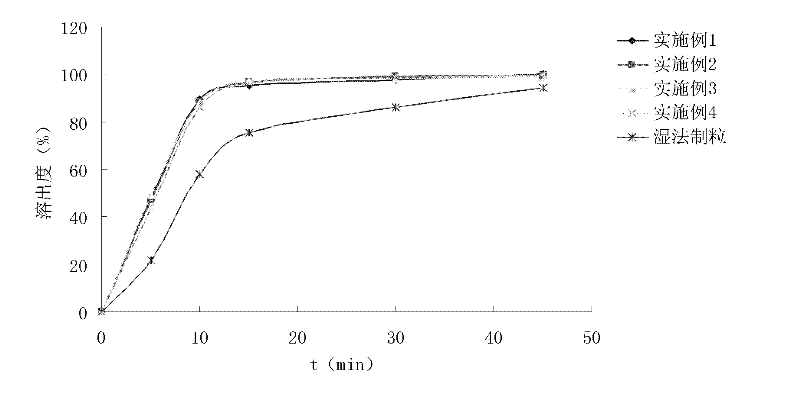
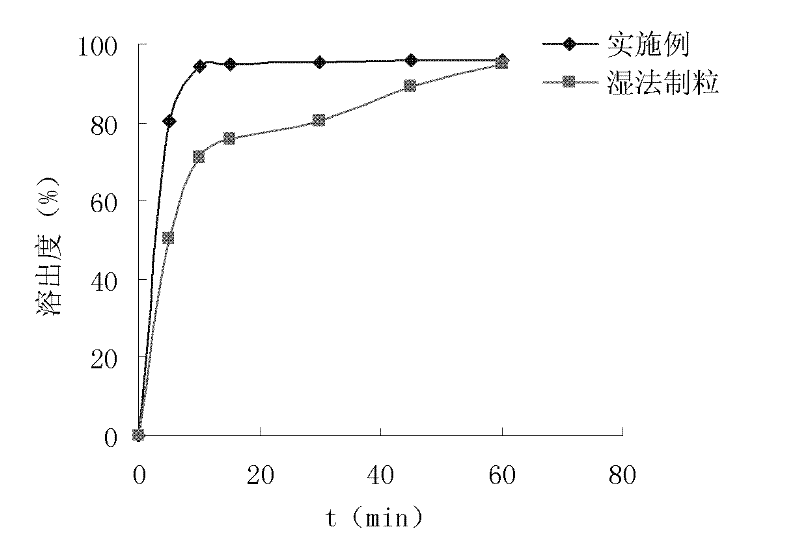
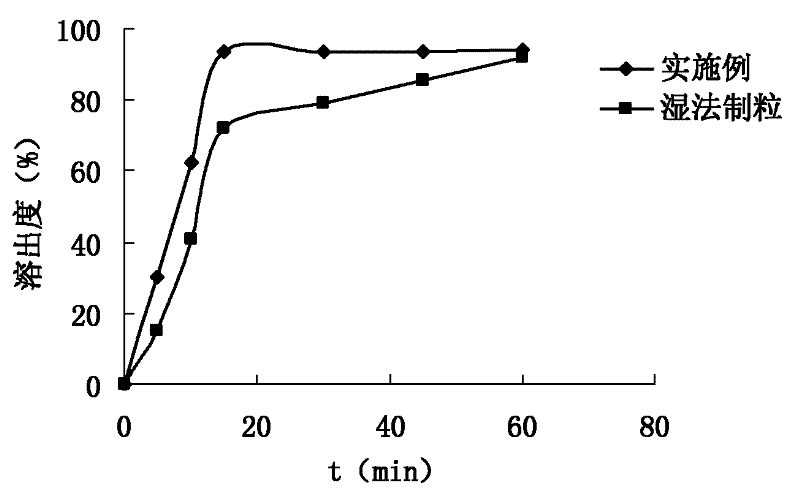
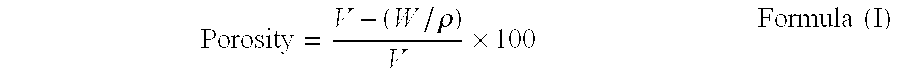Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
145results about How to "Low friability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Quick-disintegrating tablet in buccal cavity and manufacturing method thereof
InactiveUS20030099701A1Good molding effectEasily crystallizesBiocideOrganic active ingredientsMedicineDiluent
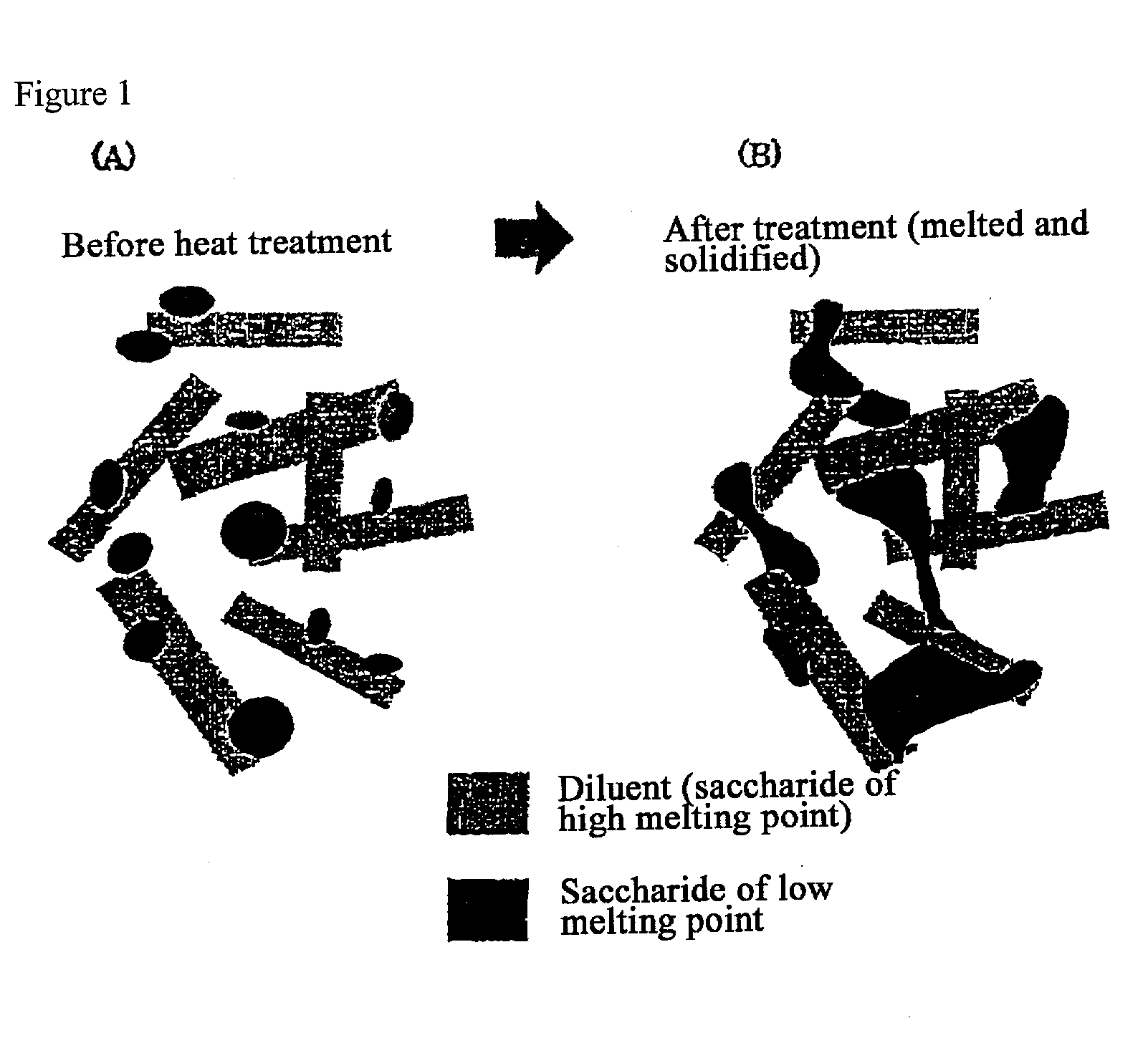
The present invention relates to a quick-disintegrating tablet in the buccal cavity comprising a drug, a diluent, and a saccharide with a relatively lower melting point than the drug and the diluent, which is obtained by uniformly mixing the saccharide with a low melting point in the tablet so that a bridge will be formed between said drug and / or said diluent particles by the product of melting and then solidification of this saccharide with a low melting point. Moreover, the present invention relates to a method of manufacturing a quick-disintegrating tablet in the buccal cavity comprising a drug, a diluent and a saccharide with a relatively lower melting point than the drug and the diluent, which comprises (a) the process whereby tablet starting materials including a drug, a diluent, and a saccharide with a relatively lower melting point than the drug and the diluent are molded under the low pressure necessary for retaining the shape of a tablet, (b) the process whereby the molded product obtained in process (a) is heated to at least the temperature at which this saccharide with a low melting point will melt, and (c) the process whereby the molded product obtained in process (b) is cooled to at least the temperature at which the molten saccharide with a low melting point solidifies. The present invention presents a quick-disintegrating tablet in the buccal cavity that can be used for practical purposes in that it has almost the same properties as conventional oral pharmaceutical tablets, that is, it has sufficient tablet strength that it can be used with automatic unit dosing machines, and it is produced by conventional tableting machines, and a manufacturing method thereof. Moreover, the present invention presents a quick-disintegrating tablet in the buccal cavity which, in comparison to conventional quick-disintegrating tablets in the buccal cavity, has increased tablet strength and an improved friability without prolonging the disintegration time in the buccal cavity, and a manufacturing method thereof.
Owner:ASTELLAS PHARMA INC
Celecoxib-containing solid dispersion and preparation method thereof
InactiveCN103371976AImprove liquidityEasy to shapeOrganic active ingredientsPowder deliveryVitrificationPolyethylene glycol
The invention provides a celecoxib-containing solid dispersion and a preparation method thereof. The celecoxib-containing solid dispersion comprises celecoxib and copovidone. The celecoxib-containing solid dispersion provided by the invention has the advantages of high rigidity, good friability and moderate glass temperature, can be suitable for large-scale industrialized production and overcomes the defects of low glass temperature, soft material, easy melting and bonding, difficulty in crushing and the like of a celecoxib solid dispersion which takes polyethylene glycol as a carrier.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Use of solid carrier particles to improve the processability of a pharmaceutical agent
ActiveUS20090324729A1Improved physical propertyLow hygroscopicityBiocideOrganic active ingredientsCytochromeMedicine
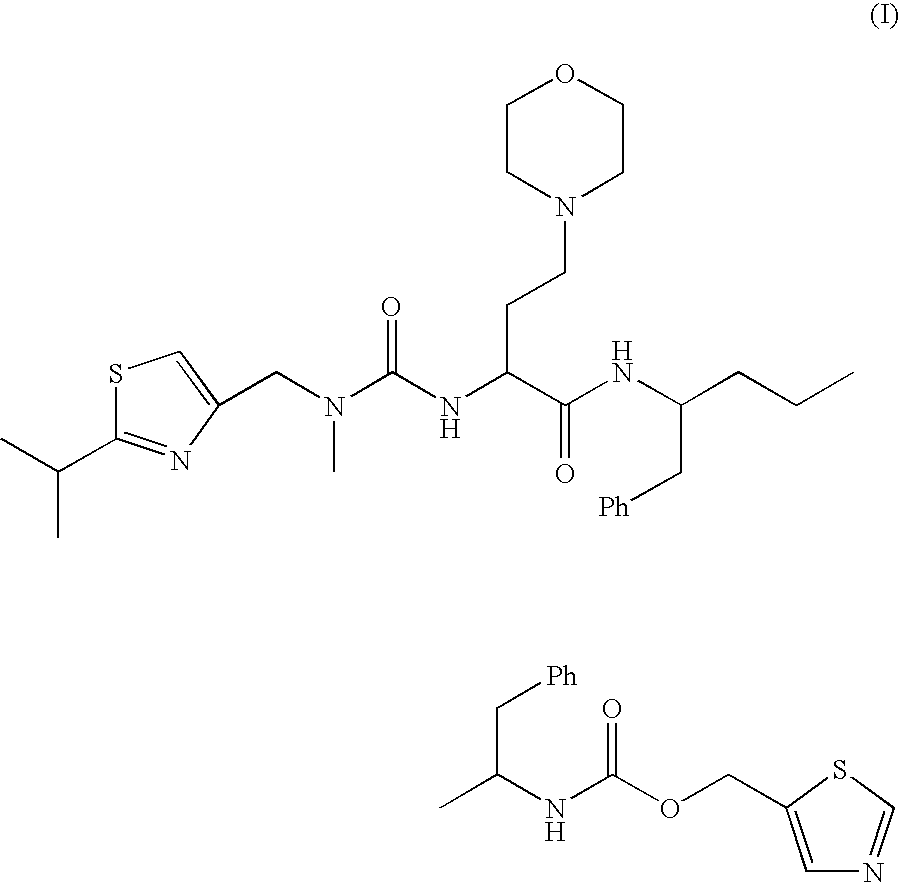
The invention provides a composition comprising, a compound of formula (I):or a pharmaceutically acceptable salt thereof and a plurality of solid carrier particles, as well as methods for using the composition to inhibit the activity of cytochrome P-450.
