in advantages.
Owner:ZHEJIANG ANGLIKANG PHARMA
Process for the preparation of immobilized recombinant penicillin acylase catalyst from Achromobacter sp. CCM 4824 expressed in E. coli BL 21 CCM 7394 and its use for the synthesis of beta-lactam antibiotics
ActiveCN101802212BImprove synthesis abilityExtended active timeFermentationAmpicillinBeta lactam antibiotic
The present invention discloses isolation of Penicillin Acylase (PA) from Achromobacter sp CCM 4824 expressed in recombinant strain E. coli BL21 CCM 7394 bearing the recombinant plasmid pKXIP1 and processing of PA into biocatalyst useful for the industrial synthesis of antibiotics. More particularly the invention discloses a synthesis of semi-synthetic &bgr;-lactam antibiotics in the reaction mixture consisting of activated acyl-donor (D-p-hydroxyphenylglycine methyl ester or amide for Amoxicillin and Cefadroxil; D-phenylglycine methyl ester or amide for Ampicillin and Cephalexin) and nucleophile (6-APA or 7-ADCA) catalyzed by PA obtained from recombinant E. coli BL21 CCM 7394 as the biocatalyst.
Owner:FERMENTA BIOTECH
Method for detecting polymer impurities in cefadroxil raw material medicine and preparation of cefadroxil raw material medicine
PendingCN113607870AEasy to separateStrong specificityComponent separationMonopotassium phosphateSilica gel
Owner:NAT INST FOR FOOD & DRUG CONTROL
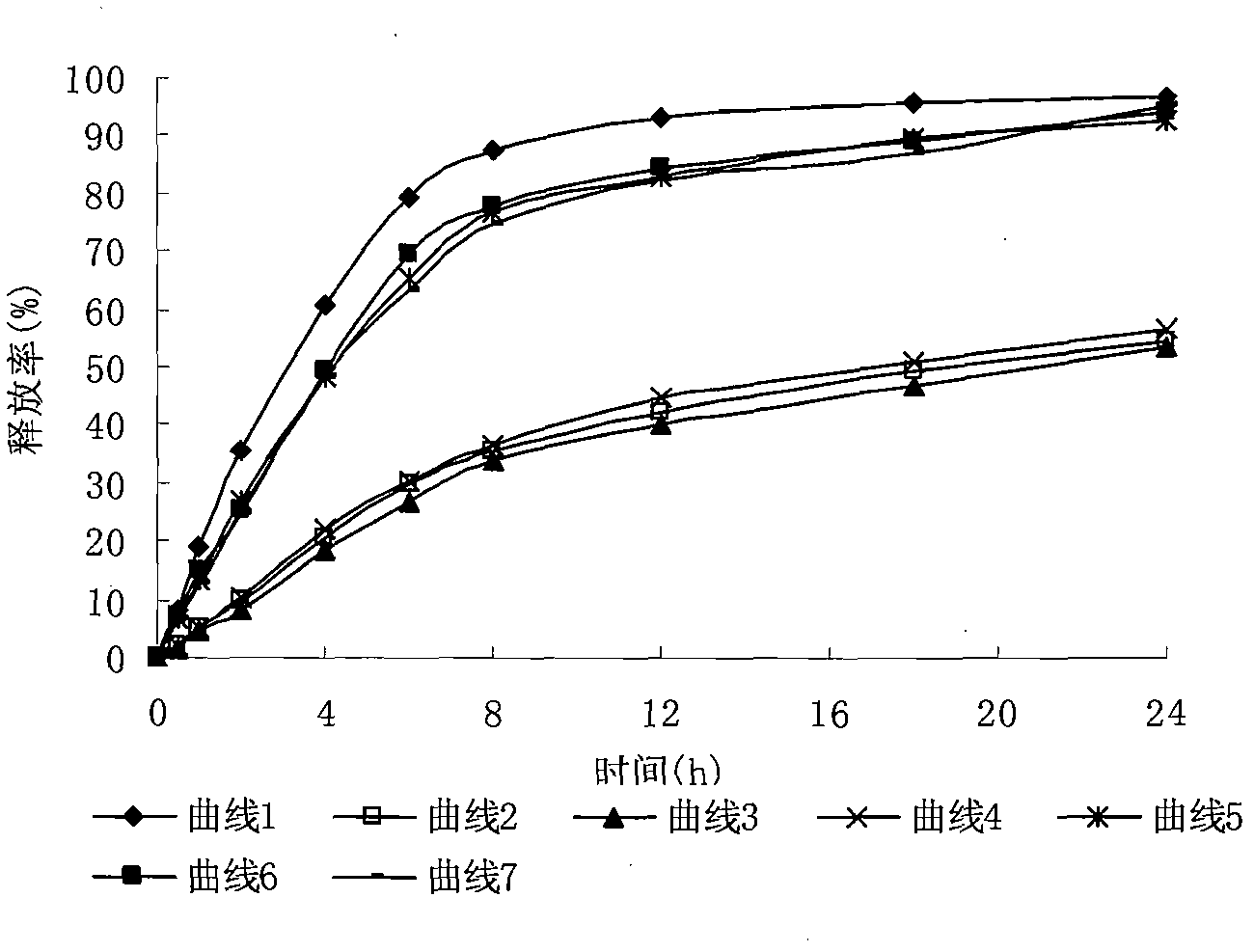
Cefadroxil liposome solid preparation
InactiveCN102327221ALow costHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsSide effectMedicine
The invention provides a cefadroxil liposome solid preparation. The cefadroxil liposome solid preparation consists of the following components in part by weight: 125 parts of cefadroxil, 90 to 120 parts of phosphatidylinositol, 30 to 40 parts of dimyristoyl phosphatidylcholine, 50 to 70 parts of cholesterol and 300 to 500 parts of other auxiliary materials which are used commonly and pharmaceutically. The liposome solid preparation is high in entrapment rate, and has uniform grain sizes, and medicines can be kept for a long time in blood circulation; and the quality of the preparation products is improved, the toxic and side effects are reduced, the equipment of a preparation method is simple and is easy to operate, and the cefadroxil liposome solid preparation is suitable for large-scaleindustrial production.
