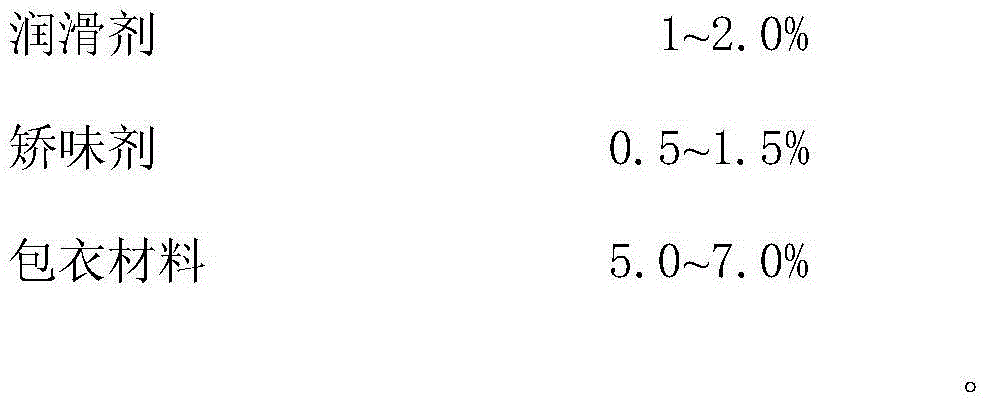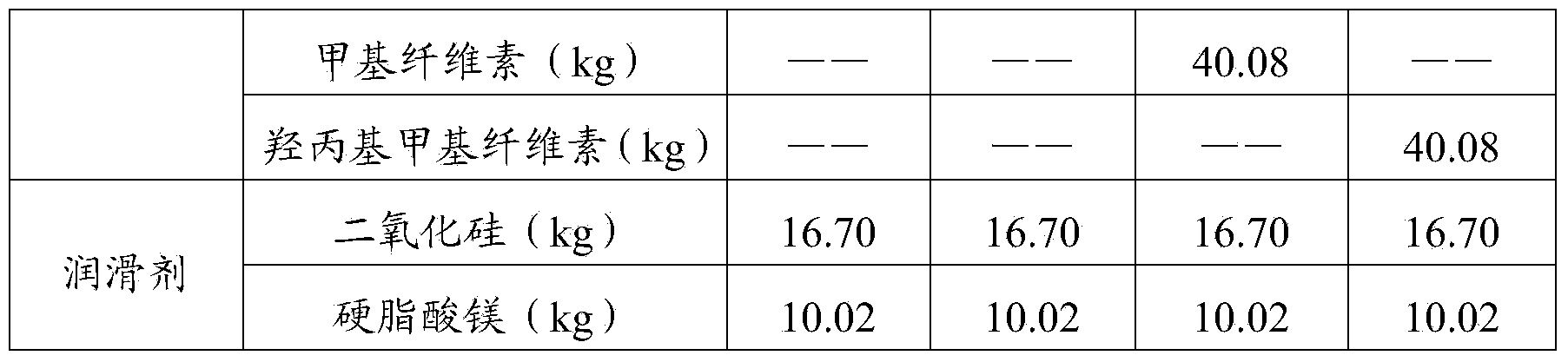Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Product tablet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
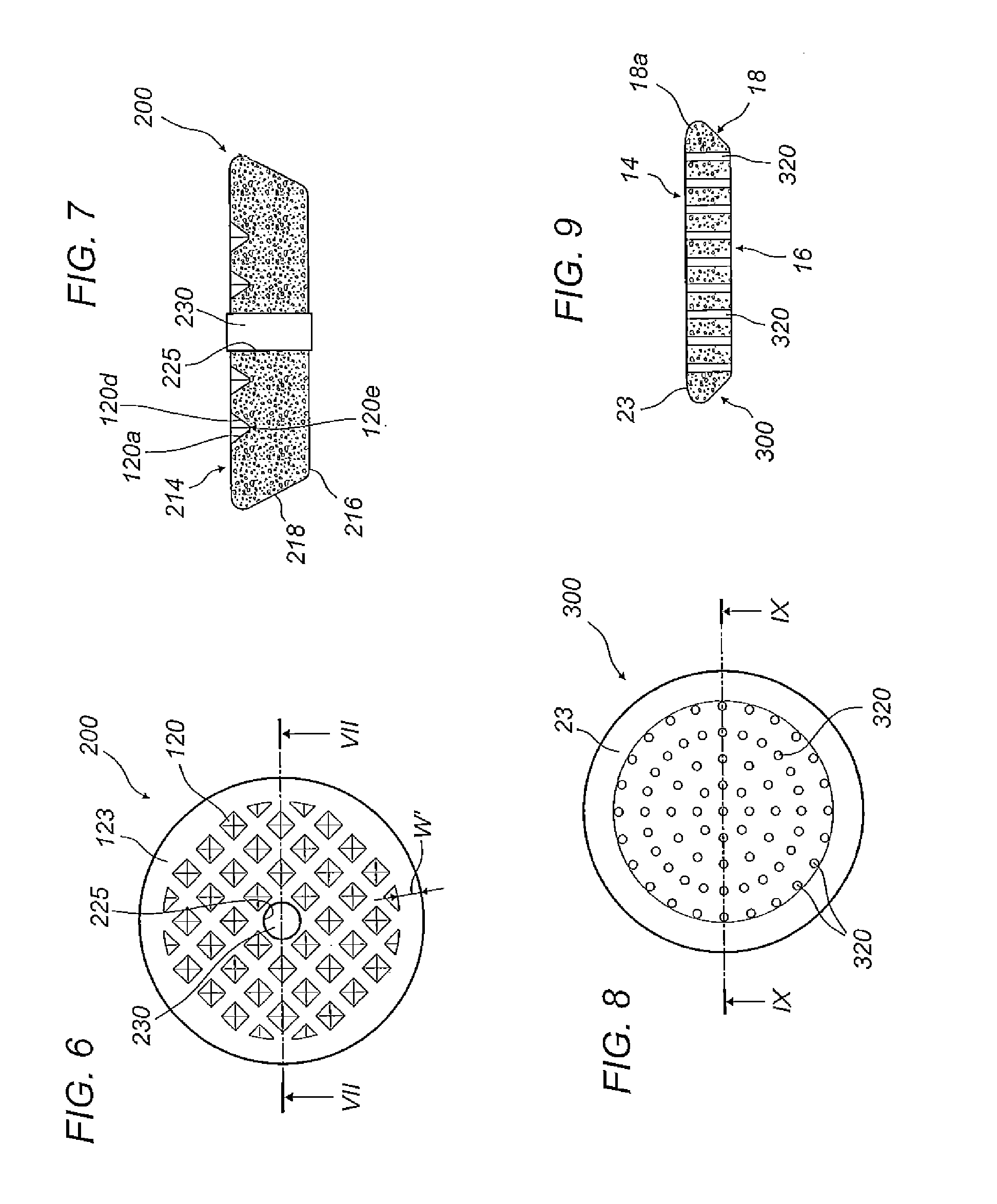
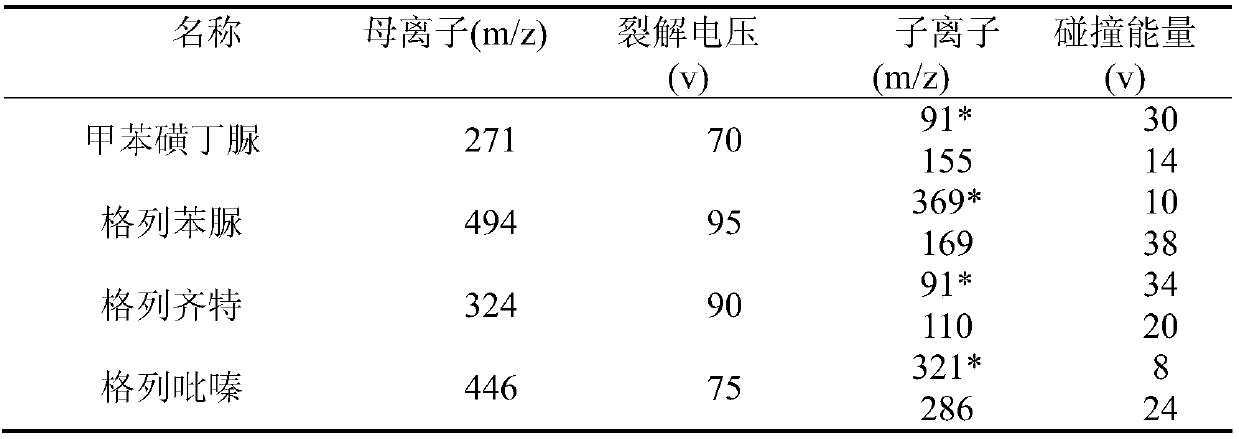
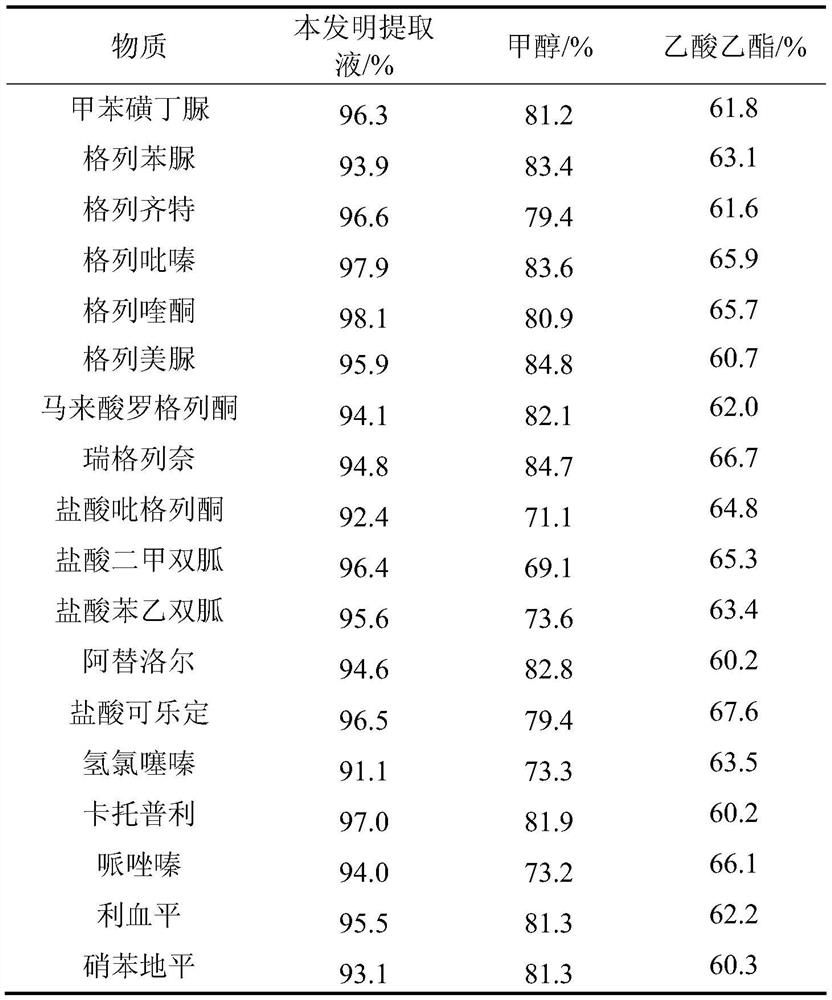
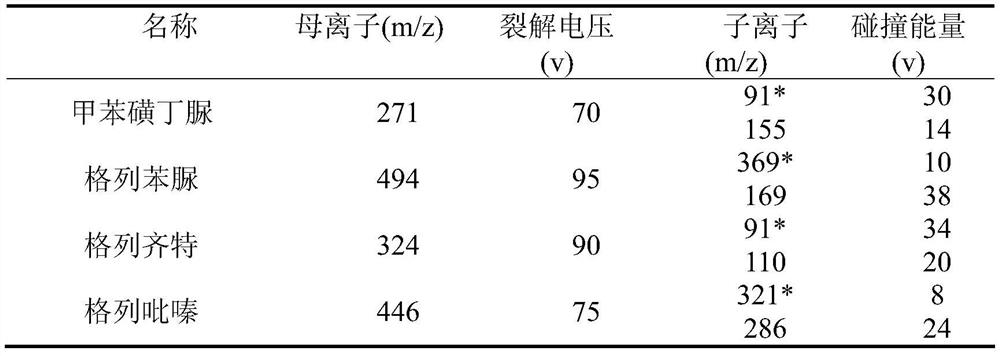
Method for detecting residual quantity of 34 illegally-added medicines in weight-losing health food
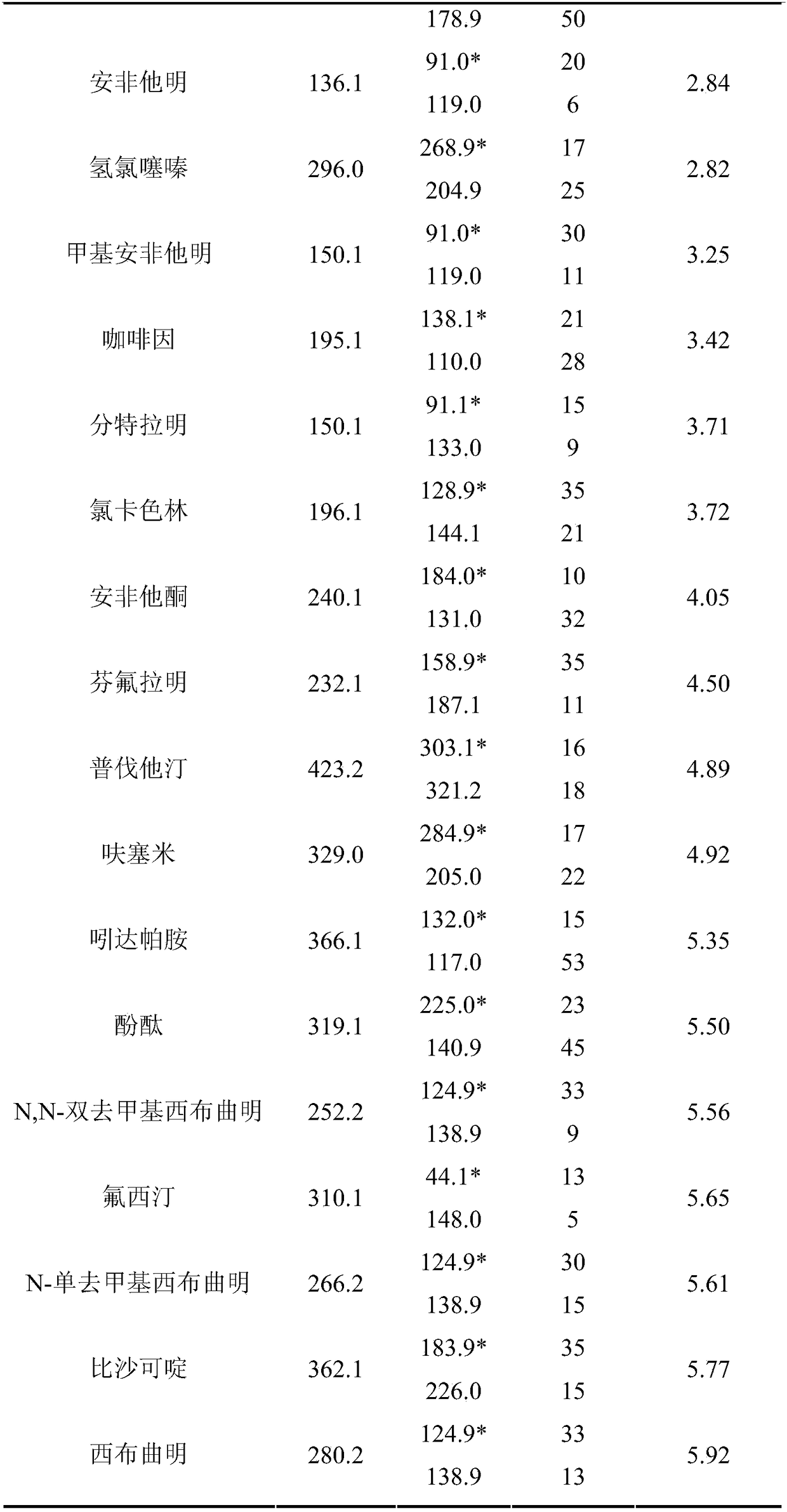
ActiveCN108061777AHigh extraction rateAchieving Simultaneous DetectionComponent separationOctanolHplc mass spectrometry
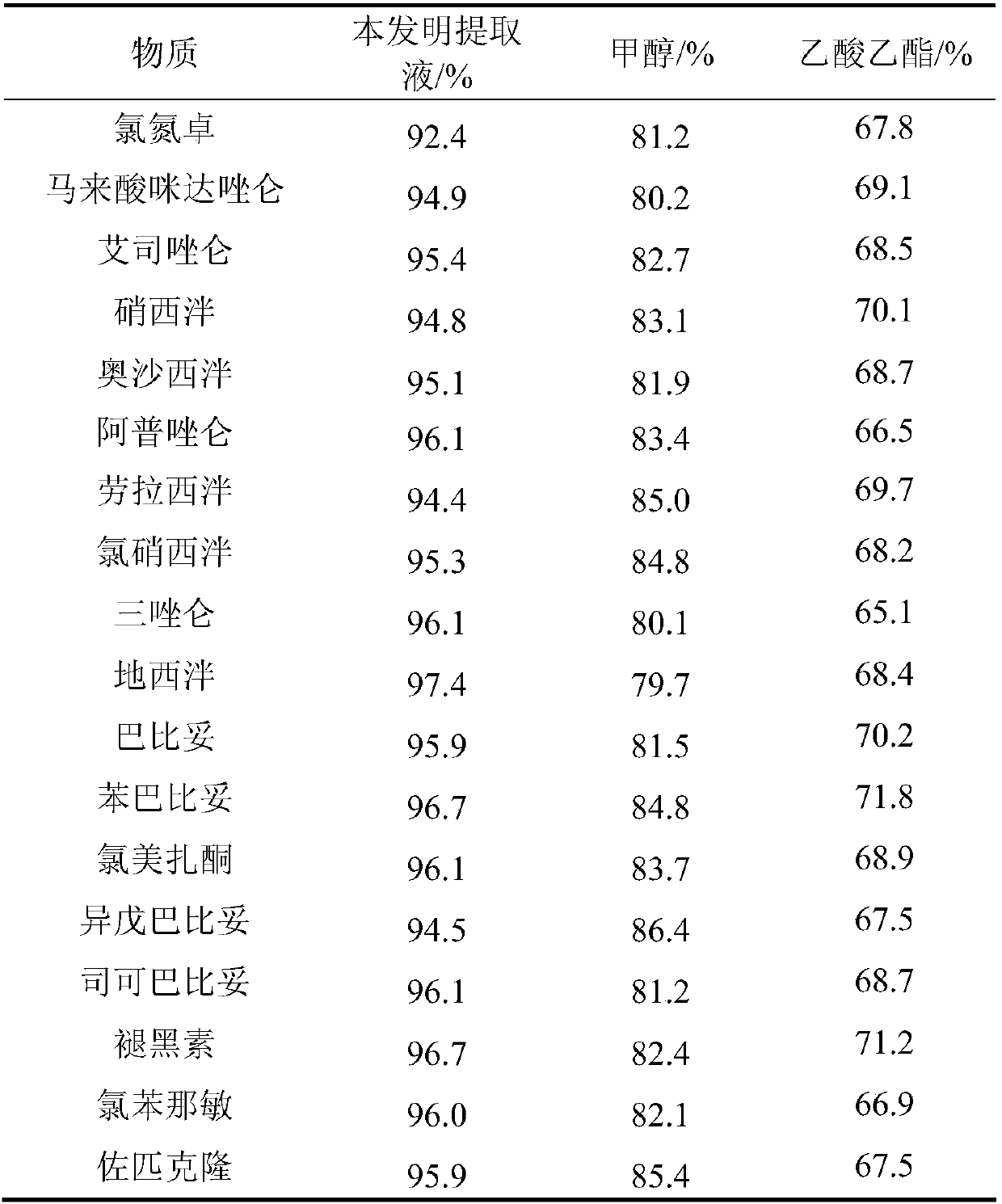
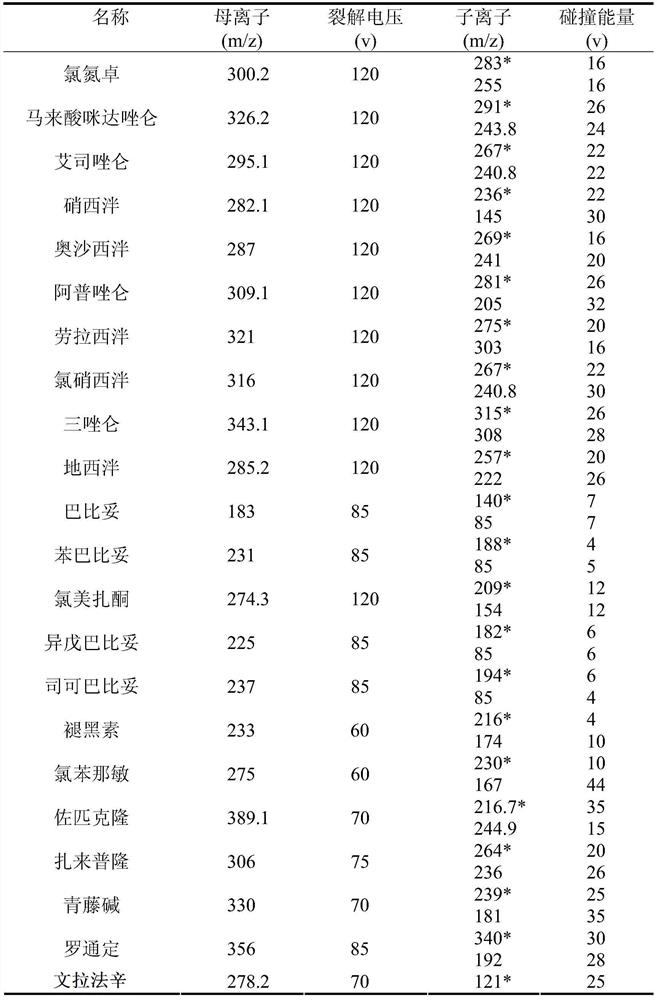
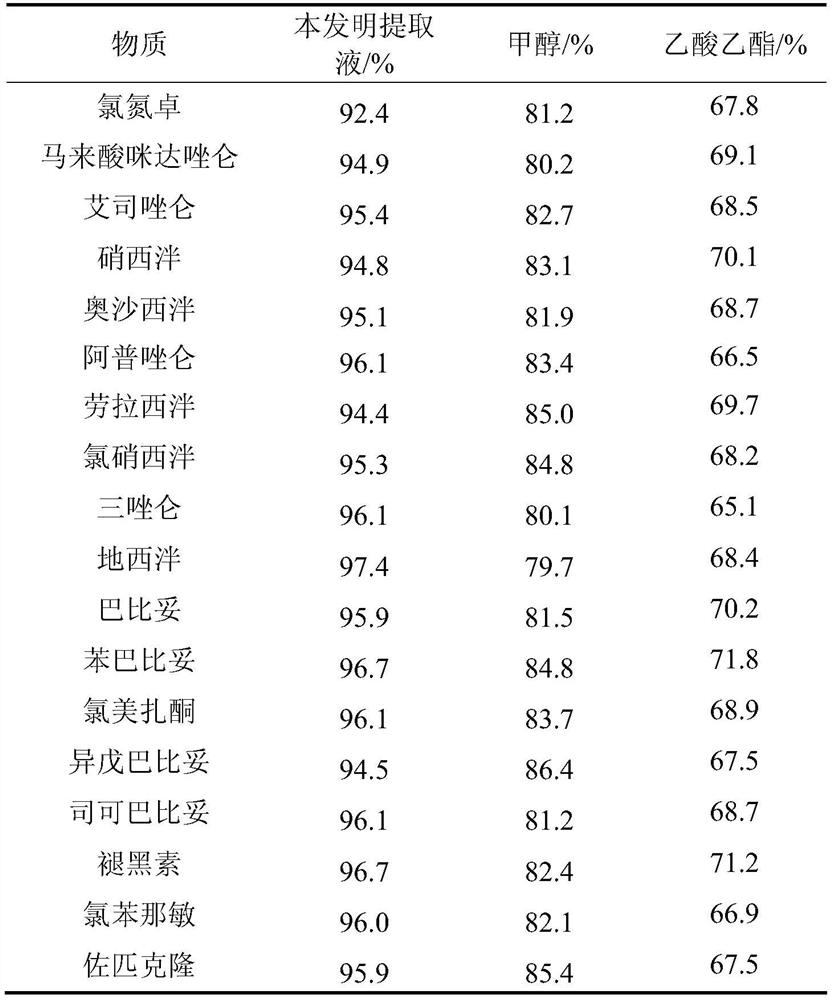
The invention relates to the technical field of medicine residue detection. An objective is to provide a method for fast detecting the residual quantity of 34 illegally-added medicines in a weight-losing health food. The method is fast and accurate, and is high in sensitivity and wide in application range. According to the technical scheme, the method comprises: 1) preparing a self-made extractedliquid, which comprises refluxing a mixture of methanol, acetone and octanol polyoxyethylene ether at 60-65 DEG C for 30 min and performing cooling for later use; 2) taking health product tablets, capsules or particles, performing pulverization, accurately weighing 3-10 g of the sample, putting the sample in a centrifuge tube provided with a plug, adding 20-60 mL of a self-made extracted liquid, performing mixing in a vortex oscillator, performing supersonic wave extraction for 2-10 min, performing centrifugation, taking 1 mL of the supernatant out, blow-drying the supernatant, and redissolving the obtained product with 2 mL of 50% of an acetonitrile aqueous solution; and 3) filtering the redissolved solution via a filter membrane with the micropore size being 0.22 mum, and measuring the filtrate via HPLC-MS / MS (that is high performance liquid chromatography and mass spectrum and secondary mass spectrum).