Owner:GILEAD SCI INC
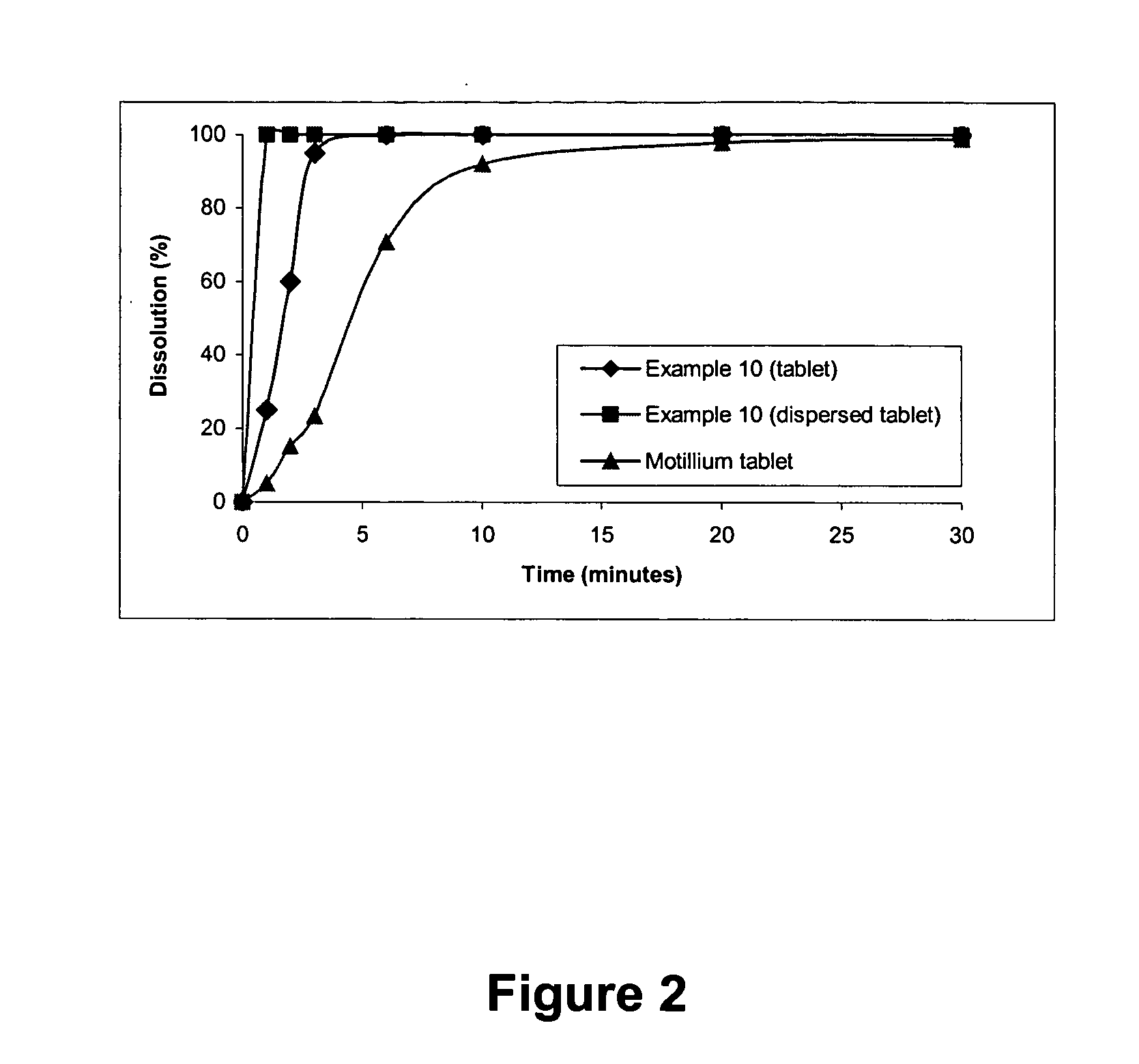
Fast water-dispersible domperidone tablets
InactiveUS20060051414A1Improves Structural IntegrityPleasant tasteBiocideDispersion deliverySolubilityWater dispersible
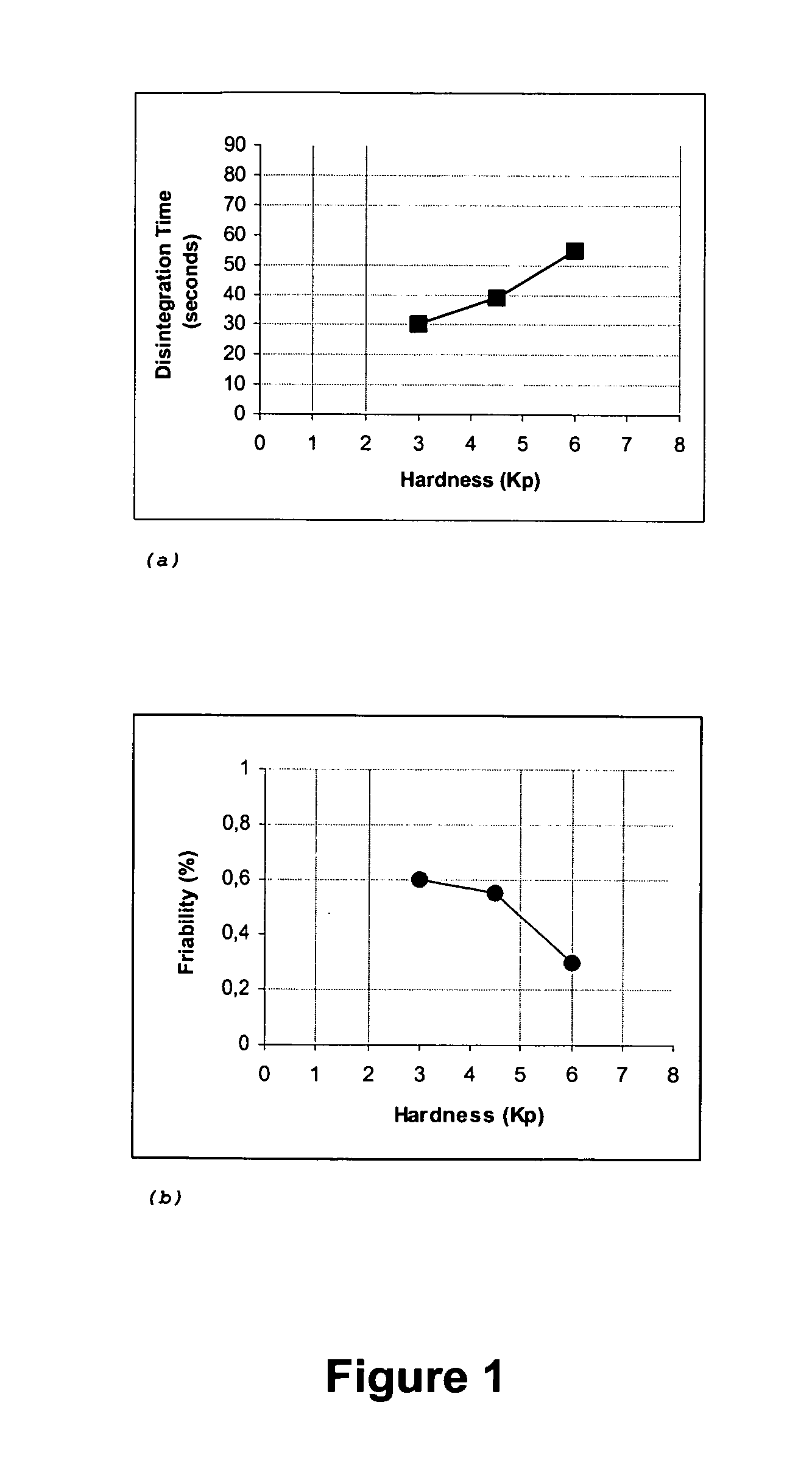
The present invention relates to fast water-dispersible tablets containing domperidone for oral administration. The formulations comprise domperidone or pharmaceutically acceptable salts thereof, about 60-80% of a “auxiliary” granulate (w / w), and about 10-30% of microcrystalline cellulose (w / w), expressed in relation to the total weight of the tablets, a sweetener, a flavouring agent and a lubricant. The “auxiliary” granulate is obtained by wet granulation of D-mannitol and maize starch gum in a high shear granulator, it facilitates the flowability and the compressibility of the mixture and, because of its high solubility in water, contributes to the fast dispersion of the tablet. The formulations have an enhanced structural integrity, for instance having a friability lower than 1.0% and hardness values between 3 and 6 Kp, and are able to disperse in water within 3 minutes, preferably within 2 minutes and most preferably within 1 minute, to provide a dispersion that passes through a 710 μm diameter mesh size sieve and presents a pleasant taste and the absence of perceptible granules in the mouth. This invention also refers to the process for the preparation of said pharmaceutical preparations.
Owner:LAB MEDINFAR PROD FARMS
Pharmaceutical composition with sodium lauryl sulfate as an extra-granular absorption/compression enhancer and the process to make the same
InactiveUS20050051922A1Improve compressibilityImprove hardnessOrganic active ingredientsWood working apparatusDosage formMedicine
A process for preparing a pharmaceutical dosage form or core wherein an absorption / compression agent is introduced into the formulation extra-granularly, and a pharmaceutical tablet prepared by said process.