Owner:HAINAN MEIDA PHARMA
Ambroxol cefadroxil dispersible tablets
InactiveCN101204379AFast absorptionImprove bioavailabilityAntibacterial agentsOrganic active ingredientsMedicineActive component
The invention discloses a compound dispersible tablet, which contains active components of cefadroxil and ambroxol hydrochloride, and the pharmacologically-acceptable excipient. The compound dispersible tablet is used for curing the upper respiratory tract infection.
Owner:BEIJING D VENTUREPHARM TECH DEV
Cefadroxil dispersible tablets and preparation method therefor
InactiveCN111467316ASmooth releaseRaw materials are easy to getAntibacterial agentsOrganic active ingredientsDrugs preparationsMagnesium stearate
The invention belongs to the technical field of pharmaceutical preparations and particularly relates to cefadroxil dispersible tablets and a preparation method therefor. A prescription for the cefadroxil dispersible tablets per 10,000 tablets comprises the following ingredients by weight: 5,000g of cefadroxil, 1,000g-1,300g of filler, 500g-800g of disintegrant and 20g-100g of magnesium stearate. The preparation method comprises the steps of weighing the cefadroxil, the filler and the disintegrant according to prescription amounts, carrying out uniform mixing, then, adding the lubricant, i.e.,magnesium stearate, carrying out uniform mixing, then, carrying out tabletting by a tabletting machine, thereby obtaining the cefadroxil dispersible tablets. The cefadroxil dispersible tablets prepared by the preparation method are uniform in unit-time drug release, readily available in raw materials and controllable in quality. According to the preparation method, the cefadroxil dispersible tablets are produced by employing a mixed-powder direct-tabletting process, the process is simple, the quality is stable, the operation is easy, the method is applicable to large-scale production, and therisk of degradation of the cefadroxil is avoided.