Owner:浙江公正检验中心有限公司 +1
Product tablet and related pack
Owner:BELLOLI GIANPAOLO +2
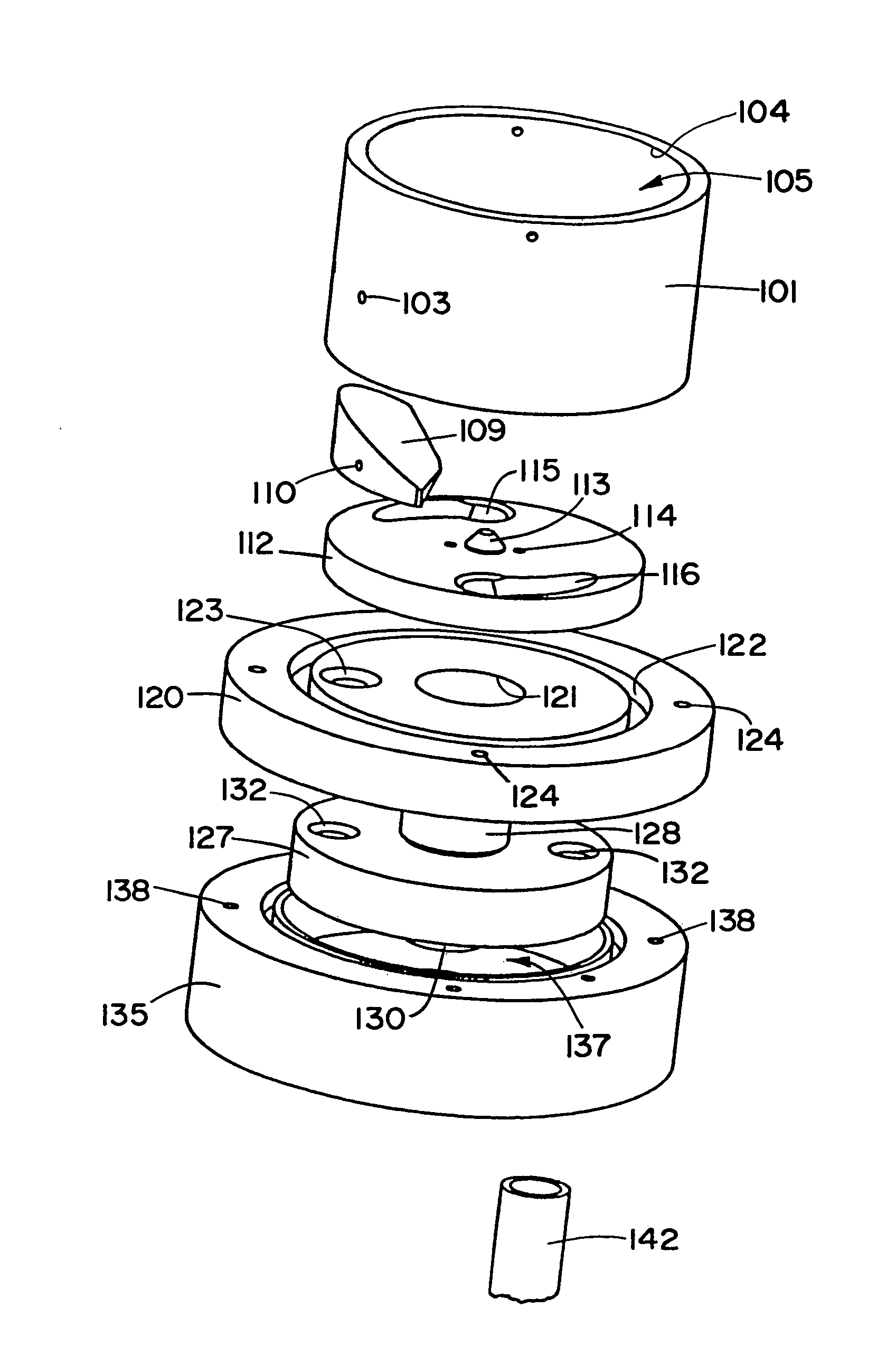
Tablet dispenser with isolated product hopper
ActiveUS7896195B2Avoid contactSmall article dispensingTableware washing/rinsing machine detailsEngineeringMechanical engineering
Owner:ECOLAB USA INC
Production method of bee pollen tablet for treating fatty liver
InactiveCN102114058BAnthropod material medical ingredientsDigestive systemFreeze-dryingAbsorption effect
The invention provides a production method of a bee pollen tablet for treating fatty liver, relating to production technologies of product tablets. The production method comprises the following steps of: performing air stream pulverization and wall breaking on impurities-removed bee pollen; soaking in water at normal pressure and temperature; performing ultrasonic treatment; centrifuging by a refrigerated centrifuge with the rotation speed of 8000-10000rpm, taking supernatant; separating the supernatant at the ambient temperature of -5 to 5 DEG C by a molecular sieve of below 2800D to obtain water-soluble bee pollen of which the molecular weight is smaller than 2800D; performing vacuum freeze drying on the water-soluble bee pollen of which the molecular weight is smaller than 2800D to form lybee pollen lyophilized powder with the moisture content below 4%; and mixing the lyophilized powder of bee pollen with water soluble starch and tabletting. According to the production method provided by the invention, the effective components in the bee pollen are extracted by water firstly and then prepared into lyophilized powder, thus being conductive to the subsequent tabletting processing, the tablet is convenient to take, the product activity is kept, and the medicament is not liable to oxidative deterioration and has a good absorption effect and rehydration.
Owner:周斌 +1
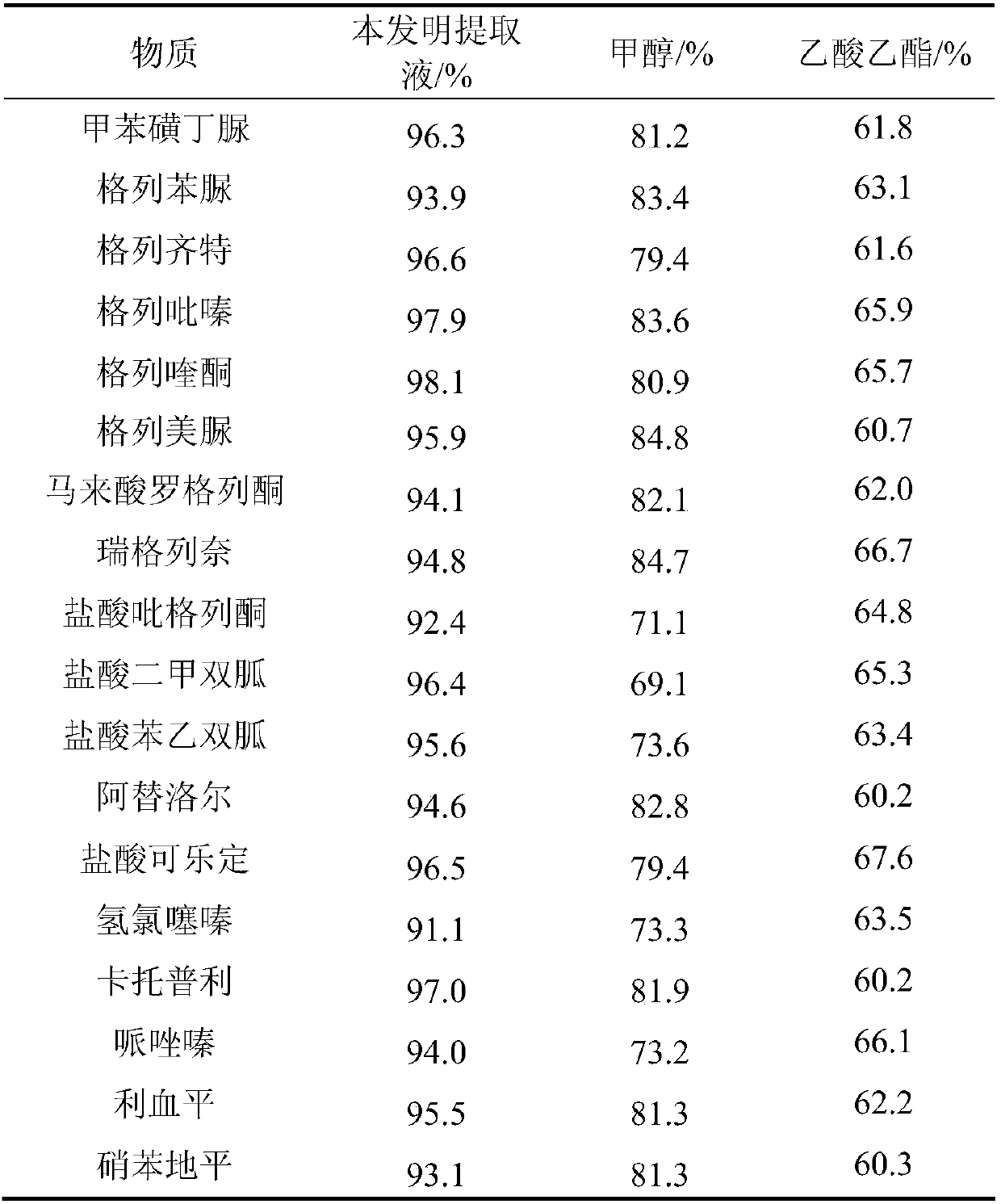
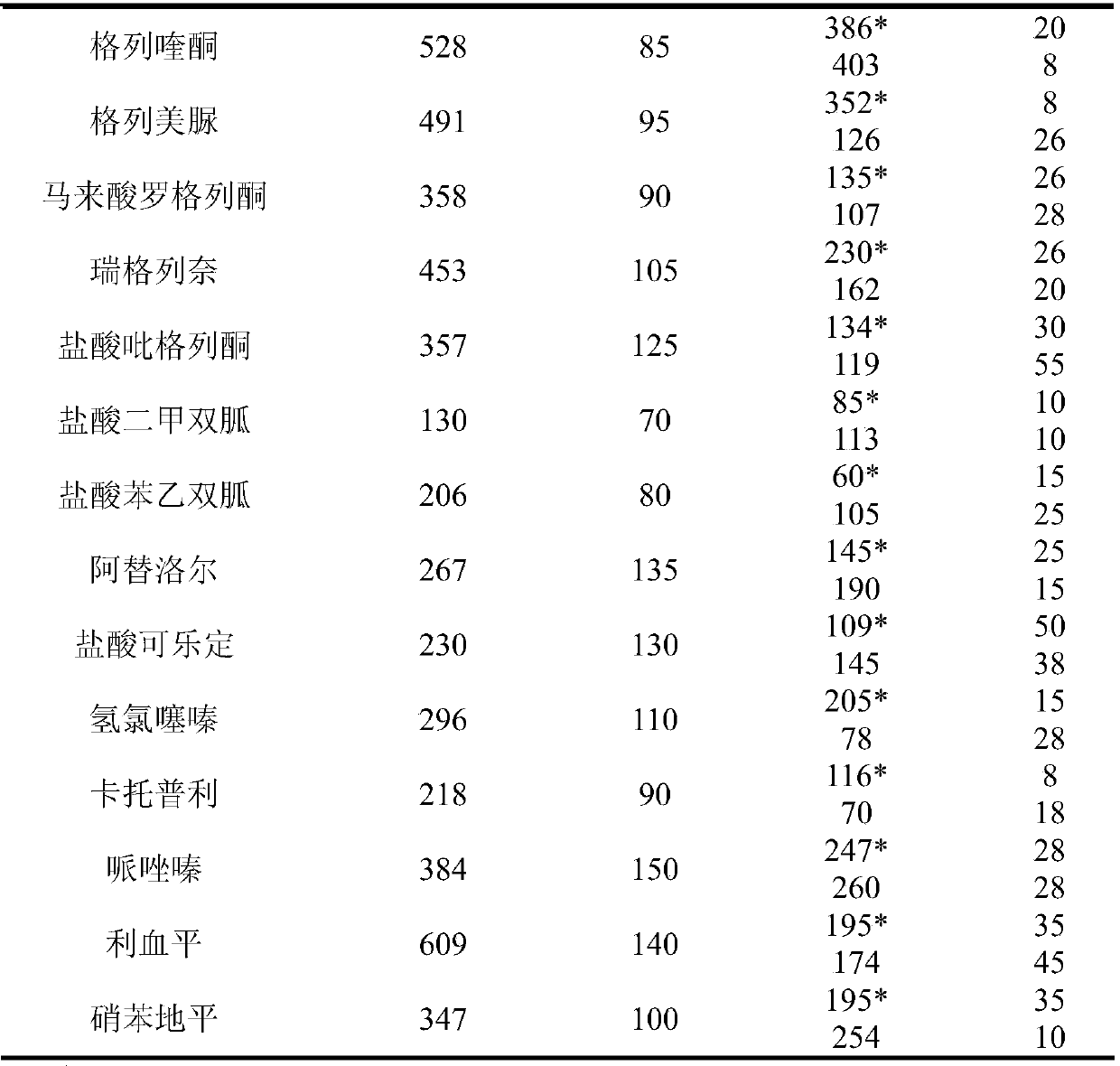
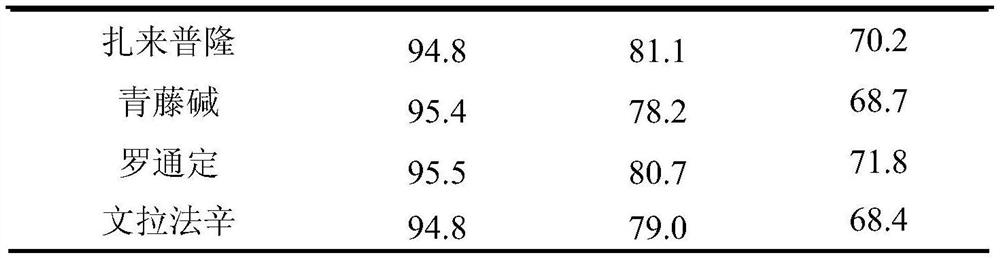
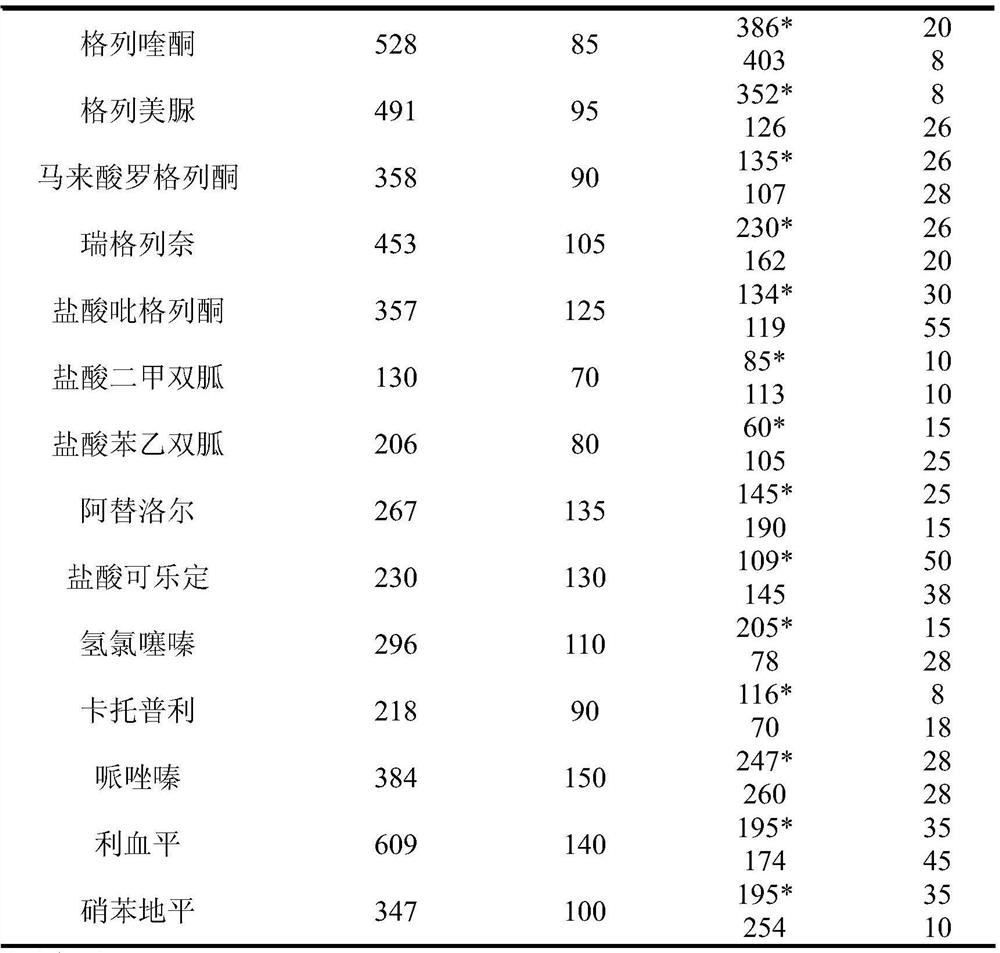
Method for detecting residue of 18 illegally-added blood-glucose-reducing and antihypertensive drugs in health product
ActiveCN107741465AHigh extraction rateImprove detection efficiencyComponent separationSpectroscopyEthyl acetate
The invention relates to the technical field of drug residue detection and aims at providing a method for detecting residue of 18 illegally-added blood-glucose-reducing and antihypertensive drugs in ahealth product, which has the characteristics of rapidness, accuracy, simplicity and low cost. According to the technical scheme, the method for detecting the residue of the 18 illegally-added blood-glucose-reducing and antihypertensive drugs in the health product comprises the following steps: (1) preparing homemade extract, namely refluxing a mixture of methyl alcohol, ethyl acetate and decyl alcohol polyoxyethylene ether for 30 minutes at the temperature of 60-65 DEG C, and cooling for later use; (2) taking health product tablets, capsules or granules, breaking, accurately weighing 3-10g of sample, putting into a centrifugal tube provided with a plug, then adding 20-60mL of homemade extract, mixing, carrying out ultrasonic extraction for 2-10 minutes, carrying out centrifugal separation, then taking 1mL of supernatant, carrying out blow drying with nitrogen, and redissolving with 1mL of 50% acetonitrile aqueous solution; and (3) filtering the redissolved solution by virtue of a 0.22 microns microporous membrane, and carrying out HPLC-MS / MS (high performance liquid chromatography+mass spectroscopy+secondary mass spectroscopy detection) determination on filtrate.