Owner:ANDRX LABS
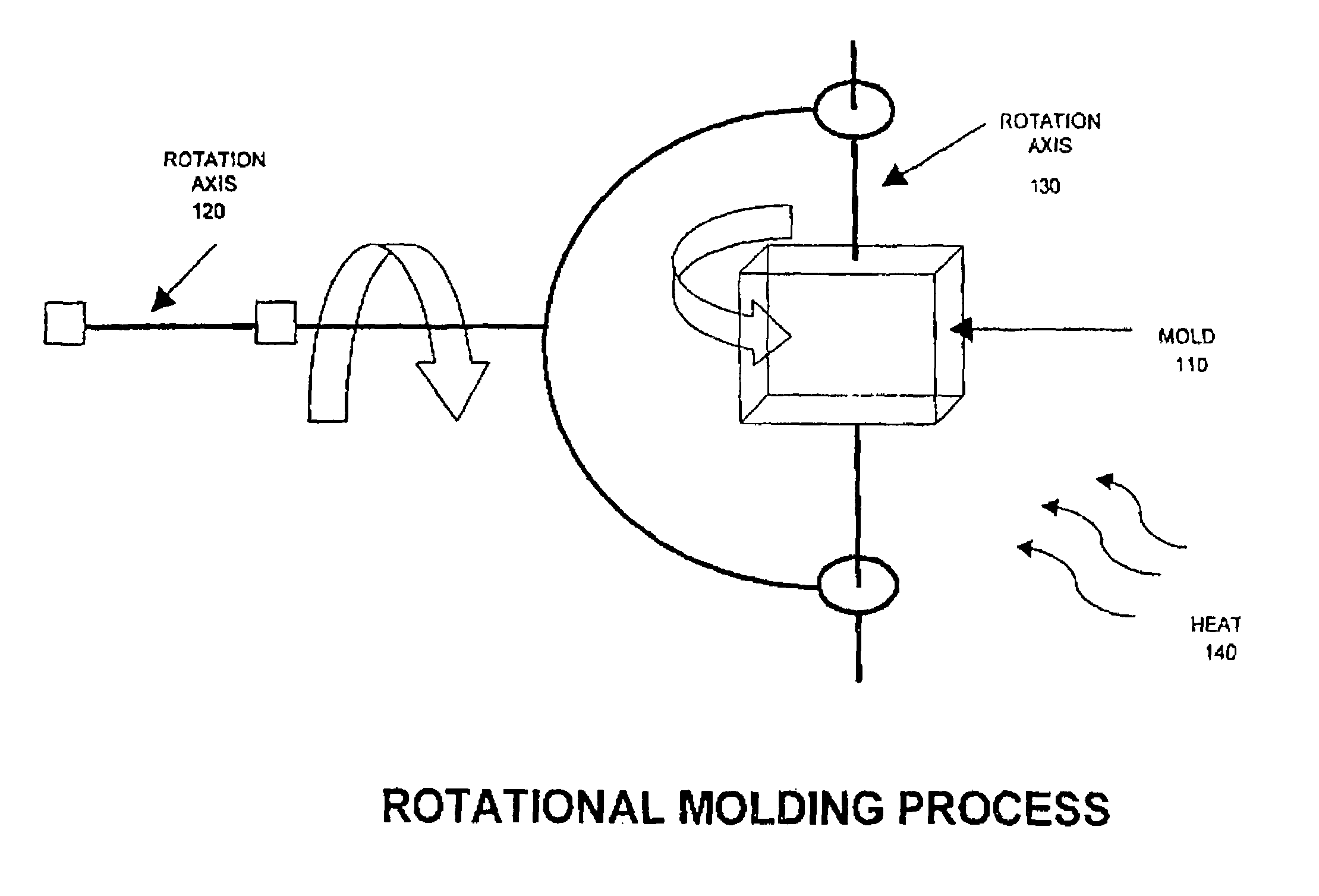
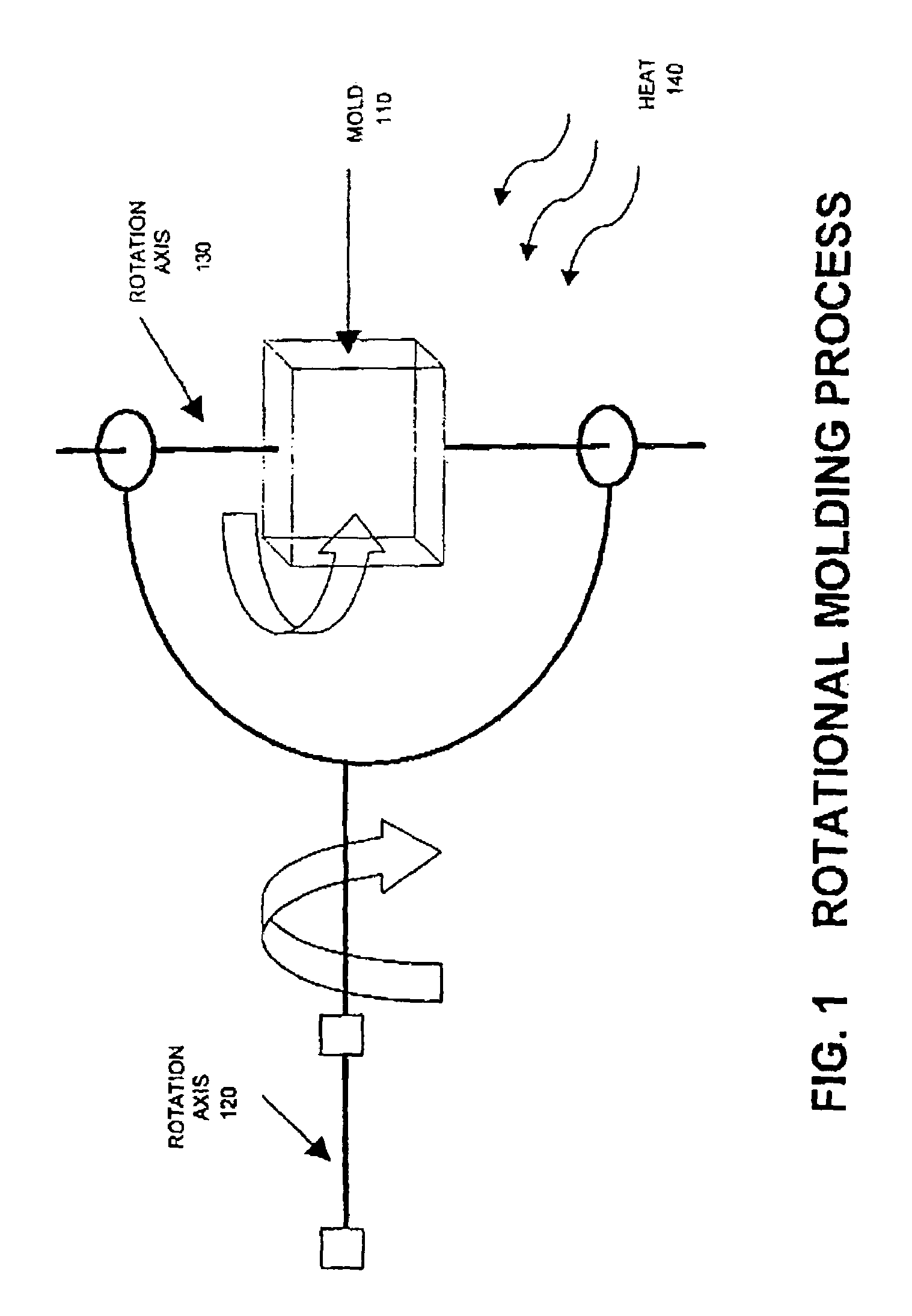
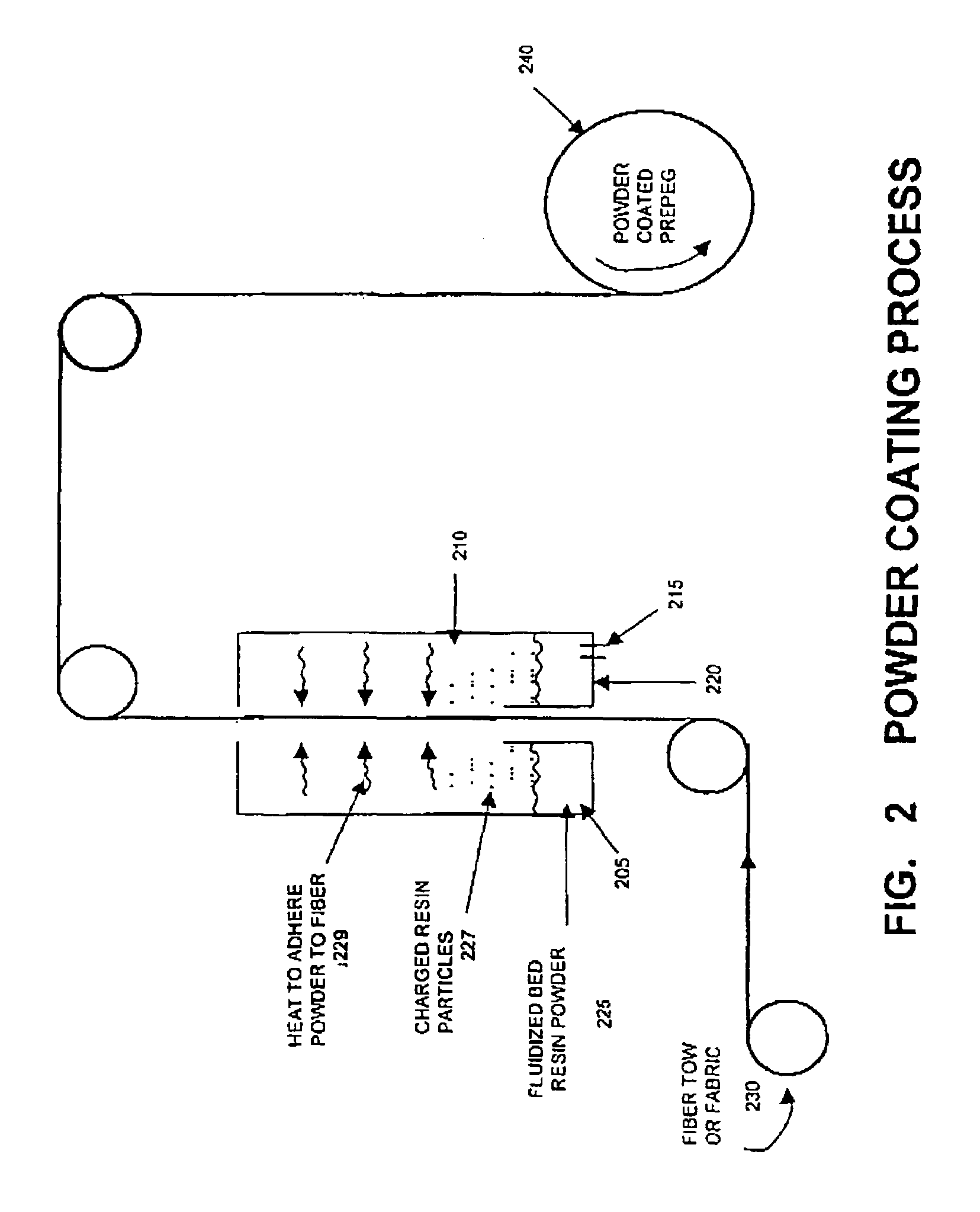
Blends containing macrocyclic polyester oligomer and high molecular weight polymer
InactiveUS7151143B2Improve handlingImproved processibilitySolid electrolyte fuel cellsCoatingsPolyesterThermoplastic
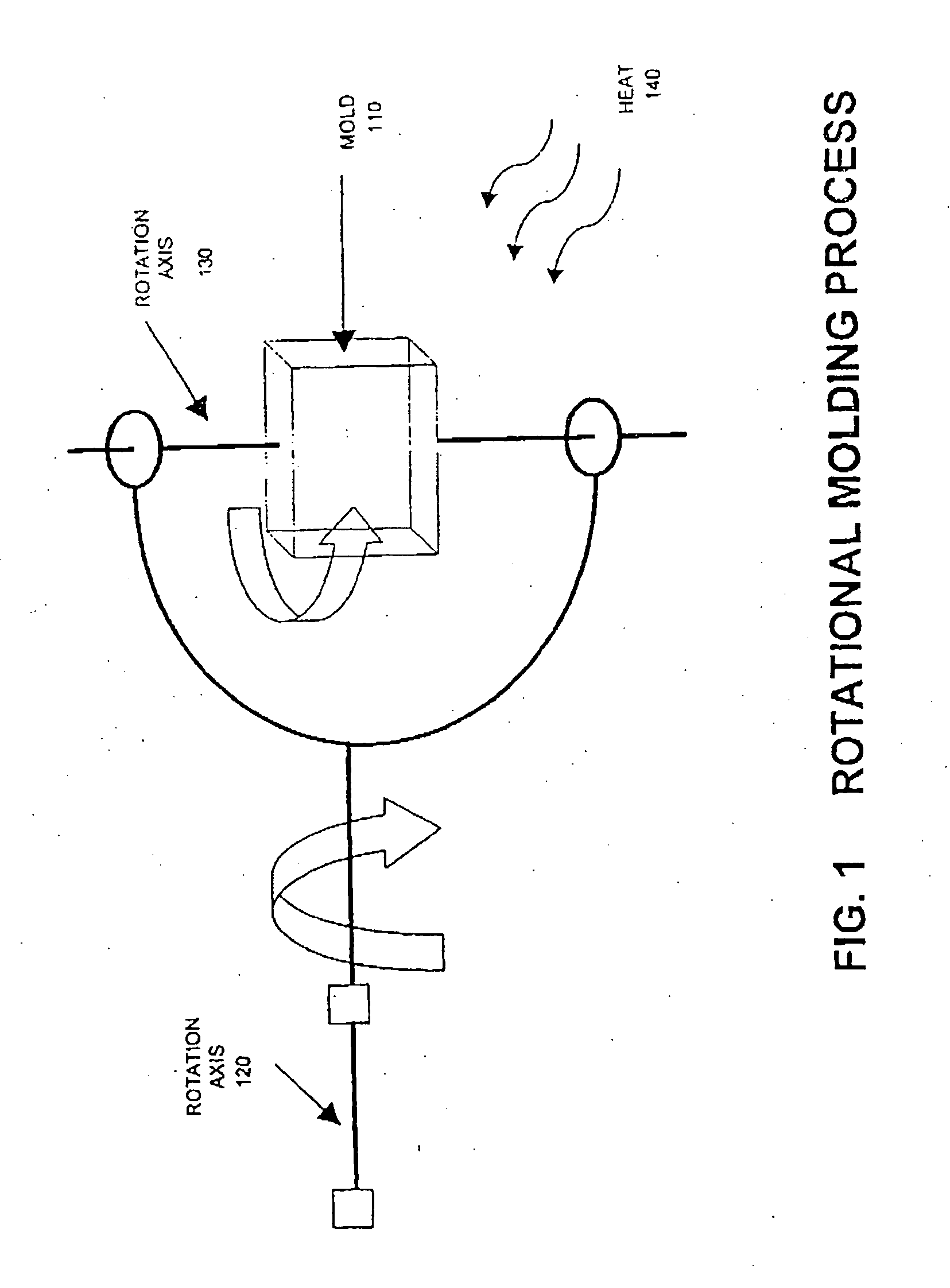
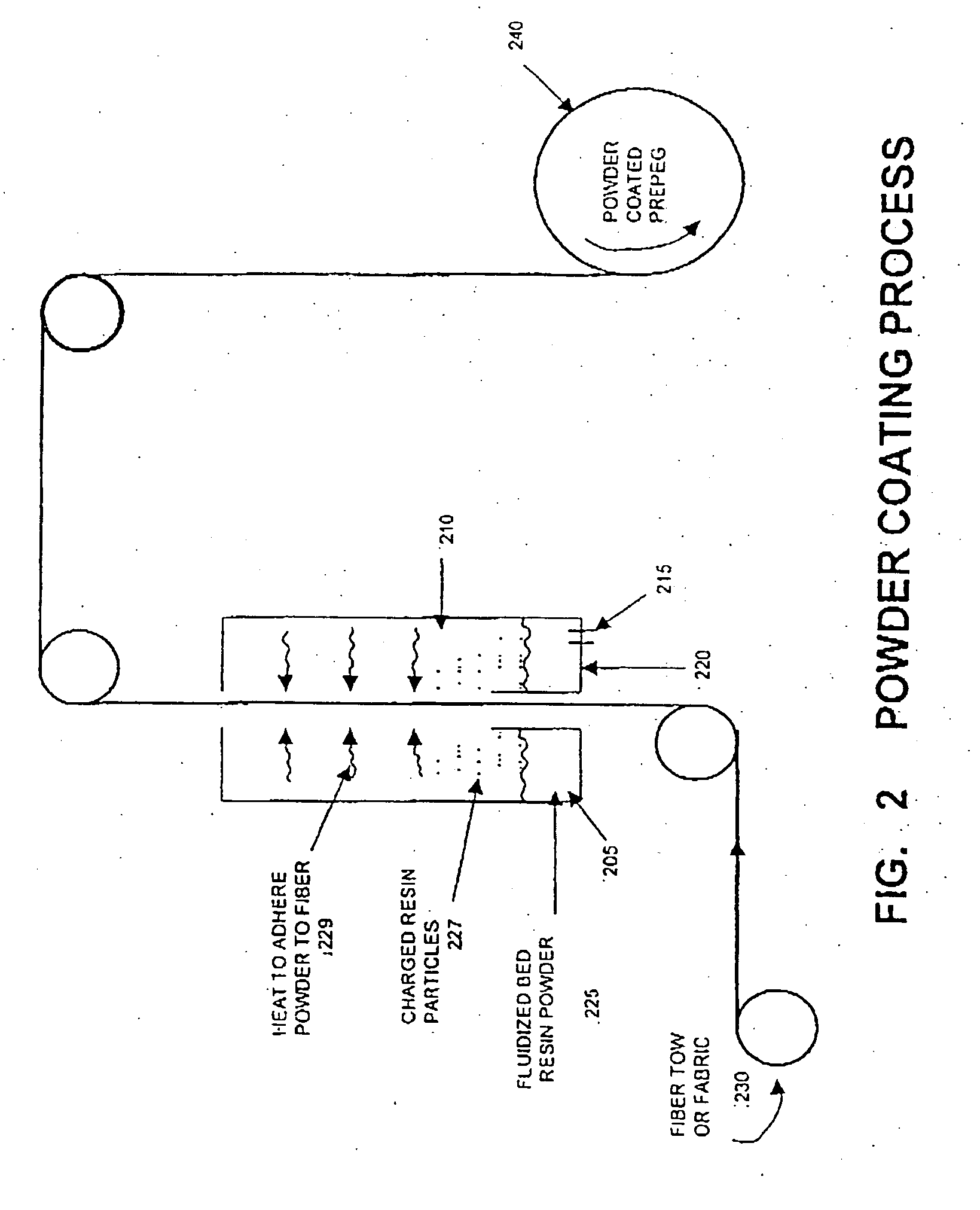
The invention provides blends of macrocyclic polyester oligomer (MPO) with high-molecular-weight polymer and polymerization catalyst as friable, one-component, ready-to-polymerize materials with long shelf life. The invention also provides methods for preparation and use of the blend materials. The blends are used, for example, in the production of thermoplastics via low-pressure processes, such as rotational molding and powder coating, without modification of existing equipment. The blends are particularly useful where it is desired to exploit the ability to polymerize and crystallize MPO isothermally, but where the melt viscosity of unfilled MPO is too low for use in existing equipment.
Owner:CYCLICS CORP
Moxifloxacin hydrochloride pharmaceutical composition and its preparation method
ActiveCN102204911AGood compressibilityHigh hardnessAntibacterial agentsHydroxy compound active ingredientsMANNITOL/SORBITOLPharmaceutical formulation