Owner:REYOUNG PHARMA
Method for measuring cefadroxil concentration in plasma through hygroplasm combination
InactiveCN109682913AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventMass spectrometry detector
The invention discloses a method for measuring cefadroxil concentration in plasma through hygroplasm combination. A hygroplasm combination system is adopted for measurement. The method comprises the steps that firstly, a to-be-measured sample is taken, a certain quantity of mixed organic solvent is added for conducting extraction twice, and after pretreatment, through chromatographic column separation, a mass spectrometry detector is used for detection. The method is quick to use, accurate, high in sensitivity and easy and convenient to operate, and the basis is provided for plasma concentration measurement of cefadroxil. The linear range of a plasma standard curve is within 0.1-60 mug / mL, the within-run precision and between-run precision RSD are smaller than + / -15%, and the method is suitable for measurement of cefadroxil concentration in plasma.
Owner:徐州立兴佳正医药科技有限公司
Preparation method of 3-ethyl cefadroxil
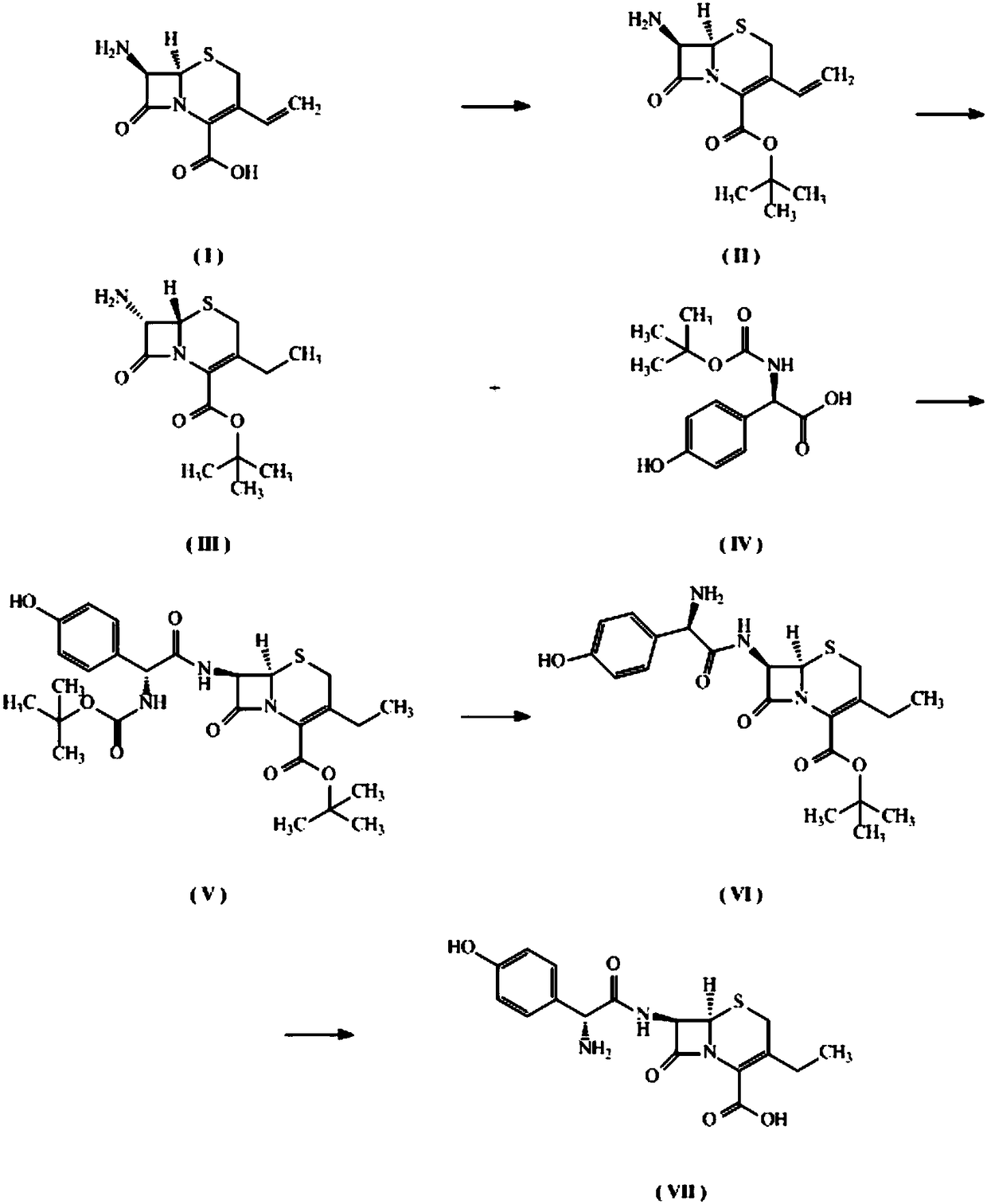
The invention discloses a preparation method of 3-ethyl cefadroxil, and belongs to the field of drug synthesis. 7-amino-3-vinyl-8-oxo-5-thia-1-azabicyalo[4.2.0]oct-2-ene-2-carboxylate is taken as a raw material to synthesize the 3-ethyl cefadroxil by five-step reaction. According to the preparation method, the process design is reasonable, the operability is high, reaction conditions are simpler,the yield is high, and large-scale production can be realized. The 3-ethyl cefadroxil prepared by the method provides an important basis for performing scientific evaluation of quality, safety and efficiency, pharmacological research, pharmacokinetic research and the like on the 3-ethyl cefadroxil, also can serve as a possible new drug to be further researched and developed for treating urinary system, respiratory tract, skin, five sense organs, gastrointestinal tract infections and the like caused by staphylococcus, streptococcus, pneumococcus, escherichia coli and the like, and has importantapplication value.