Owner:浙江公正检验中心有限公司 +1
High-performance film coating premix capable of covering peculiar smell, and preparation method thereof
InactiveCN102327245AReduce permeabilityDeodorize and enhance aromaPharmaceutical delivery mechanismPolymer scienceSilicon dioxide
The invention provides high-performance film coating premix capable of covering peculiar smell and a preparation method thereof, belongs to the technical field of pharmaceutical excipient production, and solves the defects that Chinese medicinal tablets and health care product tablets have unacceptable pungent smell in the prior art. The high-performance film coating premix mainly comprises hydroxypropyl methyl cellulose, ethyl cellulose, polyacrylic resin, and polyethylene glycol; the hydroxypropyl methyl cellulose has a particularly low viscosity level, the polyacrylic resin is one or more of polyacrylic resin No.IV resin, and the polyethylene glycol is polyethylene glycol 400, polyethylene glycol 4000, polyethylene glycol 6000 or polyethylene glycol 8000; and flow aid is one or two of silica or magnesium stearate and the like, and a coloring agent is one or more of various color lake or ferric oxide, and natural essence is one or more of hawthorn essence, orange essence, apple essence and the like. The high-performance film coating premix is mainly used for Chinese medicinal tablets and health care product tablets.
Owner:连云港康力特药业有限公司
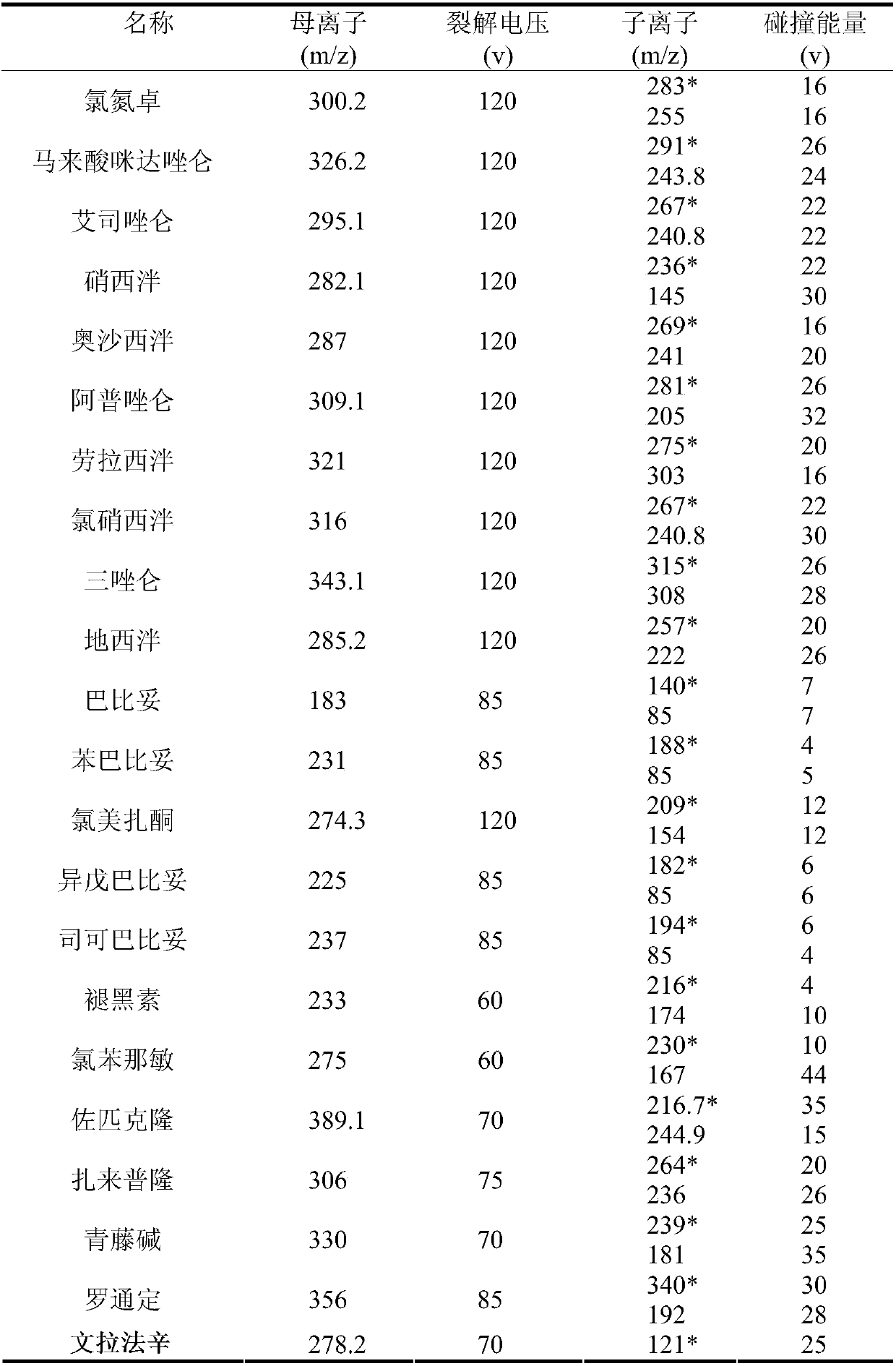
Method for detecting residue of 22 illegally-added nerve-calming drugs in health product
ActiveCN107741464AHigh extraction rateAchieving Simultaneous DetectionComponent separationSpectroscopyEthyl acetate
The invention relates to a drug residue detection technology and aims at providing a method for detecting residue of 22 illegally-added nerve-calming drugs in a health product, which has the characteristics of rapidness, accuracy, simplicity and low cost. According to the technical scheme, the method for detecting the residue of the 22 illegally-added nerve-calming drugs in the health product comprises the following steps: (1) preparing homemade extract, namely refluxing a mixture of methyl alcohol, ethyl acetate and capryl alcohol polyoxyethylene ether for 30 minutes at the temperature of 60-65 DEG C, and cooling for later use; (2) taking health product tablets, capsules or granules, breaking, accurately weighing 3-10g of sample, putting into a centrifugal tube provided with a plug, thenadding 20-60ml of homemade extract, mixing, carrying out ultrasonic extraction for 2-10 minutes, carrying out centrifugal separation, then taking 1mL of supernatant, carrying out blow drying with nitrogen, and redissolving with 1mL of 50% methanol aqueous solution; and (3) filtering the redissolved solution by virtue of a 0.22 micron microporous membrane, and carrying out HPLC-MS / MS (high performance liquid chromatography+mass spectroscopy+secondary mass spectroscopy detection) determination on filtrate.