The invention provides a moxifloxacin hydrochloride pharmaceutical composition and its preparation method. The composition comprises moxifloxacin hydrochloride, mannitol and other excipients. The preparation method employs a dry-method granulating technology for preparing medicament particles, and then preparing a medicinal preparation.
Owner:CHINA RESOURCES SAIKE PHARMA
Soft chewable tablets
InactiveUS7029699B2Reduce fracturesTaste of may become dullPowder deliveryPharmaceutical containersBULK ACTIVE INGREDIENTActive ingredient
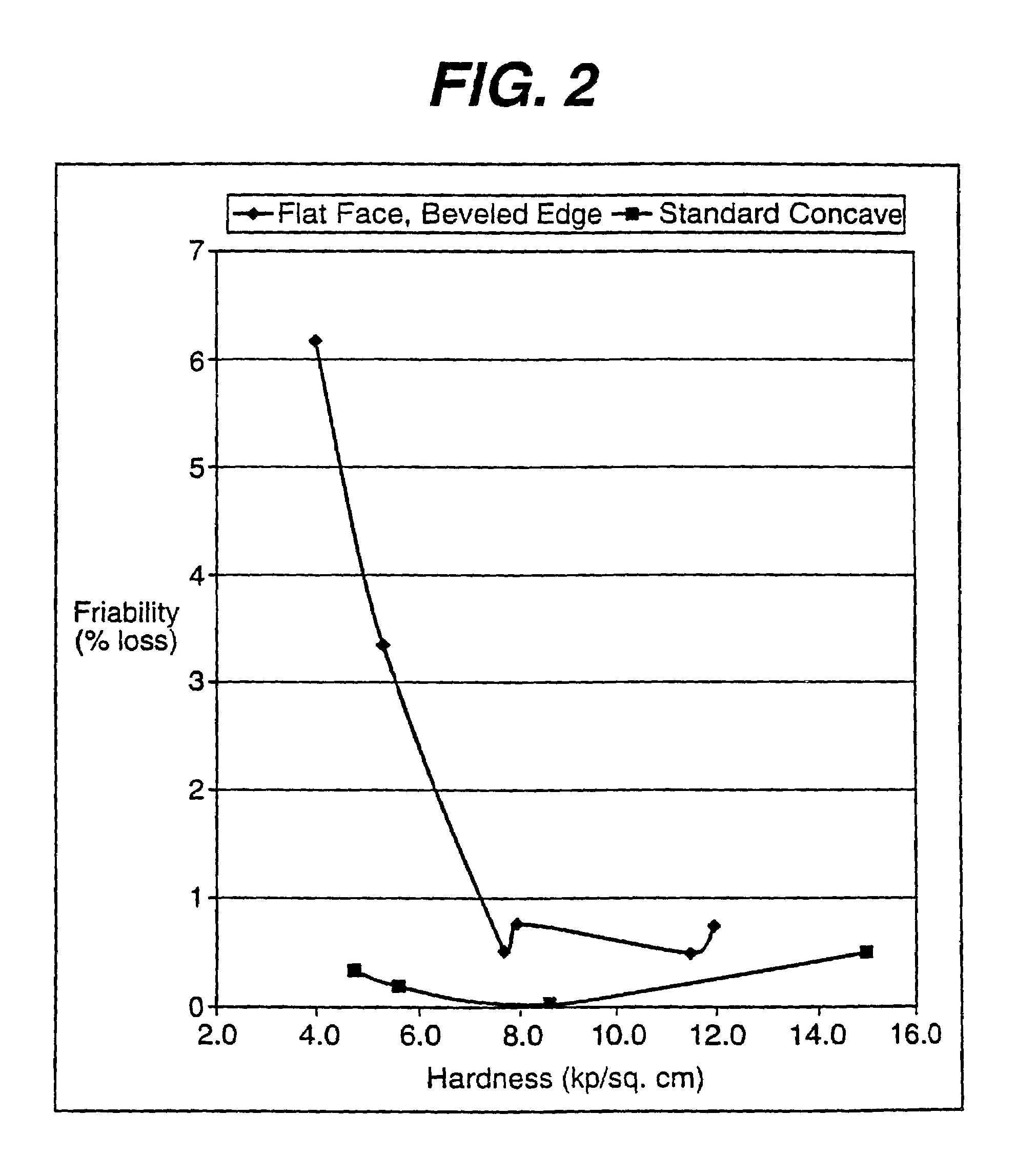
The present invention relates to a compressed, chewable tablet containing at least one active ingredient, a water-disintegratable, compressible carbohydrate and a binder. These components are dry blended and compressed into convex-shaped tablet having a hardness of about 2 to about 11 kp / cm2 and friability less than 1%.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Pharmaceutical compositions and methods for their preparation
InactiveUS20150004239A1Good physical propertiesIncrease the load valueBiocideAntiviralsSolid particleSolid core
The invention provides solid particles comprising: a) a solid core that comprises an active pharmaceutical agent and b) a coating of Compound 2: (2) or a pharmaceutically acceptable salt of thereof on the core, as well as compositions comprising such particles, and methods for treating diseases (e.g. HIV infection) with such particles.