Owner:TLC NANJING PHARMA RANDD CO LTD
Process for the preparation of immobilized recombinant penicillin acylase catalyst from achromobacter sp. ccm 4824 expressed in e. coli bl 21 ccm 7394 and its use for the synthesis of beta-lactam antibiotics
The present invention discloses isolation of Penicillin Acylase (PA) from Achromobacter sp CCM 4824 expressed in recombinant strain E. coli BL21 CCM 7394 bearing the recombinant plasmid pKXIP1 and processing of PA into biocatalyst useful for the industrial synthesis of antibiotics. More particularly the invention discloses a synthesis of semi-synthetic β-lactam antibiotics in the reaction mixture consisting of activated acyl-donor (D-p-hydroxyphenylglycine methyl ester or amide for Amoxicillin and Cefadroxil; D-phenylglycine methyl ester or amide for Ampicillin and Cephalexin) and nucleophile (6-APA or 7-ADCA) catalyzed by PA obtained from recombinant E. coli BL21 CCM 7394 as the biocatalyst.
Owner:FERMENTA BIOTECH
Cefadroxil dry suspension and preparing method
ActiveCN1282458CImprove solubilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsSolubilityPatient compliance
A cefadroxil dry suspension and a preparation method thereof belong to the technical field of medicine and are used for solving the problems of improving the water solubility and bioavailability of cefadroxil. It consists of the main drug cefadroxil and auxiliary materials. The content of each main and auxiliary material in the total prescription by weight unit is as follows: cefadroxil 80-120, inclusion agent 16-60, water-soluble filler 440-520, disintegrant Solution 55-70, suspending agent 5-10, binder 30-45. The product of the invention has the characteristics of high drug solubility and bioavailability, good chemical stability, good taste, convenient taking, strong patient compliance and the like. The invention is made into a dry suspension dosage form, which can be widely distributed in the gastrointestinal tract, has more absorption points, more sufficient absorption, and reduces the local stimulation of the drug on the gastrointestinal tract.
Owner:CSPC OUYI PHARM CO LTD
Penicillin g Acylase Mutant and Its Application in the Synthesis of Cephalosporin Antibiotics
Owner:ZHEJIANG APELOA TOSPO PHARMA +1
A kind of cefadroxil tablet and preparation method thereof
ActiveCN104800177BDisintegrates quicklyEvenly dispersedAntibacterial agentsOrganic active ingredientsDissolutionTableting
The invention provides a cefadroxil tablet, which is prepared by compressing cefadroxil granules, microcrystalline cellulose and a lubricant after mixing; the cefadroxil granules include the following components: 250 parts by weight of cefadroxil; 10-20 parts by weight of crystalline cellulose; 2-4 parts by weight of starch; 4-6 parts by weight of sodium carboxymethyl starch. Compared with the prior art, the cefadroxil tablet provided by the invention uses sodium starch glycolate as a disintegrating agent, which has a unique swelling effect, and microcrystalline cellulose is added respectively in the granulation and tabletting process, so that the main drug The dispersion of cefadroxil is more uniform, so that not only the tablet core of the product has a better disintegration effect, but also the granules can disintegrate rapidly, and the product has a high dissolution rate and a short disintegration time limit. Experimental results show that the dissolution rate of the cefadroxil tablet provided by the invention is 87%-103%, and the disintegration time limit is 4min-6min.