Owner:浙江公正检验中心有限公司 +1
Cefadroxil tablet and preparation method thereof
ActiveCN104800177ADisintegrates quicklyEvenly dispersedAntibacterial agentsOrganic active ingredientsCarboxymethyl starchCellulose
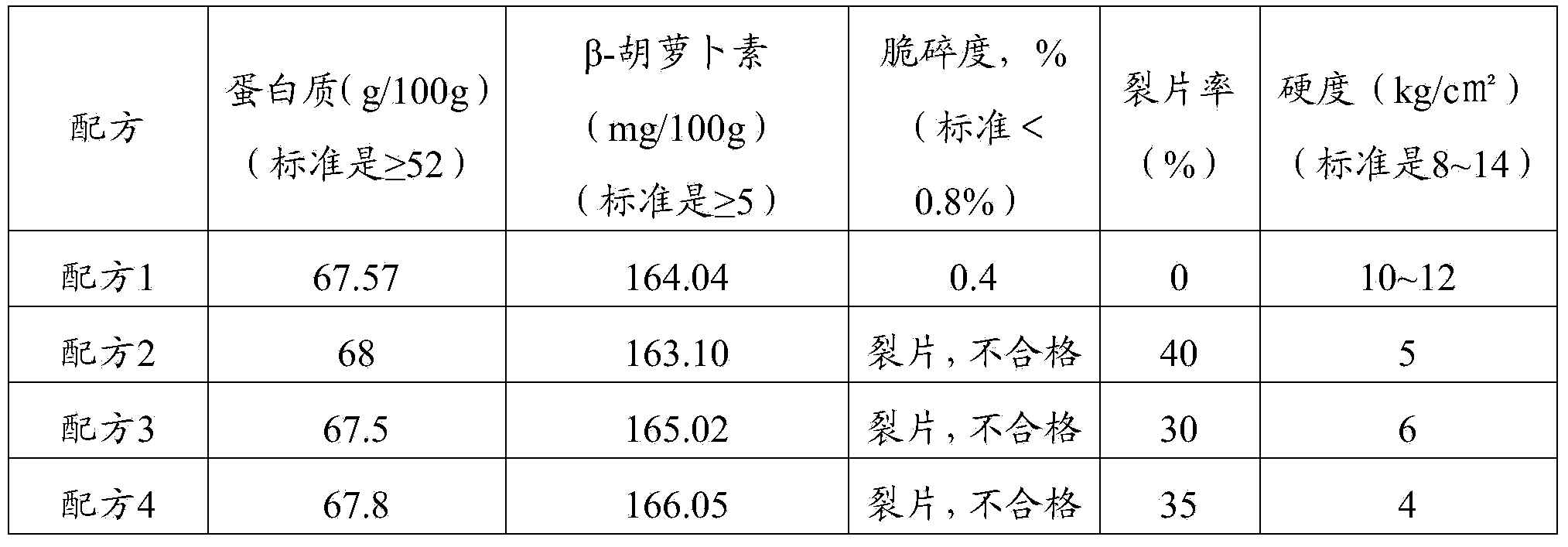
The invention provides a cefadroxil tablet. The cefadroxil tablet is prepared by mixing and tabletting cefadroxil particles, micro-crystal cellulose and a lubricating agent. The cefadroxil particle comprises the following ingredients: 250 parts by weight of cefadroxil, 10 to 20 parts by weight of micro-crystal cellulose, 2 to 4 parts by weight of starch, and 4 to 6 parts by weight of carboxymethyl starch sodium. Compared with the prior art, the cefadroxil tablet adopts the carboxymethyl starch sodium as a disintegrating agent which is unique in swelling effect; moreover, the micro-crystal cellulose is respectively added in the granulating and tabletting process, so that the cefadroxil is more uniformly dispersed, a product tablet core has good disintegrating effect, the particles can be rapidly disintegrated, the dissolution rate of the product is high, and the disintegrating time is short. The experiment result shows that the dissolution rate of the cefadroxil tablet is 87 to 103 percent, and the disintegrating time is 4 to 6 minutes.
Owner:HUNAN KELUN PHARMA
Evaluation method of oxygen-containing functional group on surface of active carbon
InactiveCN104359907AReasonable designMaterial analysis by observing effect on chemical indicatorActivated carbonImage resolution
The invention relates to a performance research method of an active carbon material, and in particular to an evaluation method of an oxygen-containing functional group on a surface of active carbon. The method comprises the following steps: (1) qualitatively judging the type of the oxygen-containing functional groups on the surfaces of to-be-tested carbon samples under different technical conditions by adopting a Fourier transform infrared spectrometer, wherein the testing conditions are as follows: the mass ratio of KBr to the carbon samples is 200:1, the thickness of a product tablet is 0.8mm-1.2mm, the scanning range is 4000cm<-1> to 450cm<-1>, the scanning speed is 0.20cm / s, the scanning time is 1min, the scanning times is 4, the unit of longitudinal coordinates is T% and the resolution ratio is 4.0cm<-1>; judging whether the surfaces of the to-be-tested carbon samples have the oxygen-containing functional groups such as carboxyl, lactone groups and phenolic hydroxyl groups or not according to a spectral peak, if so, executing step (2).
Owner:SHANXI XINHUA CHEM
Method for preparing LMO-YSZ composite solid electrolyte by microwave combustion supporting method
ActiveCN109942293AEasy to operateLow costFinal product manufactureFuel cellsPorosityComposite electrolyte
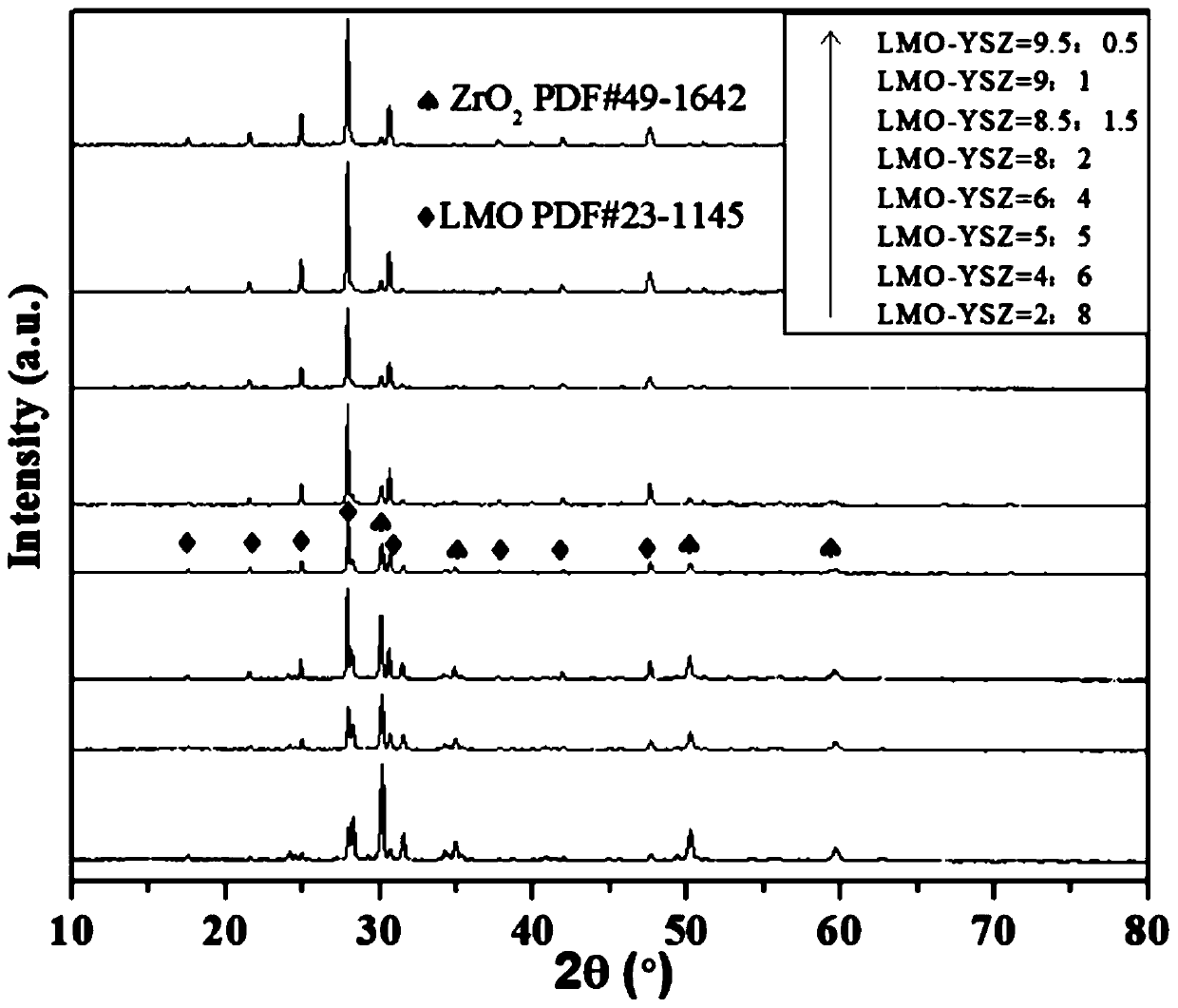
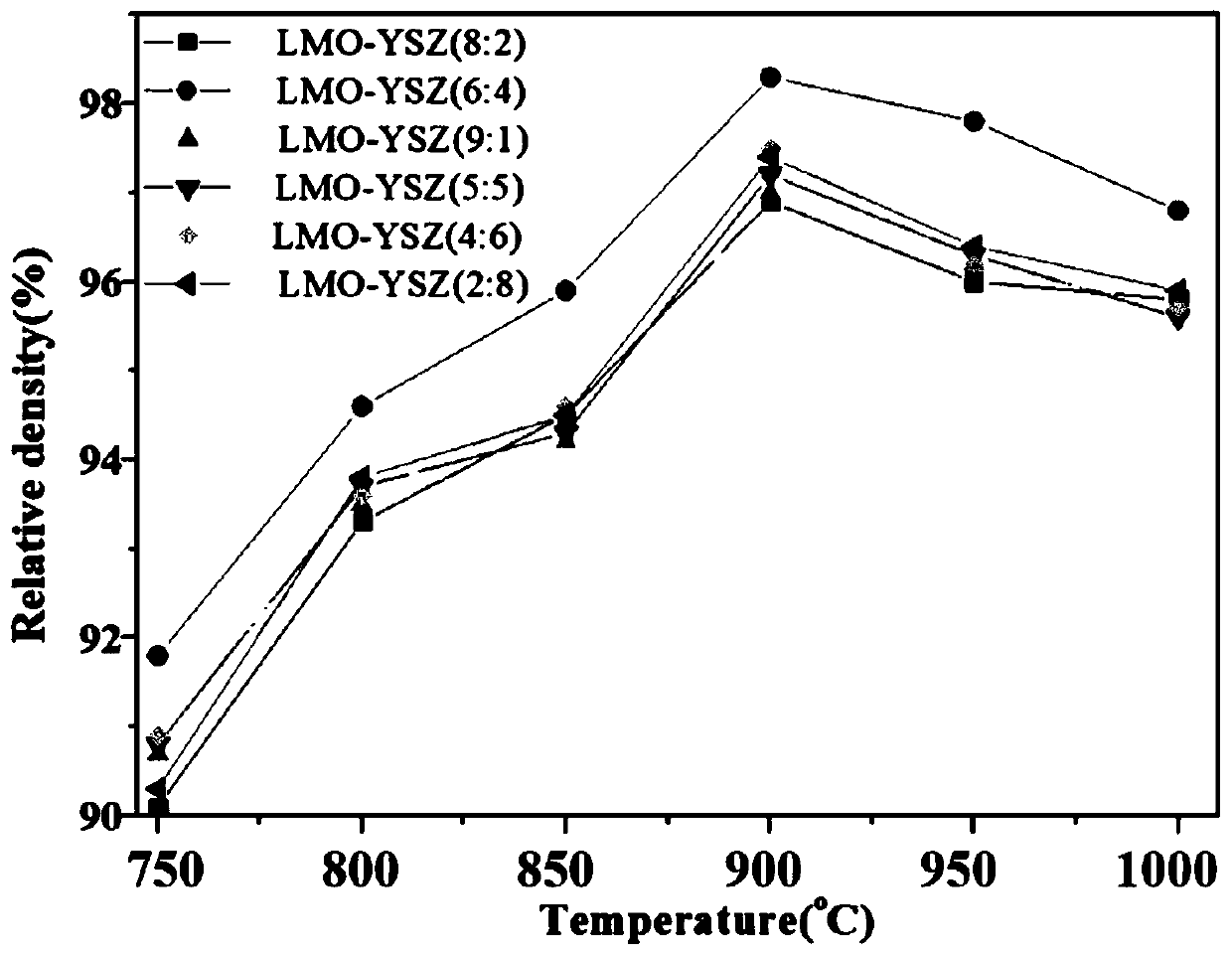
The invention provides a method for preparing LMO-YSZ composite solid electrolyte by a microwave combustion supporting method, and relates to the technical field of solid electrolyte ceramic materialpreparation. The method comprises the following steps: firstly, preparing lanthanum molybdate LMO from lanthanum nitrate and ammonium molybdate by a microwave combustion supporting method; then, preparing yttrium-based zirconia YSZ from zirconium nitrate and yttrium nitrate by the microwave combustion supporting method; and finally preparing the LMO-YSZ composite solid electrolyte by ball-millingand mixing. The LMO-YSZ composite electrolyte prepared by adopting the microwave combustion supporting method has good sintering performance in a product tablet sintered at 900 DEG C, and the porosityis less than 5.5 percent; along with constant increasing of LMO proportion, the oxygen ion conductivity shows a trend of gradually increasing, and when the temperature is 800 DEG C, the sample of LMO-YSZ=(6:4) has a maximized conductivity which is 0.0199S / cm and the activation energy of 1.232eV.