Owner:GILEAD SCI INC
Dechlorinating tablet and method of manufacture
InactiveUS20050139805A1Decreases friability of tabletProlong dissolution timeOther chemical processesSpecific water treatment objectivesSulfite saltSodium sulfite
A dehalogenating agent is disclosed that is useful for treating aqueous media. The agent contains at least one sulfite salt, such as sodium sulfite. In the agent, the salt is in mixture with a lubricant, an excipient and a saccharide binder. Then a dry, blended mixture of the agent components is directly compressed to form tablets not only of desirable strength and hardness, but also of desirably controlled dissolution rate.
Owner:EXCEL TECH INT CORP
Keratin-Based Powders and Hydrogel for Pharmaceutical Applications
InactiveUS20080089930A1High strengthIncreased formationCosmetic preparationsPowder deliveryFiberSkin treatments
Owner:KERAPLAST TECH LTD
Hollow capsule and preparation method thereof
ActiveCN102836142AEliminate quality problemsEliminate security concernsPharmaceutical non-active ingredientsCapsule deliveryAmyrisKappa-Carrageenan
The invention discloses a hollow capsule, which comprises the following components in parts by weight: 10 to 30 parts of kappa-carrageenan, 90 to 180 parts of water-soluble crosslinked starch, and 20 to 40 parts of plasticizer. The invention also discloses a preparation of the hollow capsule, which comprises the following steps of: 1) dissolving gum, wherein carrageenan and water-soluble crosslinked starch are stirred, water at a temperature of between 70 and 90 DEG C is added into the mixture, the plasticizer in the amount of the prescription is added and completely dissolved, and the mixture is stirred uniformly; 2) culturing the gum, wherein the gum liquid obtained in the step 1) is cooled to a temperature of between 40 and 45 DEG C, the gum liquid is insulated and stood for 30 to 60 minutes, and the gum liquid is put into a gum soaking tank; and 3) soaking the gum, wherein when the temperature of the gum liquid in the gum soaking tank is cooled to 35 DEG C, operations of gum soaking, drying, shell pulling out, cutting and buckling are automatically carried out through a capsule template. According to the hollow capsule disclosed by the invention, the transparency and toughness are remarkably improved, the friability is remarkably reduced, and the product yield is greatly increased.
Owner:青岛晓丛生物科技有限公司
Lelrozol tablet and preparation method thereof
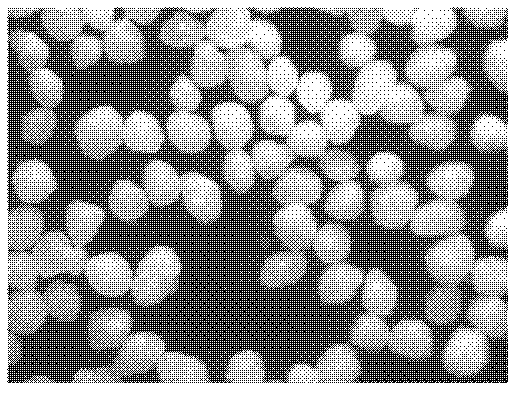
ActiveCN107737112AEvenly dispersedImprove liquidityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineSmallerThan
The invention belongs to the technical field of medicine and particularly relates to a lelrozol tablet and a preparation method thereof. The lelrozol tablet is prepared from lelrozol and silicified microcrystalline cellulose, wherein the content of the silicified microcrystalline cellulose accounts for 20 to 60 percent of total weight of the tablets. The particle size D(v, 0.9) of the lelrozol issmaller than or equal to 60mu m, preferably the particle size D(v, 0.9) of the lelrozol is smaller than or equal to 40mu m and more preferably, the particle size D (v, 0.9) of the lelrozol is smallerthan or equal to 8mu m. According to the preparation method of the lelrozol tablet, disclosed by the invention, the silica microcrystalline cellulose is used, so that the trazodone is uniformly dispersed in the mixed powder; in addition, the lelrozol tablet has good fluidity, and the powder can be directly pressured into tablets, so that the technique is simplified, the time is saved and the laborintensity is low; besides, after a crude drug is crushed, the particle size is obviously reduced; by controlling the content of a disintegrating agent, the dissolution rate of the prepared lelrozol tablet is significantly increased, 85 percent or above can be reached in 15 minutes and thereby the lelrozol tablet is rapidly dissolved out.
Owner:HAINAN JINRUI PHARMA CO LTD
Xylitol particles capable of being directly pressed into tablets and preparation method thereof
The invention relates to xylitol particles capable of being directly pressed into tablets and a preparation method thereof, belonging to the technical field of functional sugar, and the xylitol particles comprise the following raw materials by weight ratio: 95-120kg of crystal xylitol and 0.1-5kg of adhesive, wherein the adhesive adopts one of polydextrose or sodium carboxymethyl cellulose in cellulose ether or hydroxypropyl methyl cellulose in methyl cellulose, the viscosity number of the adhesive is 500-2000mPa.S, the using amount of the sodium carboxymethyl cellulose and the methyl cellulose is 0.1-5kg, the using amount of the polydextrose is 3-5kg, and the using amount of water is 20-50kg. The preparation method comprises the following steps: 1) mixing; 2) preparing solution; 3) granulating; and 4) pressing into the tablets, adding the obtained particles into 1% of magnesium stearate, uniformly mixing, pressing into the tablets by using a tablet press machine, and obtaining high-purity xylitol tablets. The prepared high-purity xylitol tablets have the advantages of good stability, high dissolution rate, low friability, high hardness and the like.
Owner:FUTASTE PHARM CO LTD
Micro-pill and preparation method thereof
ActiveCN102525943ALow friabilityHigh yieldPharmaceutical product form changeGranular deliveryAdditive ingredientExcipient
The invention belongs to the field of pharmacy and discloses a micro-pill and a preparation method thereof. The micro-pill contains the following ingredients in parts by weight: 0 to 100 of active compounds and 0 to 100 of excipient. The proportion of active compounds and the proportion of excipient are not 0 at the same time and the sum of the two is equal to 100, or the proportion of active compounds and the proportion of excipient are proportionally increased or reduced and the proportion of excipient is not 0 when the active compounds are in a liquid state. The content of surfactants is 0.1% to 8% the total weight of active compounds and excipient, and the content of humectants is 0.1% to 30% the total weight of active compounds and excipient. The micro-pill has the advantages of low friability, high yield, small particle size and smooth surface; and can be further processed to micro-pill with functional coating for target drug delivery or micro-pill for drug delivery under special physiological environment conditions.
Owner:JINLING PHARMA
Blends containing macrocyclic polyester oligomer and high molecular weight polymer
InactiveUS20060287440A1Increases ductility and toughnessExtended shelf lifeCoatingsPolyesterThermoplastic
The invention provides blends of macrocyclic polyester oligomer (MPO) with high-molecular-weight polymer and polymerization catalyst as friable, one-component, ready-to-polymerize materials with long shelf life. The invention also provides methods for preparation and use of the blend materials. The blends are used, for example, in the production of thermoplastics via low-pressure processes, such as rotational molding and powder coating, without modification of existing equipment. The blends are particularly useful where it is desired to exploit the ability to polymerize and crystallize MPO isothermally, but where the melt viscosity of unfilled MPO is too low for use in existing equipment.
Owner:CYCLICS CORP
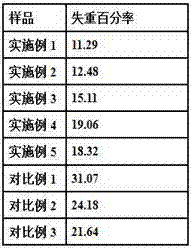
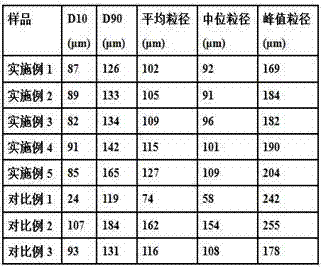
Hydrocolloid stabilized dehydrated food foam
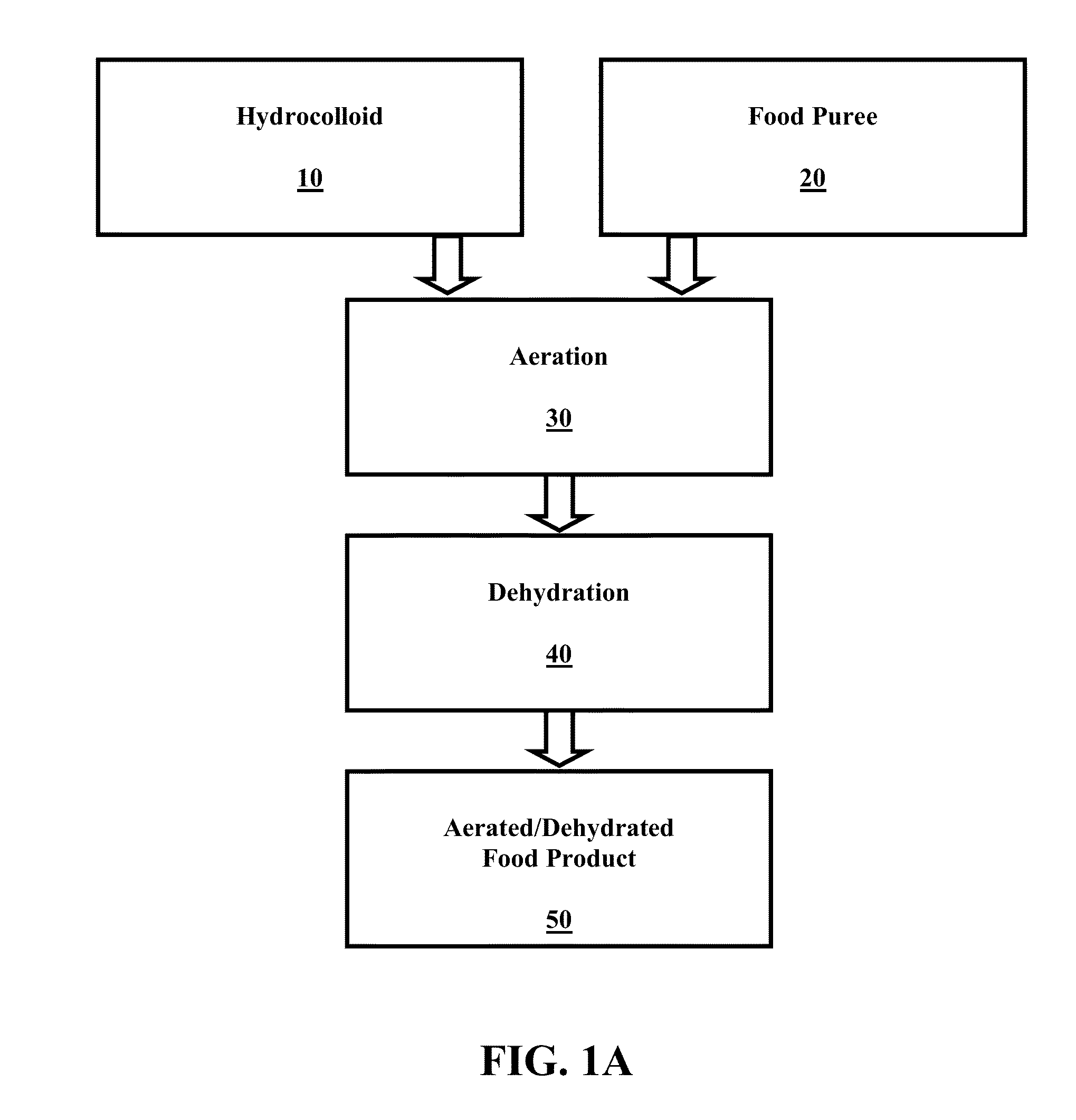
InactiveUS20140072672A1Shorten dehydration timeMore surface areaReady-for-oven doughsFruit and vegetables preservationFood productsAeration
The present invention relates to a dehydrated and aerated food product. The dehydrated and aerated food product includes a plant puree mixture and a hydrocolloid. The plant puree mixture and hydrocolloid combine to form a dehydrated and aerated plant puree foam having a hydrocolloid-based aeration network dispersed throughout the plant puree mixture. The present invention also relates to methods of making and using the dehydrated and aerated food product, and combination food products that include the dehydrated and aerated food product.