Owner:HUNAN KELUN PHARMA
A kind of extraction recovery method of penicillin sulfoxide
ActiveCN105418640BRealize zero pollutionQuality improvementAntibacterial agentsOrganic active ingredientsUpper urinary tract infectionPenicillin G sulfoxide
Owner:NORTH CHINA PHARMA COMPANY +1
An indirect competition ELISA kit for detecting cephalosporin antibiotics in food of animal origin and its application
ActiveCN107014993BIncreased cross-reactivityIncreased sensitivityMaterial analysisElisa kitCefotaxime
The invention discloses an indirect-competitive ELISA (Enzyme-linked Immunosorbent Assay) kit for detecting cephalosporin antibiotics in animal derived foods and application of the kit. The kit comprises an ELISA plate coated with a coating antigen, a cephalosporin antibiotic standard substance, a cephalosporin antibiotic general antibody, an enzyme labeled secondary antibody, a dilution buffer, a washing buffer, a substrate developing solution and a stop solution, wherein the cephalosporin antibiotic general antibody is capable of specifically identifying cefalexin, cefradine, cefadroxil, cefoperazone, cefazolin or cefotaxime and cannot identify penicillin sodium. According to the cephalosporin antibiotic general antibody prepared in the invention, the general antibody and most of cephalosporin antibiotics have high cross reaction rates, while hardly have any cross reaction for the analogue penicillin sodium containing a beta-lactam ring; and therefore, the general antibody has high specificity. The kit prepared by the general antibody has the advantages of high sensitivity, capability of detecting many types of drugs, low cost, simple operation and short detection time.
Owner:HEBEI AGRICULTURAL UNIV.
Preparation method of cefadroxil
ActiveCN109852660BHigh Catalytic Synthesis EfficiencyHigh synthetic efficiencyHydrolasesFermentationPhosphateSolvent
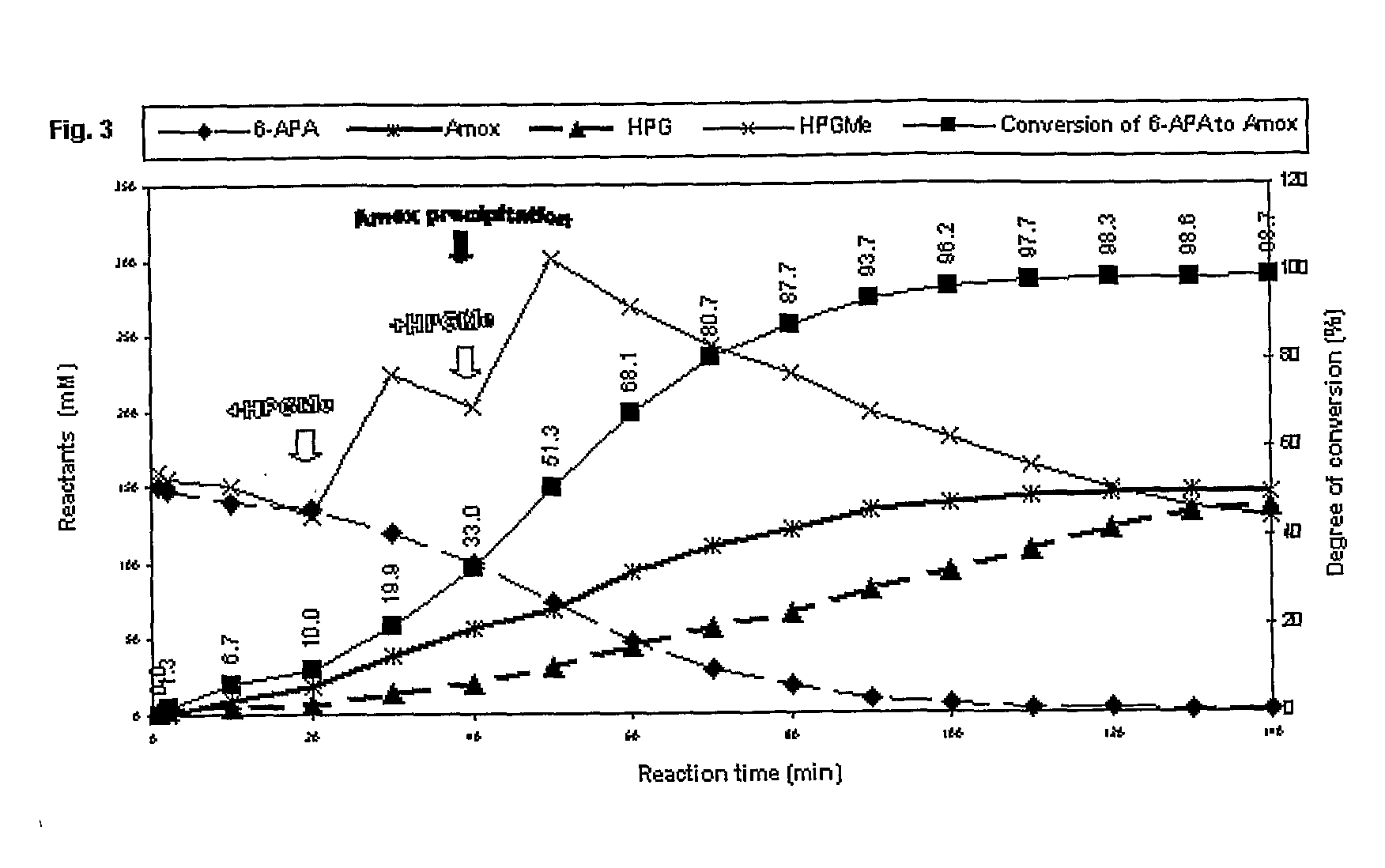