Owner:HEFEI UNIV
Health-care product tablet for treating gastritis and preparation method thereof
InactiveCN102949667AIncrease the totalFunction increaseDigestive systemDrageesRadix Astragali seu HedysariBlood drug concentration
The invention discloses a health-care product tablet for treating gastritis and a preparation method of the heal-care product tablet. The heal-care product tablet contains the following raw material medicinal components in parts by weight: 30 parts of radix pseudostellariae, 12 parts of radix astragali, 12 parts of rhiizoma dioscoreae, 12 parts of dendrobium, 12 parts of hyacinth bean, 30 parts of fructus hordei germinatus, 12 parts of radix salviae miltiorrhizae, 30 parts of cortex albiziae, 5 parts of glycyrrhiza and 3 parts of radix curcumae. The relative tablet in the invention has the effects of tonifying the spleen and nourishing the stomach and tonifying yin and activating collaterals, and is used for treating the symptoms such as atrophic gastritis and chronic superficial gastritis. A traditional Chinese medicinal composition of the tablet has the advantages of being fast to absorb, high in bioavailability, rapid in speed of reaching the effective blood concentration, accurate in qualification and stable in quality, free from dependency and the like, and conventional equipment can finish the production of the health-care product tablet.
Owner:XIAN FUAN INNOVATION CONSULTATION
Dilapidated wall three-in-one type royal jelly product
InactiveCN103005251AImprove immunityImprove sub-health statusFood preparationBiotechnologyAnimal science
The invention provides a dilapidated wall three-in-one type royal jelly product. The product has the following main characteristics: the product comprises tablets, paste products and soft capsules. The product is characterized in that taking preparation of 10 kg dilapidated wall three-in-one type royal jelly product tablets as an example, the tablets comprise the following components by weight: 6.35-6.37 kg of royal jelly freeze-dried powder, 2.72-2.74 kg of queen bee placenta powder, 0.49-0.51 kg of selenium-enriched yeast powder, 0.25-0.27 kg of ingredient maltodextrin, 0.10-0.13 kg of microcrystalline cellulose, and 0.03-0.04 kg film-coating liquid coating premixing agent. The rest paste products and the capsules have the same components and technique with the tablets, but specific numerical values are different. The product has the advantages that the royal jelly, the selenium element and the queen bee placenta are integrated, the cellular immunity gene activating theory is combined, and all nutrition ingredients are superfinely dilapidated walls to enter human bodies in a form of micromolecules so as to accord with the human body circulating rules.
Owner:周云川
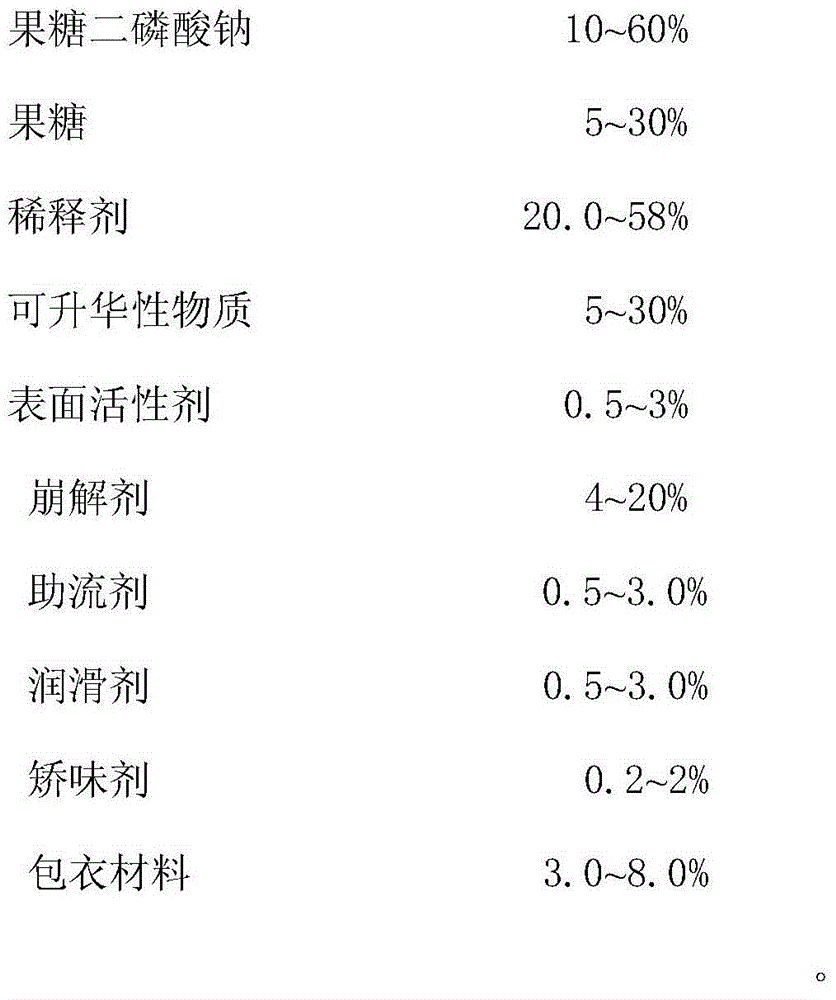
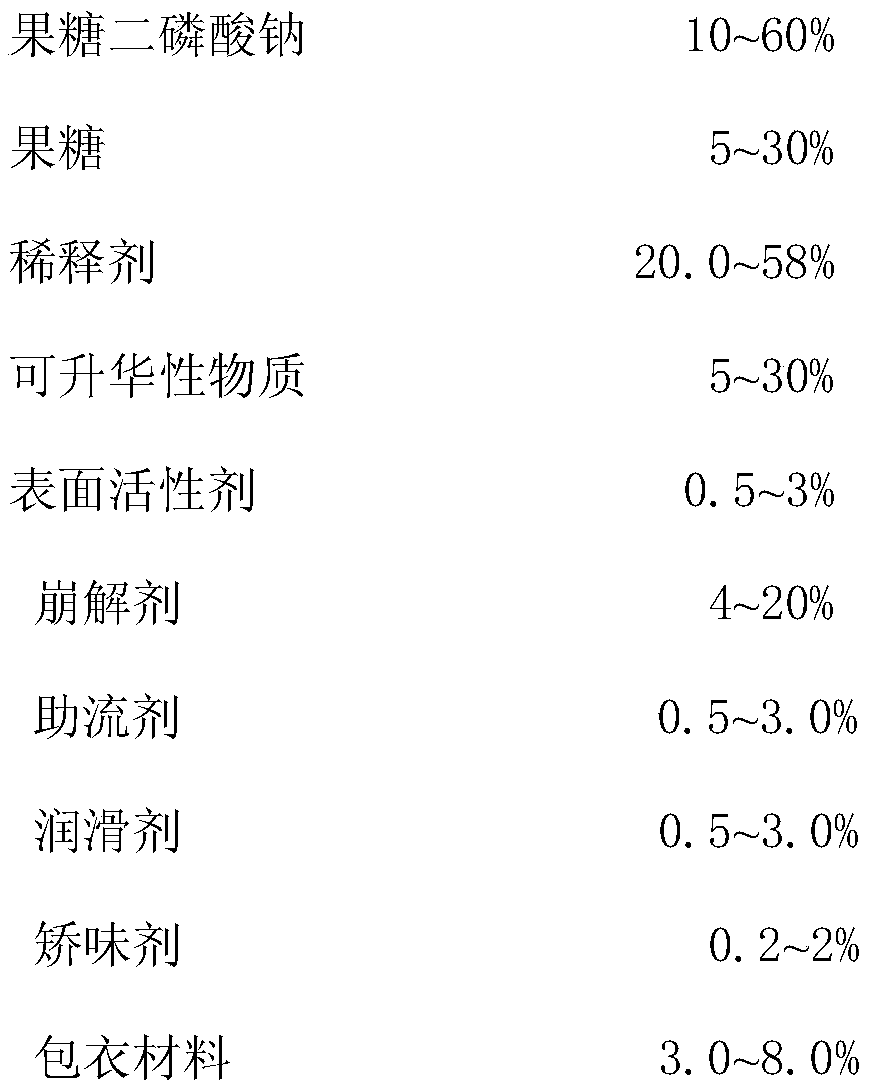
Compound sodium fructose diphosphate and fructose orally disintegrating tablets and preparation method thereof
ActiveCN105769887ADisintegrates quicklyImprove support strengthOrganic active ingredientsNervous disorderOrally disintegrating tabletBULK ACTIVE INGREDIENT
The invention provides compound sodium fructose diphosphate and fructose orally disintegrating tablets. The compound sodium fructose diphosphate and fructose orally disintegrating tablets are prepared from the following ingredients in percentage by weight: 10-60% of sodium fructose diphosphate, 5-30% of fructose, 5-30% of sublimable substance, 0.5-3% of a surfactant, 4-20% of a disintegrating agent, 0.5-3.0% of a flow aid, 0.5-3.0% of a lubricant, 20.0-58% of a diluent, 0.2-2% of a corrigent, and 3-8% of a coating material. The invention further provides a preparation method of the compound sodium fructose diphosphate and fructose orally disintegrating tablets. The preparation method comprises the following steps: mixing the active ingredients, namely sodium fructose diphosphate and fructose, and pharmaceutical excipients, wherein the sublimable substance is added into the pharmaceutical excipients, tabletting the mixture, coating the obtained semi-finished product tablets, heating the semi-finished product tablets after coating, and drying, thus the sublimable substance sublimates, a plurality of holes are formed in the tablets, and finally, the tablets capable of rapidly disintegrating and with certain strength are obtained. The orally disintegrating tablets can rapidly disintegrate, so that good clinical effects can be obtained; the preparation method is easy and convenient to operate, is suitable for industrial production, and has a large application value.
Owner:SHANGHAI SUNTECH PHARMA +1
Preparing device for migraine treatment medicine
InactiveCN108542776AImprove coordinationEfficiencyPharmaceutical product form changeIndividual articlesBottleRotating disc
The invention discloses a preparing device for migraine treatment medicine. The preparing device comprises a fixed machine frame, a feeding opening extending upwards is fixedly formed in the upper endface of the fixed machine frame, a cylindrical screening net is fixedly arranged on the right side of the feeding opening, an inverted-conical discharging cavity is communicated to the lower side ofthe screening net, a delivering pipe is arranged in the middle of the right end face of the discharging cavity, a liquid cavity is fixedly formed in the upper end of the delivering pipe, a transmission cavity is communicated to the lower end face of the discharging cavity, a horizontal rotating disc is arranged in the transmission cavity, through holes are circumferentially formed in the rotatingdisc, a horizontal conveying belt is arranged on the lower side of the left end face of the fixed machine frame, and containing bottles are arranged in the conveying belt. When the preparing device works, the device is simple in structure and easy to operate, two intermittent movement parts simultaneously rotate to cooperatively complete the operation process of medicine powder pelleting, the automatic efficiency of the device is improved, unaccepted products in finished-product tablets are reduced through the tablet screening net, and waste of medicine is reduced.
Owner:NINGBO HANGZHOU BAY NEW DISTRICT NO 9 TECH SERVICE
Health-care product tablet for treating emphysema, relieving asthma and treating chronic bronchitis and preparation thereof
InactiveCN102949644AFast absorptionImprove bioavailabilityRespiratory disorderPlant ingredientsDiseaseObstructive chronic bronchitis
The invention discloses a health-care product tablet for treating emphysema, relieving asthma and treating chronic bronchitis. The health-care product tablet comprises the following raw medicine ingredients by weight part: 30 parts of humulus scandens, 30 parts of codonopsis pilosula, 15 parts of tuckahoe, 5 parts of radix stemonae, 10 parts of radix asteris, 10 parts of semen raphani, 10 parts of semen brassicae and 30 parts of folium microcotis. The tablet related to the invention has the effects of reinforcing earth to generate metal, reducing Qi and expelling phlegm and is applied to the diseases such as the emphysema, the paracmasis of asthma and the chronic bronchitis. The traditional Chinese medicine combination of the health-care product tablet has the advantages of quick absorption, high biological availability, high effective blood concentration speed, independency, accurate quantification, stable quality, and the like. A conventional device can be used for finishing the production.