Owner:CORNELL UNIVERSITY
Tablet containing linezolid crystal form III
ActiveCN103893138AGood compressibilityModerate intensityAntibacterial agentsOrganic active ingredientsLinezolidLactose
The invention relates to a tablet containing a linezolid crystal form III. The tablet contains 67.0-75.0wt% of linezolid, 2.0-4.8wt% of lactose, 9.0-18.0wt% of microcrystalline cellulose as well as disintegrating agent, binding agent, lubricant and other medicinal excipients, wherein preferably, the tablet contains 67.0-75.0wt% of linezolid, 2.0-4.8wt% of lactose, 9.0-18.0wt% of microcrystalline cellulose, 2.0-10.0wt% of disintegrating agent, 1.2-4.0wt% of binding agent and 0.3-2.0wt% of lubricant. The tablet containing the linezolid crystal form III of the prescription, in an extremely limited additive adding range, guarantees characteristics of good compressibility, excellent formability and rapid dissolution.
Owner:CHENGDU GUOHONG PHARMA
Procaterol hydrochloride granule and preparation method thereof
ActiveCN107184555APromote meltingGood disintegrationOrganic active ingredientsInorganic non-active ingredientsMoisture absorptionDissolution
The invention belongs to the field of medicine, and relates to a procaterol hydrochloride granule and a preparation method thereof. The procaterol hydrochloride granule is prepared from procaterol hydrochloride and auxiliary materials at least prepared from lactose, hydroxypropyl methylcellulose, croscarmellose sodium, calcium chloride and palmityl diethylester aspartate. The granule is prepared by a fluidization spraying method; the calcium chloride is used as a master batch; a suspension prepared from the procaterol hydrochloride, mannitol, hydroxypropyl methylcellulose, croscarmellose sodium and palmityl diethylester aspartate is sprayed onto the surface of the calcium chloride master batch; the procaterol hydrochloride granule is obtained. The granule has the advantages that the dissolution is fast; the throat clamping is avoided; the content uniformity is high; the flowability is high; the moisture absorption performance and the friability are low; the stability is high. The preparation method has the advantages that the process is simple; the requirements on equipment are lower; the energy consumption is lower; the industrial production is convenient.
Owner:HEILONGJIANG LONGDE PHARMA CO LTD
Soluble milk-based tablet surface-treated with carbohydrate
The present invention relates to a method for the manufacture of soluble milk-based tablets and in particular to milk-based tablets surface-treated with a carbohydrate. The invention also relates to the use of a concentrated carbohydrate solution to re-duce a friability of a milk-based tablet. The soluble milk-based tablet surface-treated with the carbohydrate has applications in the beverage industry.
Owner:NESTEC SA
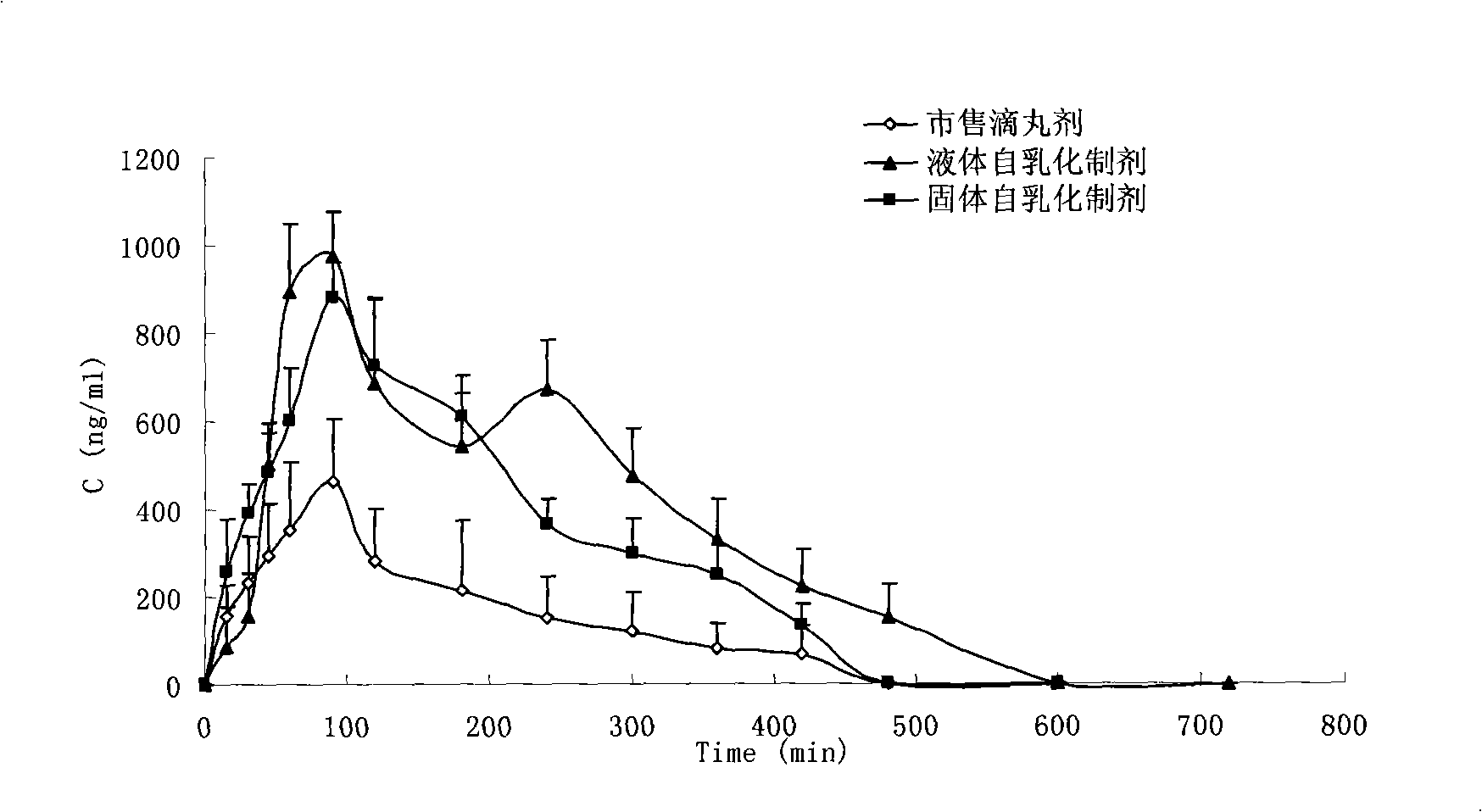
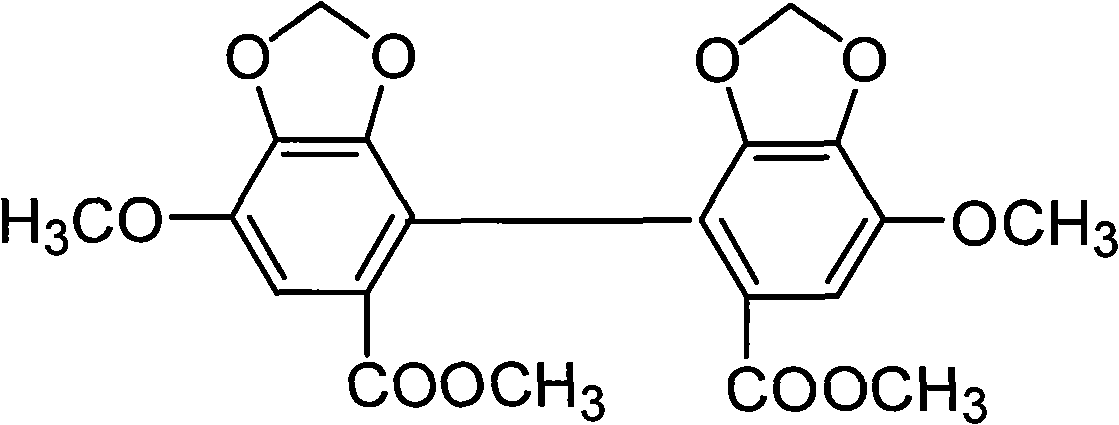
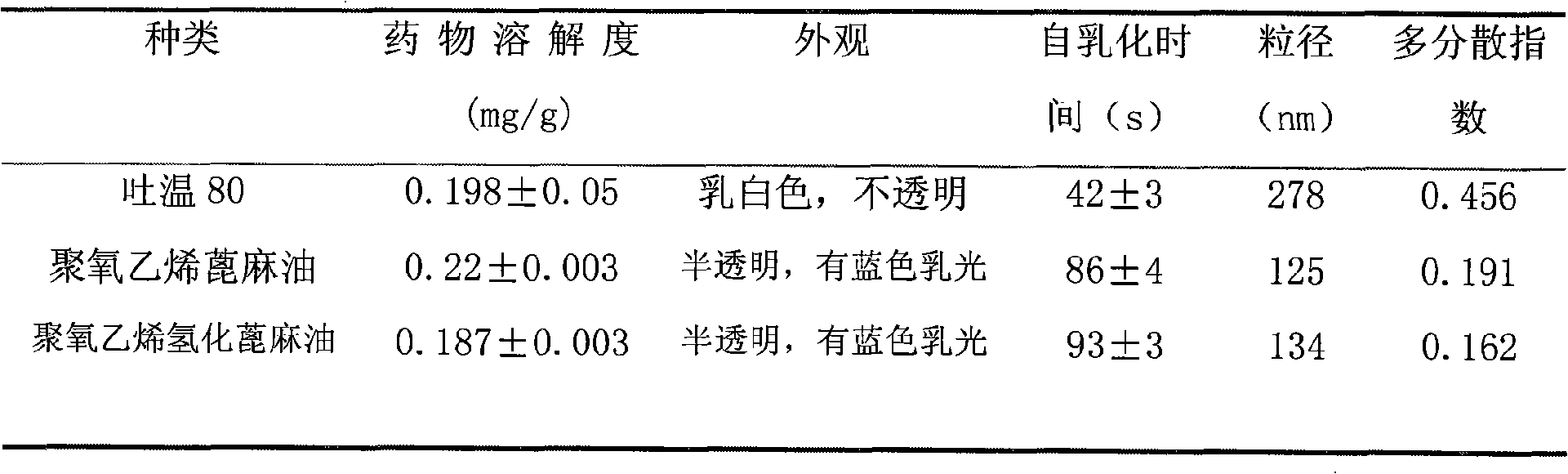
Self-emulsifying formulation of biphenyldicarboxylate and preparation method thereof
InactiveCN101401788AImprove solubilityImprove dissolution rateDigestive systemEmulsion deliverySolubilityPolyoxyethylene castor oil
The invention relates to the field of pharmaceutical preparation, in particular to a bifendate self-emulsifying preparation and a method for preparing the same. The bifendate self-emulsifying preparation is characterized by adopting the mixture of polyethylene glycol-12-hydroxy-stearic acid ester and polyoxyethylene hydrogenated castor oil or the mixture of the polyethylene glycol-12-hydroxy-stearic acid ester and polyoxyethylene castor oil as an emulsifier. The bifendate self-emulsifying preparation is emulsified spontaneously to form micro-emulsion droplets of which the particle diameter is less than 200 nanometers after oral administration, thereby enhancing the solubility and the dissolving speed of the bifendate so as to improve the bioavailability of bifendate. At the same time, the bifendate self-emulsifying preparation prepared by adding a solid adsorption material into a liquid self-emulsifying preparation not only effectively improves the defects of liquid preparations in production, storage, administration and the like, but also significantly improves the stability of drugs with a simple and practicable preparation process.