A method for preparing cefadroxil, comprising the steps of: adding a predetermined volume of ionic liquid and a phosphate buffer solution to form a co-solvent in a water-bath constant temperature reactor; adding D-HPGM and 7-ADCA of a predetermined molar ratio to the co-solvent and stirring it, the molar ratio of D-HPGM and 7-ADCA is 1:1~1:2.0; the concentration of 7-ADCA is 0.1~1.0mol / L; Magnetically immobilize penicillin G acylase, and keep stirring to make the magnetically immobilized penicillin G acylase fully contact with D-HPGM and 7-ADCA until the end of the reaction; The upper phosphate buffer solution of cefadroxil is layered with the lower ionic liquid, and the pH of the separated phosphate buffer solution is adjusted to 4.0-5.5, so that cefadroxil crystals are precipitated and dried.
Owner:NINGXIA UNIVERSITY
Cefadroxil oral disintegrant tablet, and its prepn. method
ActiveCN1311830CPromote dissolutionQuickly exert the therapeutic effect of the whole bodyAntibacterial agentsOrganic active ingredientsMedicineWater soluble
An oral disintegrating tablet of dracefal contains dracefal and the medicinal excipient (water-soluble filler, disintegrant, lubricant, wetting agent, or adhesive). Its preparing process is also disclosed. Its advantages are high disintegrating speed, quickly taking its effect, less residue and low by-effect.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Cefadroxil chewable tablets and preparation method thereof
ActiveCN102247331BQuickly exert the therapeutic effect of the whole bodyQuick effectAntibacterial agentsOrganic active ingredientsMentholHigh absorption
The invention relates to cefadroxil chewable tablets and a preparation method thereof. The cefadroxil chewable tablets provided by the invention comprise the following raw material components in parts by weight: 110-550 parts of cefadroxil, 240-1,050 parts of mannitol, 110-500 parts of sucrose, 15-90 parts of maltodextrin, 0.5-5 parts of aspartame, 10-70 parts of sweet orange powder essence, 0.5-5 parts of menthol, a proper amount of water and 3-25 parts of magnesium stearate. The cefadroxil chewable tablets provided by the invention have high absorption speed, no lingering bitterness and excellent mouthfeel.
Owner:金鸿药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com