Owner:XIAN FUAN INNOVATION CONSULTATION
Health-care product tablet for treating hypertension and preparation method thereof
InactiveCN102949602ALow absorption rateSignificant cholesterol-lowering effectDrageesCardiovascular disorderTraditional medicineBioavailability
The invention discloses a health-care product tablet for treating hypertension and a preparation method of the health-care product tablet. The health-care product tablet contains the following raw material medicinal components in parts by weight: 30 parts of concha haliotidis, 30 parts of raw oyster shell, 15 parts of radix paeoniae alba, 15 parts of radix achyranthis bidentatae, 12 parts of ramulus uncariaecum uncis, 3 parts of lotus plumule, 10 parts of poria cocos, 10 parts of rhizoma alismatis and 10 parts of rhizoma gastrodiae. The related health-care product tablet in the invention has the effects of calming the liver and suppressing yang, and is used for treating the hypertension with hyperactivity of liver-yang. A traditional Chinese medicinal composition of the health-care product tablet has the advantages of being fast in absorption, high in bioavailability, long in time of maintaining the effective blood concentration, accurate in qualification and stable in quality, and free from dependency, and conventional equipment can finish the production of the health-care product tablet.
Owner:XIAN FUAN INNOVATION CONSULTATION
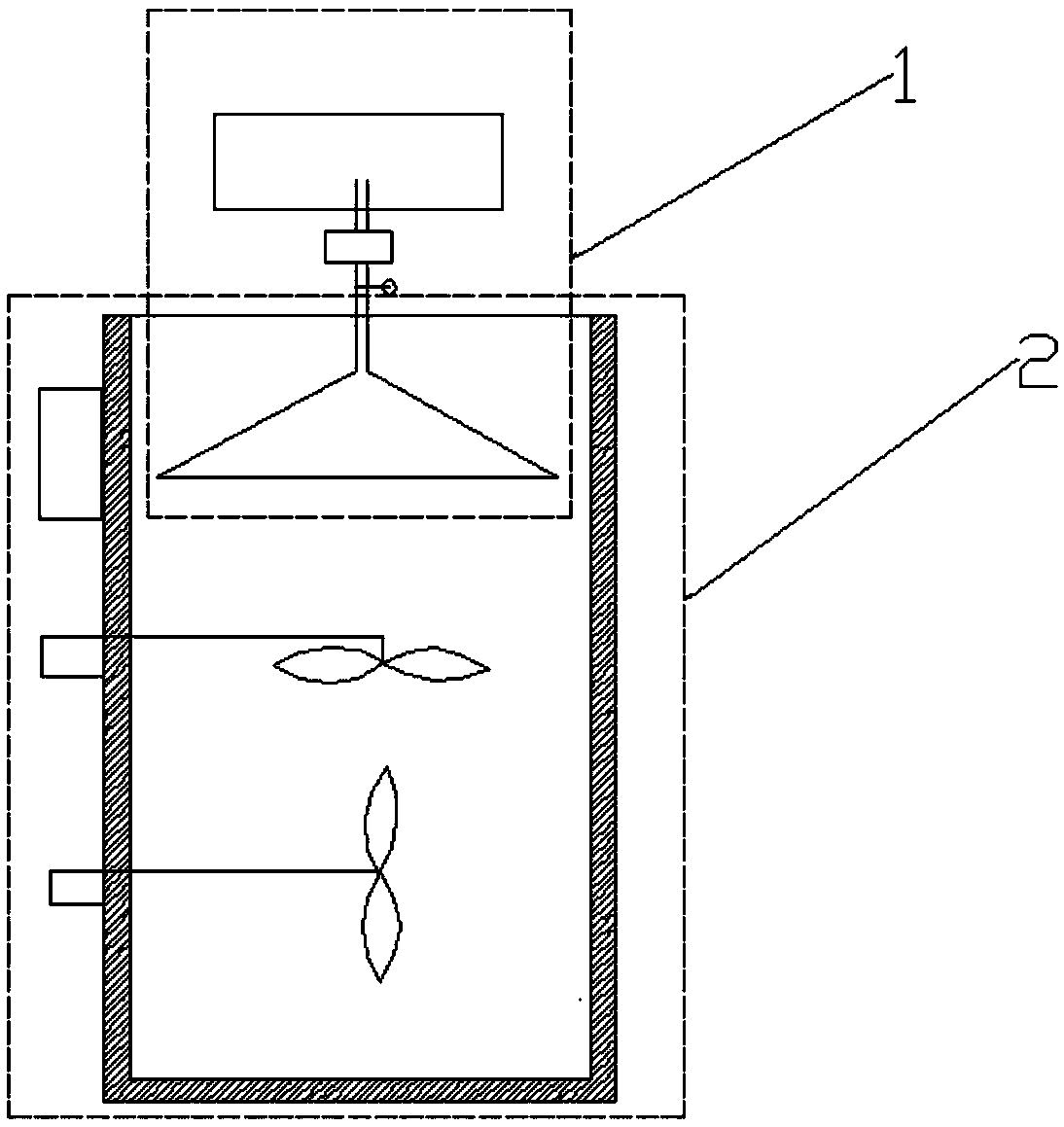
Micro-powder encapsulating and material mixing device for improving content uniformity of clonidine hydrochloride in zhenju antihypertensive tablets and material mixing method
ActiveCN109092191AGood content uniformityImprove mixing uniformityOrganic active ingredientsTransportation and packagingMedicineProduct tablet
The invention discloses a micro-powder encapsulating and material mixing device for improving the content uniformity of clonidine hydrochloride in zhenju antihypertensive tablets and a material mixingmethod. The material mixing method comprises the following steps: firstly, a clonidine hydrochloride solution is prepared by dissolving clonidine hydrochlorid with an ethanol solution, and is uniformly sprayed to a medicine mixing container containing other auxiliary materials while rotating at high speed in a dosing nozzle, furthermore, a transverse cutting blade and a longitudinal cutting bladeopposite to the dosing nozzle in the rotation direction are arranged in the medicine mixing container, and the medicine mixing container synchronously rotates at the high speed reversely while the materials are mixed until the solution is completely sprayed, the ethanol solution is volatilized in the high-speed rotating container, and the clonidine hydrochloride and the other raw materials form amicro-powder encapsulating state to complete the micro-powder encapsulating and material mixing process; and the micro-powder encapsulating and material mixing device for improving the content uniformity of the clonidine hydrochloride in the zhenju antihypertensive tablets and the material mixing method disclosed by the invention effectively improve the mixing uniformity and the bonding firmnessdegree of the clonidine hydrochloride, enhance the content uniformity of the clonidine hydrochloride in finished product tablets of the zhenju antihypertensive tablets, lower errors in the proceduresof artificial compounding, material mixing and the like, reduce the workload and improve the work efficiency.
Owner:ZHONGXING PHARM CO LTD JIANGSU
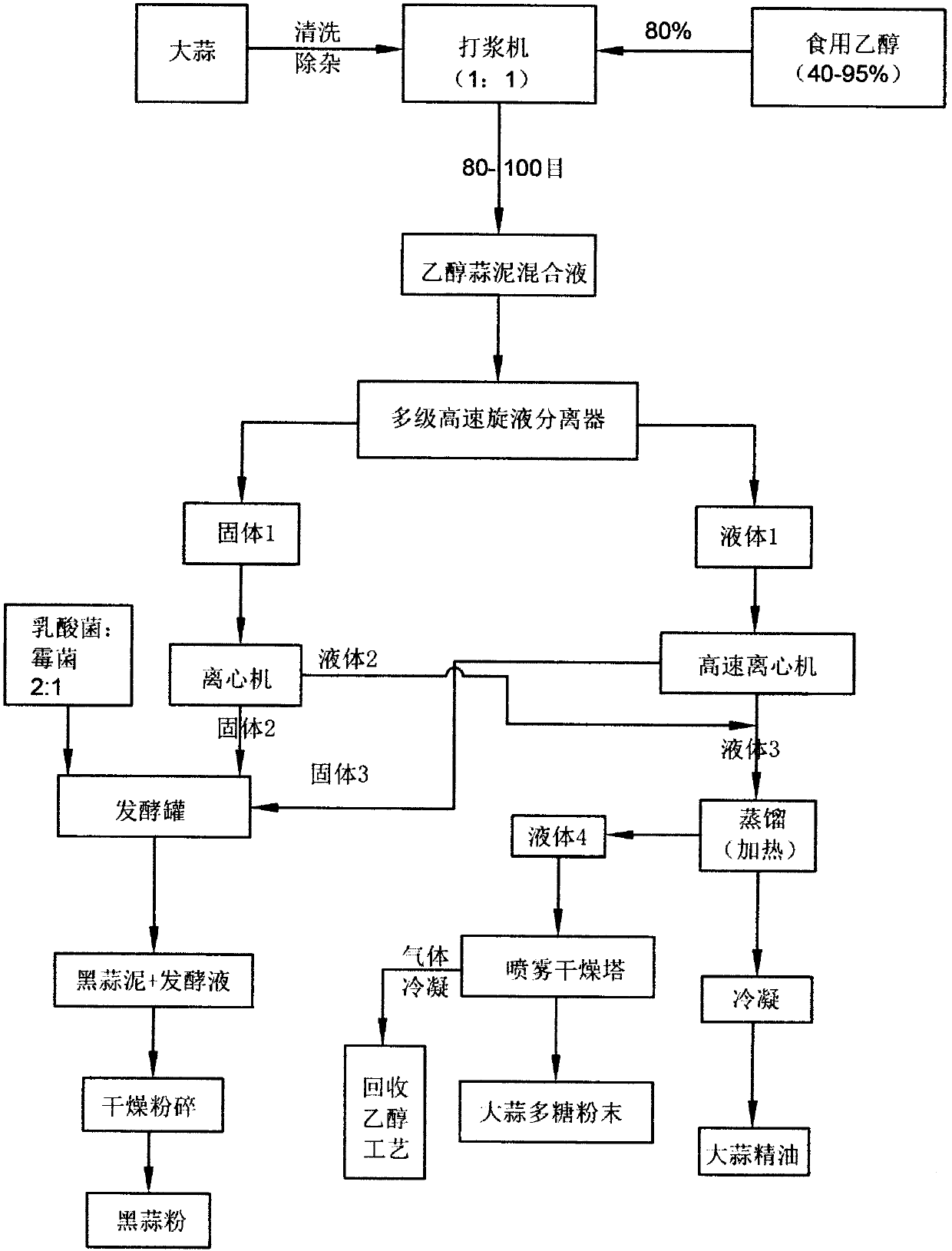
Garlic deep processing and comprehensive utilization process and device thereof
PendingCN110856543AIncrease credibilityRealize trace flowEssential-oils/perfumesBulk chemical productionGARLIC POWDERProcess engineering
The invention provides a garlic deep processing and comprehensive utilization process, which can make garlic into black garlic powder, garlic polysaccharide, garlic essential oil and other products. The whole technological process is carried out in a closed device. According to the invention, no waste gas, wastewater or pollutants are generated during the whole process, zero pollution production is realized, and the product conversion rate is high; the added value of the product is greatly improved, and the product can be further processed into garlic essential oil capsules, health product tablets, liquid agents and drugs directly facing the consumer terminal market; and garlic polysaccharide can be made into tablets and black garlic powder can be made into granules.
Owner:山东丙合生物科技有限公司
Spirulina composition and preparation method and preparation thereof
ActiveCN104382950ANot easy to splitSolve the fragileMetabolism disorderDigestive systemMedicineBULK ACTIVE INGREDIENT
The invention relates to the field of pharmaceutical preparations and particularly relates to a spirulina composition and a preparation method and a preparation thereof. The spirulina composition comprises spirulina and high-substituted hydroxypropyl cellulose. The spirulina composition and the preparation thereof provided by the invention are less prone to splitting, the problem of agglomeration of high-substituted hydroxypropyl cellulose can be thoroughly solved, a prepared spirulina health product tablet is high in forming rate, the appearance has no white spots, and the product quality is in line with requirements; and raw materials and auxiliary materials in the spirulina composition and the preparation thereof provided by the invention have high compatibility, and the content of active ingredients is less affected by a tabletting process.
Owner:BY HEALTH CO LTD
Health care product tablet used for nourishing and developing hair and preparation method thereof
InactiveCN102949654AFast absorptionImprove bioavailabilityDermatological disorderFood preparationBlood drug concentrationChinese herbology
The invention discloses a health care product tablet used for nourishing and developing hair and a preparation method thereof. The health care product tablet comprises following raw material medicine ingredients in parts by weight: 15 parts of radix rehmanniae, 9 parts of Chinese angelica, 30 parts of magnetite, 6 parts of fructus amomi, 15 parts of prepared rehmannia roots, 6 parts of ligusticum wallichii, 15 parts of yerbadetajo herb, 15 parts of mulberry fruit, 12 parts of radix paeoniae alba, 15 parts of radix polygoni multiflori preparata, 15 parts of cinnabar root poria, 9 parts of fructus chaenomelis and 15 parts of rhizoma polygonati. The tablet disclosed by the invention achieves the effects of tonifying kidneys to thrive the hair and nourishing the blood to calm the heart, and is used for treating illnesses such as alopecia, hair scarity and premature hair greyness. The traditional Chinese medicine composition disclosed by the invention has the advantages of fast absorption, high bioavailability, fast effective blood concentration reaching speed, no dependence, accuracy in quantification, stable quality and the like, and can be produced by using conventional equipment.