Owner:CHINA PHARM UNIV
Sarpogrelate hydrochloride sustained release pellet and preparation method thereof
ActiveCN102552165ALow friabilityHigh yieldOrganic active ingredientsPharmaceutical product form changeSustained release pelletsSarpogrelate Hydrochloride
The invention belongs to the field of medicinal preparation and discloses a sarpogrelate hydrochloride sustained release pellet and a preparation method thereof. The pellet is prepared by coating a sustained release coat on a medicine contained pellet core, wherein the formula of the medicine contained pellet core comprises the following raw materials in parts by weight: 75-100 parts of sarpogrelate hydrochloride and 0-25 parts of excipient, wherein the total weight part of the sarpogrelate hydrochloride and the excipient is 100 or the weight parts of the sarpogrelate hydrochloride and the excipient are increased or reduced by the same ratio; a surfactant accounts for 0.1-8% of the total weight of the sarpogrelate hydrochloride and the excipient; and a wetting agent accounts for 0.1-30% of the total weight of the sarpogrelate hydrochloride and the excipient. By using the preparation method disclosed by the invention, the medicine contained pellet core with low friability, higher yield, smaller particle size and smooth surface can be obtained; the pellet core is convenient to be further processed; according to the invention, the medicine contained pellet core is coated with the sustained release coat so as to obtain a potassium citrate sustained release pellet; and the pellet has the advantages of controllable and stable quality in vitro and sustained release characteristics in vivo.
Owner:JINLING PHARMA
Bee pollen granular preparation capable of direct compression and preparation method thereof
InactiveCN103082162AThe appearance is complete and smoothAngularFood shapingFood preparationMagnesium stearateStearic acid
The invention discloses a bee pollen granular preparation capable of direct compression and a preparation method of the bee pollen granular preparation. The bee pollen granular preparation comprises the following main ingredients: bee pollen, magnesium stearate, vitamin B2, butter essence, and silicon dioxide or a non-viscous other material. The bee pollen granular preparation which can be directly compression-molded, is good in quality and less than 97% in bee pollen content, can be simply and conveniently produced by the method disclosed by the invention, a compressed tablet is complete, smooth and clean in appearance, angular, high in hardness, and low in friability, wherein the quality of the compressed tablet is superior to that of a bee pollen compressed tablet commonly seen in the market, especially, the hardness of the tablet is more than 3 times that of the common tablet, and the industrialized mass production of the bee pollen tablet can be well achieved.
Owner:JIANGSU ALAND NOURISHMENT
Highly robust fast-disintegrating tablet and process for manufacturing the same
ActiveUS20140364513A1Easily take their medicineHigh hardnessBiocideOrganic active ingredientsMedicineHardness
Disclosed are an oral formulation which disintegrates quickly in the oral cavity; a fast-disintegrating tablet having fast disintegrability and high hardness, and a process for manufacturing the same. In addition, slightly wetted granules for manufacturing said fast-disintegrating tablet and a process for manufacturing the same are disclosed.
Owner:SAMYANG HLDG CORP
Rapidly disintegrating low friability tablets comprising silica materials
InactiveUS20070196475A1Effective quick dissolve resultLow friabilityCosmetic preparationsToilet preparationsFriabilityMedicine
This invention pertains to the ability to provide rapidly disintegrating tablets through the inclusion of a silica material in combination with other common tablet components. Such a silica material must exhibit a sufficiently low surface area in order to boost the ability of the table to separate quickly when introduced into a user's mouth cavity. Such a tablet is dimensionally stable prior to use (low friability) and, when immersed in water the tablet disintegrates therein in less than about 60 seconds.
Owner:WITHIAM MICHAEL C +2
Method for preparing voglibose particles
InactiveCN108635332ASimple production processShorten production timeOrganic active ingredientsMetabolism disorderAdhesiveDiluent
