Preparation method of 3-ethyl cefadroxil
A technology of ethyl cefadroxil and tert-butylation, which is applied in the field of preparation of 3-ethyl cefadroxil, can solve the problems of reducing drug efficacy, complex production process of cefadroxil, harm to human body, etc., and achieve operability Strong, high-purity, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
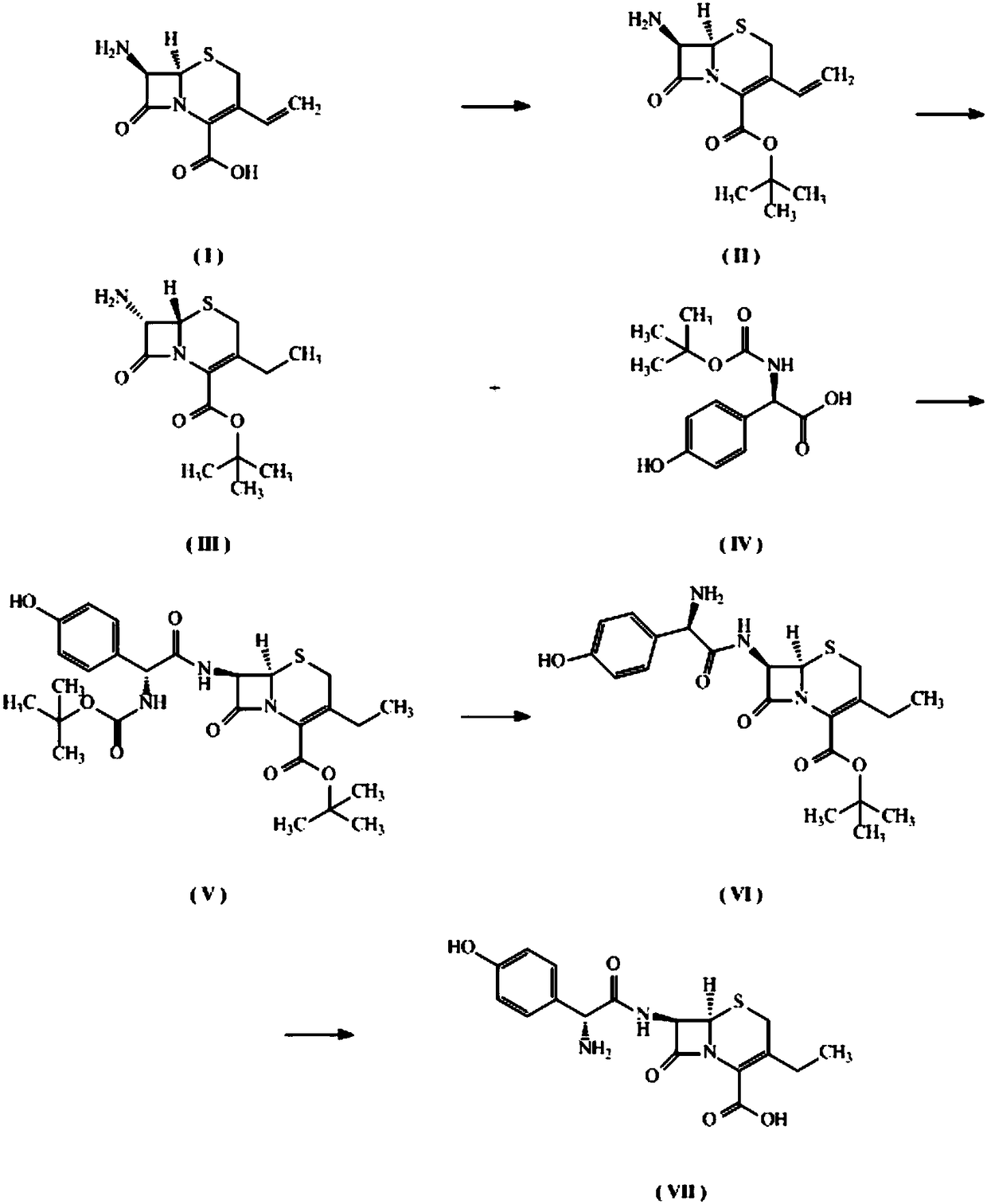
[0027] Synthesis of compound Ⅱ
[0028] The reaction process is:
[0029]
[0030] Get 50 grams of 7-amino-3-vinyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (7-AVCA) suspended at 290 Add 54 ml of boron trifluoride diethyl ether slowly to the above solution in an ice bath, and react at 20°C for 6 hours to obtain a clear yellow solution. The reaction solution is slowly quenched with saturated sodium carbonate solution to neutral partial Alkaline (pH=9), suction filter the quenched reaction solution, extract the filtrate 3 times with 200 ml ethyl acetate, combine the organic phases, wash 3 times with 100 ml saturated brine, and wash the organic phase with anhydrous sodium sulfate Dry, filter and spin dry to obtain the crude product. The crude product was slurried with 300 ml of n-hexane to obtain compound II, 49.12 g, off-white solid, yield 78.7%.
[0031] Compound Ⅱ structural characterization results: 1 H-NMR (DMSO-d 6 )δppm: 6.72-6.79(dd,1H),5.53-5.57...
Embodiment 2
[0053] Synthesis of compound Ⅱ
[0054] The reaction process is:
[0055]
[0056] Take 10 grams of 7-amino-3-vinyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (7-AVCA) suspended in 150 In one milliliter of N,N-dimethylformamide, add 8.82 grams of sodium isooctanoate to the above reaction solution, then react at room temperature for 30 minutes to obtain a clear solution, add 12.1 grams of tert-butyl bromide to the reaction solution, and continue to react at room temperature for 12 hours , a yellow solution was obtained. Add 300 ml of water to the reaction solution, and extract 3 times with 150 ml of ethyl acetate, combine the organic phases, wash 3 times with 200 ml of saturated brine, dry the organic phase with anhydrous sodium sulfate, filter and spin dry to obtain a crude product. The crude product was purified once by column chromatography to obtain compound II, 3.8 g of off-white solid, with a yield of 30.4%.
[0057] Compound Ⅱ structural characteri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com