Owner:XIAN FUAN INNOVATION CONSULTATION
Health care product tablet for uremia and preparation method thereof
InactiveCN102949692AFast absorptionImprove bioavailabilityUrinary disorderPlant ingredientsBlood drug concentrationAtractylis ovata
The invention discloses a health care product tablet for uremia and a preparation method of the health care product tablet. The health care product tablet contains the following raw material medicinal components in parts by weight: 10 parts of cooker radix aconiti lateralis preparata, 10 parts of ramulus cinnamomi, 15 parts of pericarpium citri reticulatae, 15 parts of poria cocos, 15 parts of rhizoma atractylodis macrocephalae, 10 parts of rhizoma zingiberis recens, 30 parts of herba leonuri and 5 parts of glycyrrhiza. The related tablet in the invention with the effects of warming yang to promote diuresis is used for uremia. A traditional Chinese medicine composition of the health care product tablet has the advantages of being fast to absorb, high in bioavailability, rapid in speed of reaching the effective blood concentration, accurate in qualification, stable in quality, free from dependency and the like, and the preparation of the traditional Chinese medicine composition can be finished by conventional equipment.
Owner:XIAN FUAN INNOVATION CONSULTATION
Production method of pine pollen tablet for treating prostatic hypertrophy
ActiveCN102114056ALow costSignificant effect on hypertrophyUrinary disorderPill deliveryEccentric hypertrophySide effect
The invention provides a production method of a pine pollen tablet for treating prostatic hypertrophy, relating to production technologies of product tablets. The production method comprises the following steps of: performing air stream pulverization and wall breaking on pine pollen with impurities removed; soaking in water of normal pressure and temperature; performing ultrasonic treatment; centrifuging by a refrigerated centrifuge, taking supernatant; separating the supernatant at the ambient temperature of 1-5 DEG C to obtain water soluble matters of pine pollen of which the molecular weight is smaller than 2000D; performing vacuum freeze drying on the water soluble matters of pine pollen of which the molecular weight is smaller than 2000D to form lyophilized powder of pine pollen with the moisture content below 5%; and finally, mixing the lyophilized powder of pine pollen with lecithin, water-soluble starch and microcrystalline cellulose PH102 for preparation. The pine pollen tablet provided by the invention has no side effect, simple production process and low cost, is favorable for reducing the medicament cost and reserving the activity of functional substances, is not liable to oxidative deterioration, and has good absorption effect and good rehydration.
Owner:YANTAI NEW ERA HEALTH IND +1
Production method of pine pollen tablet for treating prostatic hypertrophy
ActiveCN102114056BLow costSignificant effect on hypertrophyPill deliveryEccentric hypertrophySide effect
The invention provides a production method of a pine pollen tablet for treating prostatic hypertrophy, relating to production technologies of product tablets. The production method comprises the following steps of: performing air stream pulverization and wall breaking on pine pollen with impurities removed; soaking in water of normal pressure and temperature; performing ultrasonic treatment; centrifuging by a refrigerated centrifuge, taking supernatant; separating the supernatant at the ambient temperature of 1-5 DEG C to obtain water soluble matters of pine pollen of which the molecular weight is smaller than 2000D; performing vacuum freeze drying on the water soluble matters of pine pollen of which the molecular weight is smaller than 2000D to form lyophilized powder of pine pollen with the moisture content below 5%; and finally, mixing the lyophilized powder of pine pollen with lecithin, water-soluble starch and microcrystalline cellulose PH102 for preparation. The pine pollen tablet provided by the invention has no side effect, simple production process and low cost, is favorable for reducing the medicament cost and reserving the activity of functional substances, is not liable to oxidative deterioration, and has good absorption effect and good rehydration.
Owner:YANTAI NEW ERA HEALTH IND +1
Detection method of 22 kinds of illegally added sedative drugs residues in health products
ActiveCN107741464BHigh extraction rateAchieving Simultaneous DetectionComponent separationSedative drugDioxyethylene Ether
The invention relates to a drug residue detection technology and aims at providing a method for detecting residue of 22 illegally-added nerve-calming drugs in a health product, which has the characteristics of rapidness, accuracy, simplicity and low cost. According to the technical scheme, the method for detecting the residue of the 22 illegally-added nerve-calming drugs in the health product comprises the following steps: (1) preparing homemade extract, namely refluxing a mixture of methyl alcohol, ethyl acetate and capryl alcohol polyoxyethylene ether for 30 minutes at the temperature of 60-65 DEG C, and cooling for later use; (2) taking health product tablets, capsules or granules, breaking, accurately weighing 3-10g of sample, putting into a centrifugal tube provided with a plug, thenadding 20-60ml of homemade extract, mixing, carrying out ultrasonic extraction for 2-10 minutes, carrying out centrifugal separation, then taking 1mL of supernatant, carrying out blow drying with nitrogen, and redissolving with 1mL of 50% methanol aqueous solution; and (3) filtering the redissolved solution by virtue of a 0.22 micron microporous membrane, and carrying out HPLC-MS / MS (high performance liquid chromatography+mass spectroscopy+secondary mass spectroscopy detection) determination on filtrate.
Owner:浙江公正检验中心有限公司 +1
Continuous ring-pull can cutting and tabletting production process
PendingCN112872721ASolve the problem that cutting cannot realize automatic feedingSolve processabilityOther manufacturing equipments/toolsTabletingIndustrial engineering
The invention relates to a continuous ring-pull can cutting and tabletting production process which comprises the following steps: a ring-pull can transfer procedure, wherein ring-pull cans output by a storage bin are clamped and stopped on a material turning plate; a ring-pull can feeding procedure, wherein a pushing assembly enables the clamped ring-pull cans to fall onto a supporting assembly; a ring-pull can supporting procedure, wherein the supporting assembly is pressed downwards to fix and support the ring-pull cans; a ring-pull can cutting procedure, wherein end openings in two sides of the ring-pull cans are cut by a cutting mechanism; a ring-pull can transverse cutting procedure, wherein the ring-pull cans are transversely cut and crevassed through a transverse cutting assembly; a first-stage flattening procedure, wherein a driving assembly drives a pressing roller to conduct first-stage flattening on the ring-pull cans; a primary tablet transferring procedure, wherein tablets subjected to primary flattening are transferred to a demising groove; a second-stage flattening procedure, wherein the tablets on the demising groove enter a roller press so as to be subjected to second-stage flattening; and a finished product tablet output procedure, wherein the finished product tablets subjected to second-stage flattening are output into a finished product tablet collecting box. According to the continuous ring-pull can cutting and tabletting production process provided by the invention, the automation degree is high, continuous automatic processing is achieved, and secondary recycling of the ring-pull cans is facilitated through finished product tabletting.
Owner:王一晓
A kind of compound fructose diphosphate sodium fructose orally disintegrating tablet and preparation method thereof
ActiveCN105769887BDisintegrates quicklyImprove support strengthOrganic active ingredientsNervous disorderAdditive ingredientOrally disintegrating tablet
The invention provides compound sodium fructose diphosphate and fructose orally disintegrating tablets. The compound sodium fructose diphosphate and fructose orally disintegrating tablets are prepared from the following ingredients in percentage by weight: 10-60% of sodium fructose diphosphate, 5-30% of fructose, 5-30% of sublimable substance, 0.5-3% of a surfactant, 4-20% of a disintegrating agent, 0.5-3.0% of a flow aid, 0.5-3.0% of a lubricant, 20.0-58% of a diluent, 0.2-2% of a corrigent, and 3-8% of a coating material. The invention further provides a preparation method of the compound sodium fructose diphosphate and fructose orally disintegrating tablets. The preparation method comprises the following steps: mixing the active ingredients, namely sodium fructose diphosphate and fructose, and pharmaceutical excipients, wherein the sublimable substance is added into the pharmaceutical excipients, tabletting the mixture, coating the obtained semi-finished product tablets, heating the semi-finished product tablets after coating, and drying, thus the sublimable substance sublimates, a plurality of holes are formed in the tablets, and finally, the tablets capable of rapidly disintegrating and with certain strength are obtained. The orally disintegrating tablets can rapidly disintegrate, so that good clinical effects can be obtained; the preparation method is easy and convenient to operate, is suitable for industrial production, and has a large application value.
Owner:SHANGHAI SUNTECH PHARMA +1
Detection method of 18 kinds of illegally added hypoglycemic and antihypertensive drug residues in health products
ActiveCN107741465BHigh extraction rateImprove detection efficiencyComponent separationDioxyethylene EtherEthyl acetate
The invention relates to the technical field of drug residue detection and aims at providing a method for detecting residue of 18 illegally-added blood-glucose-reducing and antihypertensive drugs in ahealth product, which has the characteristics of rapidness, accuracy, simplicity and low cost. According to the technical scheme, the method for detecting the residue of the 18 illegally-added blood-glucose-reducing and antihypertensive drugs in the health product comprises the following steps: (1) preparing homemade extract, namely refluxing a mixture of methyl alcohol, ethyl acetate and decyl alcohol polyoxyethylene ether for 30 minutes at the temperature of 60-65 DEG C, and cooling for later use; (2) taking health product tablets, capsules or granules, breaking, accurately weighing 3-10g of sample, putting into a centrifugal tube provided with a plug, then adding 20-60mL of homemade extract, mixing, carrying out ultrasonic extraction for 2-10 minutes, carrying out centrifugal separation, then taking 1mL of supernatant, carrying out blow drying with nitrogen, and redissolving with 1mL of 50% acetonitrile aqueous solution; and (3) filtering the redissolved solution by virtue of a 0.22 microns microporous membrane, and carrying out HPLC-MS / MS (high performance liquid chromatography+mass spectroscopy+secondary mass spectroscopy detection) determination on filtrate.
Owner:浙江公正检验中心有限公司 +1
A kind of spirulina composition and preparation method and preparation thereof
ActiveCN104382950BSolve the fragileImprove molding rateMetabolism disorderDigestive systemCelluloseDrugs preparations
The invention relates to the field of pharmaceutical preparations and particularly relates to a spirulina composition and a preparation method and a preparation thereof. The spirulina composition comprises spirulina and high-substituted hydroxypropyl cellulose. The spirulina composition and the preparation thereof provided by the invention are less prone to splitting, the problem of agglomeration of high-substituted hydroxypropyl cellulose can be thoroughly solved, a prepared spirulina health product tablet is high in forming rate, the appearance has no white spots, and the product quality is in line with requirements; and raw materials and auxiliary materials in the spirulina composition and the preparation thereof provided by the invention have high compatibility, and the content of active ingredients is less affected by a tabletting process.
Owner:BY HEALTH CO LTD
Health care product tablet used for activating meridians to stop pain and preparation method thereof
The invention discloses a health care product tablet used for activating meridians to stop pain and a preparation method thereof. The health care product tablet comprises following raw material medicine ingredients in parts by weight: 15 parts of Chinese angelica, 15 parts of codonopsis pilosula, 5 parts of frankincense, 5 parts of myrrh, 25 parts of prepared rehmannia roots, 15 parts of radix paeoniae rubra, 15 parts of thunberg fritillary bulbs and 5 parts of liquorice. The tablet disclosed by the invention achieves the effects of promoting blood circulation to remove blood stasis and activating the meridians to stop pain, and is used for treating illnesses such as back and leg pain and sciatica. The traditional Chinese medicine composition disclosed by the invention has the advantages of fast absorption, high bioavailability, fast effective blood concentration reaching speed, no dependence, accuracy in quantification, stable quality and the like, and can be produced by using conventional equipment.
Owner:XIAN FUAN INNOVATION CONSULTATION
Health care product tablet for prostatic diseases and preparation method thereof
InactiveCN102949614ASweet and blandFast absorptionUrinary disorderAluminium/calcium/magnesium active ingredientsRadix Astragali seu HedysariBioavailability
The invention discloses a health care product tablet for the prostatic diseases and a preparation method of the health care product tablet. The health care product tablet contains the following raw material medicinal components in parts by weight: 30 parts of radix astragali, 10 parts of margarita, 12 parts of semen vaccariae, 20 parts of talc, 10 parts of caulis akebiae, 15 parts of poria cocos, 5 parts of glycyrrhiza and 30 parts of corn stigma. The related tablet in the invention with the effects of tonifying and activating qi and benefiting micturition is used for treating benign prostatic hyperplasia, etc. A traditional Chinese medicine composition of the health care product tablet has the advantages of being fast to absorb, high in bioavailability, rapid in speed of reaching the effective blood concentration, accurate in qualification, stable in quality, free from dependency and the like, and the preparation of the health care product tablet can be finished by conventional equipment.
Owner:XIAN FUAN INNOVATION CONSULTATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com