The invention provides a method for preparing voglibose particles. The particles are prepared through a one-step prilling process,the friability is low,and the roundness is good. Voglibose can be dissolved out fast,the content uniformity is good,and the voglibose particles are suitable for being further prepared into capsules,tables (including dispersible tablets and orally disintegrating tablets)and granules. According to the preparation idea,the voglibose is added into a polar solvent containing a water-soluble adhesive,and the mixture is sprayed into a one-step pelletizer containing a diluent and a disintegrating agent in a top spraying prilling mode. The preparation method is easy to operate,the labor intensity is reduced,and the preparation method is suitable for large-scale production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Starch-based vacant capsule and preparation process thereof
ActiveCN104800186AEliminate the trouble of heating gelatinizationHigh strengthPharmaceutical non-active ingredientsCapsule deliveryKonjac glucomannanChinese pharmacopoeia
The invention belongs to the field of medicine preparation and relates to a starch-based vacant capsule and a preparation process thereof. The starch-based vacant capsule comprises components in parts by weight as follows: 40-70 parts of high-amylose starch, 20-60 parts of high-amylopectin starch, 5-15 parts of pregelatinized starch, 5-20 parts of konjac glucomannan and 60-90 parts of deionized water. The starch-based vacant capsule has the characteristics of low cost, good quality and high water-retaining property, meets the standard of Chinese pharmacopoeia, has excellent properties of short disintegration time, low friability and the like, has a better effect in practical application and is suitable for industrial production.
Owner:HUNAN ER KANG PHARMA
Pramipexole-contained pharmaceutical composition capable of being dispersed in mouth
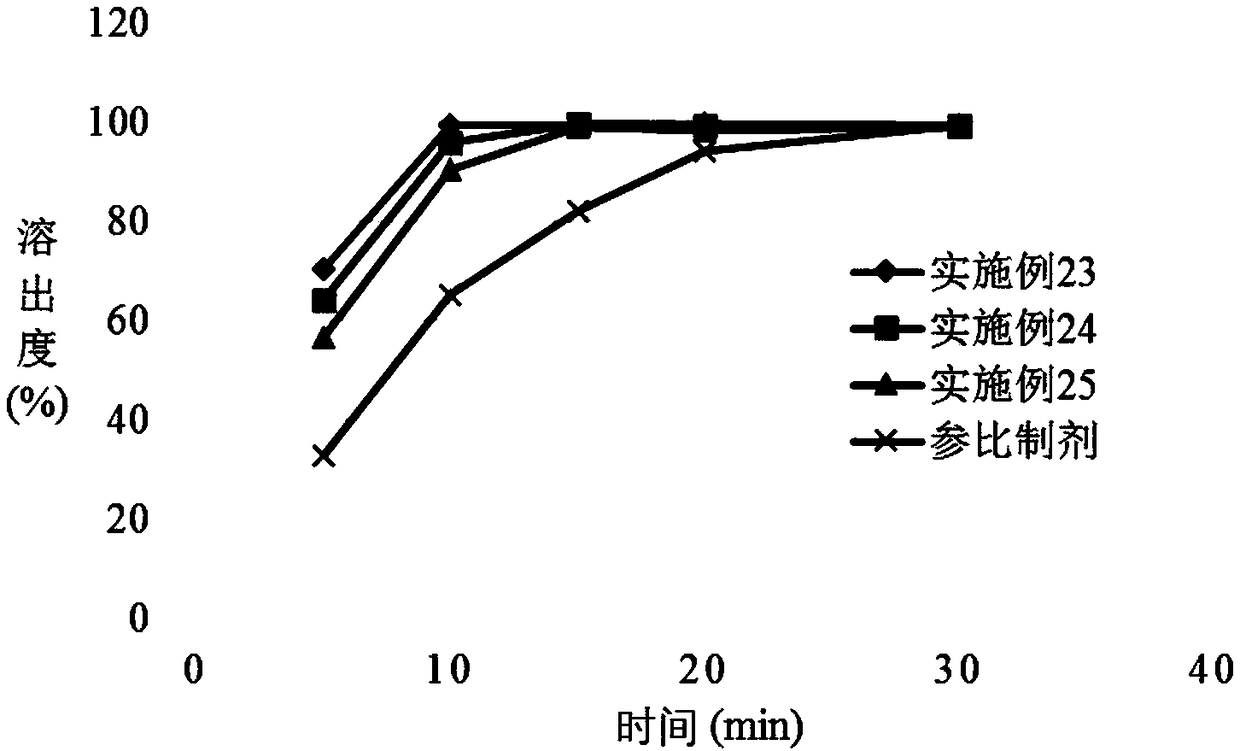
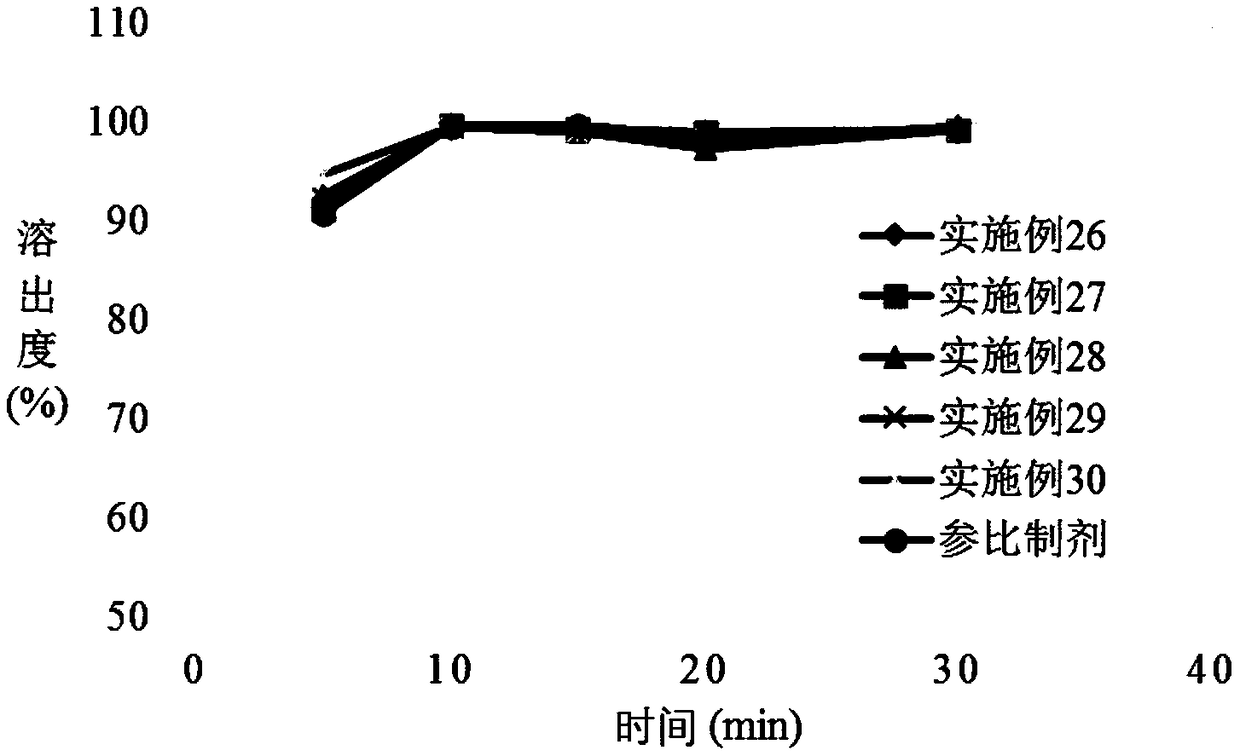
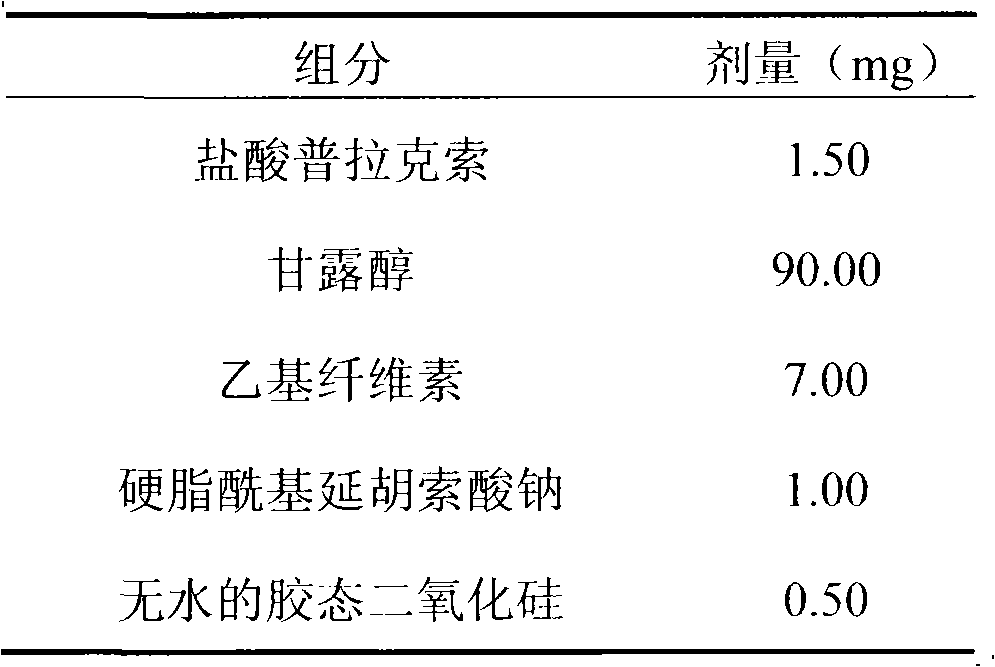
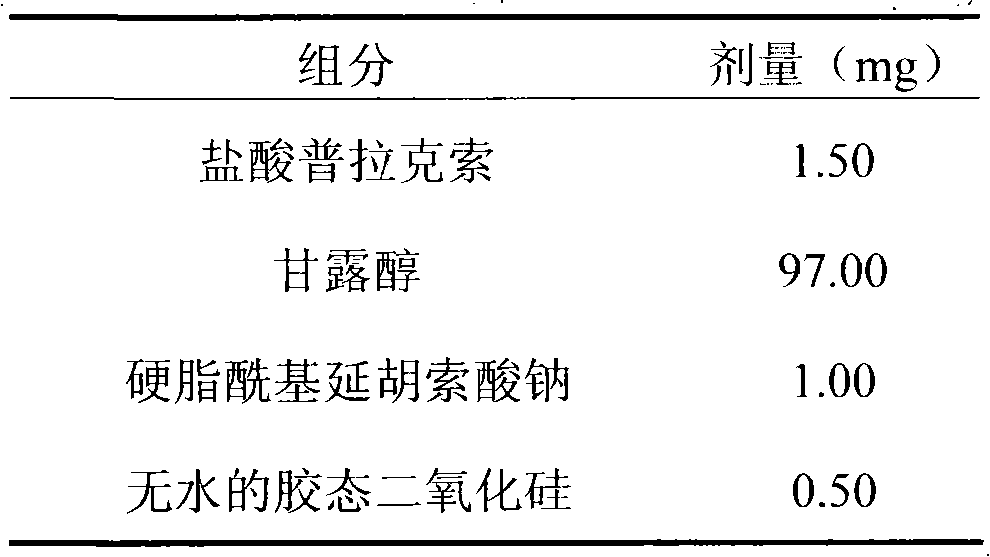
InactiveCN101766605AWide range of hardnessLow friabilityOrganic active ingredientsPill deliveryMedicinePramipexole
The invention relates to a pramipexole-contained pharmaceutical composition capable of being dispersed in a mouth, which is characterized by containing pramipexole or medicinal salt thereof or pharmaceutically acceptable auxiliary materials. The invention is used for the technical field of medicines.
Owner:BEIJING D VENTUREPHARM TECH DEV
Preparation method of artificial stone for building external walls
The invention discloses a preparation method of an artificial stone for building external walls. The method comprises the steps of: mixing quartzite, reflective powder, and epoxy modified polyester resin evenly, spreading the mixture evenly on a die to obtain a first prefabricated material; mixing unsaturated polyester resin, benzoyl peroxide, dodecyl mercaptan and phenyltriethoxysilane, adding aluminum hydroxide, mica powder, modified collagen protein, a pigment, metakaolin, water glass, lignocellulose, carbon fiber, steel fiber, limestone, fly ash and water, mixing the substances evenly, and spreading the mixture uniformly on the first prefabricated material so as to obtain a second prefabricated material; and sending the second prefabricated material into a press to conduct vacuum pressing, then sending the product into a drying room to perform heat insulation, carrying out natural cooling to room temperature, and then conducting die removal, polishing and cutting to obtain the artificial stone for building external walls.
Owner:ANHUI GUANGYAN NEW MATERIAL TECH
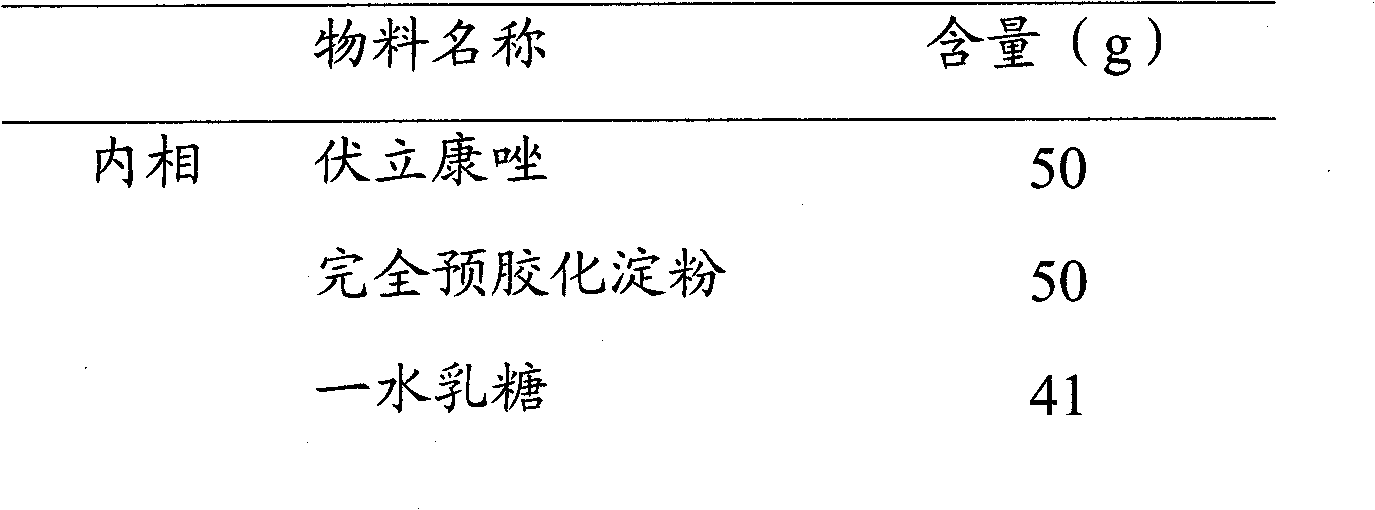
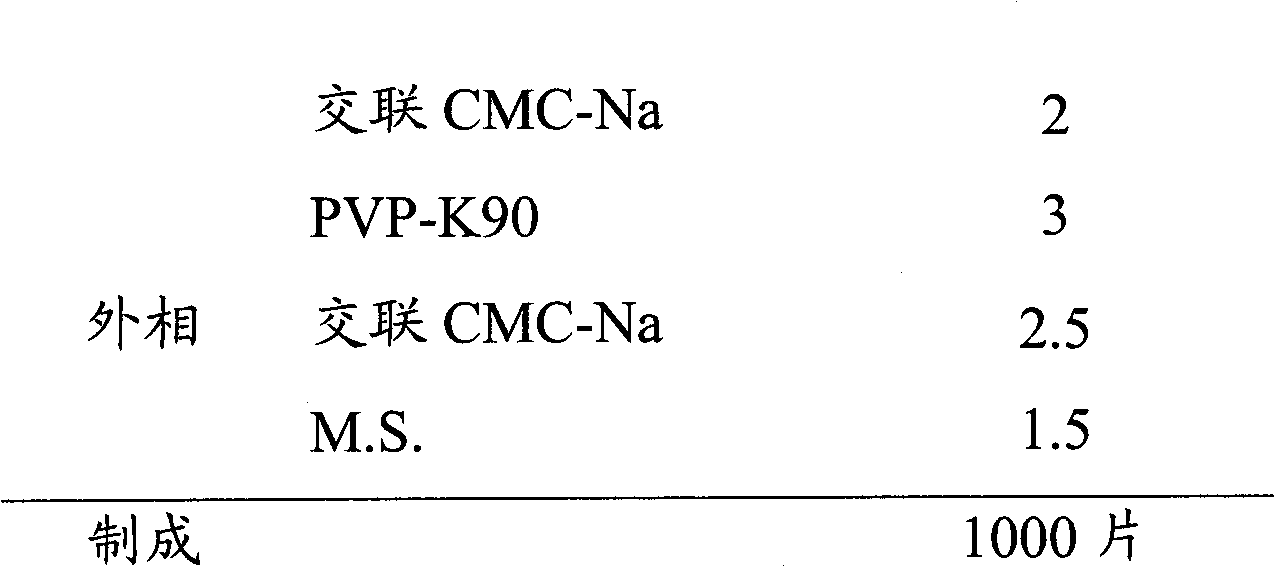
Voriconazole composition and preparation method thereof
The invention relates to a voriconazole tablet, wherein the tablet core of the tablet comprises lactose hydrous, pregelatinized starch, croscarmellose sodium, povidone K90 and magnesium stearate. The prepared tablet core can reach higher hardness, is low in friability, facilitates coating, and can maintain the physical form during packaging and transport process; and although the hardness of the tablet core is higher, the dissolution rate of the tablet is not influenced, and the tablet can be disintegrated within about 20 minutes or a shorter time.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com