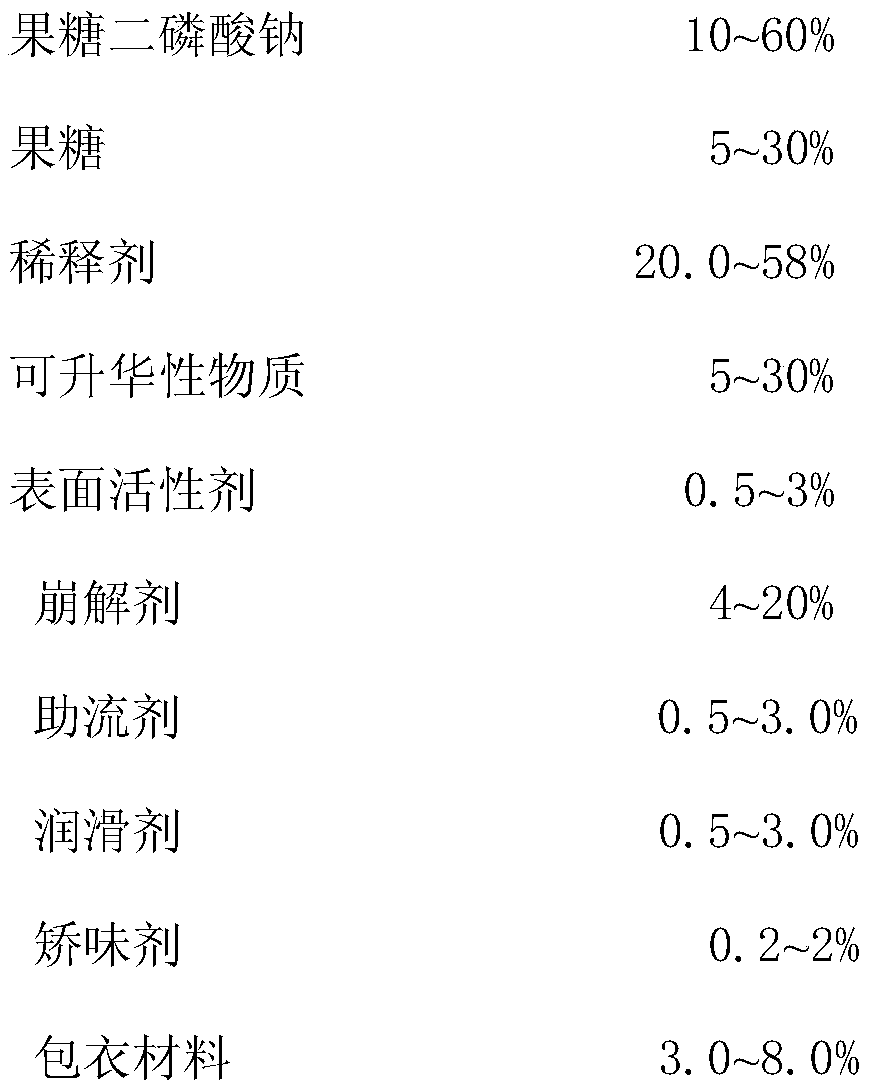
A kind of compound fructose diphosphate sodium fructose orally disintegrating tablet and preparation method thereof
A technology of sodium fructose diphosphate and orally disintegrating tablets is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., which can solve the problems of high production cost, long disintegration time, large equipment investment, etc. Hardness, good clinical effect, friability reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 compound fructose diphosphate sodium fructose orally disintegrating tablet:
[0034]
[0035] Note: The borneol is removed during heating and drying.
[0036] Preparation:
[0037] (1) Separately pulverize sodium fructose diphosphate, fructose and borneol, and pass each through a 60-mesh sieve.
[0038] (2) Weigh 70 g of sucralose, 70 g of colloidal silicon dioxide, 140 g of sodium lauryl sulfate and 300 g of microcrystalline cellulose and mix them.
[0039] (3) Add 2500g of sodium fructose diphosphate, 1250g of fructose, 1830g of microcrystalline cellulose, 800g of crospovidone, and 700g of borneol to the above mixture, mix well, then add 40g of magnesium stearate and mix well, and obtain the total powder before tableting. mixture.
[0040] (4) Put the total mixture into a tablet press, and press into tablets to obtain compound fructose diphosphate sodium fructose tablets (10,000 tablets).
[0041] (5) Coating the above-mentioned tablet in a coating...
Embodiment 2
[0045]
[0046]
[0047] NOTE: The borneol is removed in drying.
[0048] Preparation:
[0049] (1) Separately pulverize sodium fructose diphosphate, fructose and borneol, and pass each through a 60-mesh sieve.
[0050] (2) Weigh 70 g of sucralose, 70 g of colloidal silicon dioxide, 140 g of sodium lauryl sulfate and 300 g of microcrystalline cellulose and mix them.
[0051] (3) Add 2,500 g of sodium fructose diphosphate, 1,250 g of fructose, 1,830 g of microcrystalline cellulose, 800 g of crospovidone, and 1,050 g of borneol to the above mixture, mix well, then add 40 g of magnesium stearate, mix well, and obtain the total powder before tableting. mixture.
[0052] (4) Put the total mixture into a tablet press, and press into tablets to obtain compound fructose diphosphate sodium fructose tablets (10,000 tablets).
[0053] (5) Coating the above tablet in a coating pan to obtain compound fructose diphosphate sodium fructose orally disintegrating tablets.
[0054] The...
Embodiment 3
[0057]
[0058]
[0059] NOTE: The borneol is removed in drying.
[0060] Preparation:
[0061] (1) Separately pulverize sodium fructose diphosphate, fructose and borneol, and pass each through a 60-mesh sieve.
[0062] (2) Weigh 70 g of sucralose, 70 g of colloidal silicon dioxide, 140 g of sodium lauryl sulfate and 300 g of microcrystalline cellulose and mix them.
[0063] (3) Add 2,500 g of sodium fructose diphosphate, 1,250 g of fructose, 1,830 g of microcrystalline cellulose, 800 g of crospovidone, and 1,400 g of borneol to the above mixture, mix well, then add 40 g of magnesium stearate, mix well, and obtain the total powder before tableting. mixture.
[0064] (4) Put the total mixture into a tablet press, and press into tablets to obtain compound fructose diphosphate sodium fructose tablets (10,000 tablets).
[0065] (5) Coating the above tablet in a coating pan to obtain compound fructose diphosphate sodium fructose orally disintegrating tablets.
[0066] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com