Lelrozol tablet and preparation method thereof
A technology of letrozole tablets and letrozole, which is applied in the field of medicine, can solve the problems of unsatisfactory content uniformity, loss of material dust pollution, unfavorable scale-up production, etc., and achieves small dissolution differences, low labor intensity, and reduced fragility sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, letrozole tablet
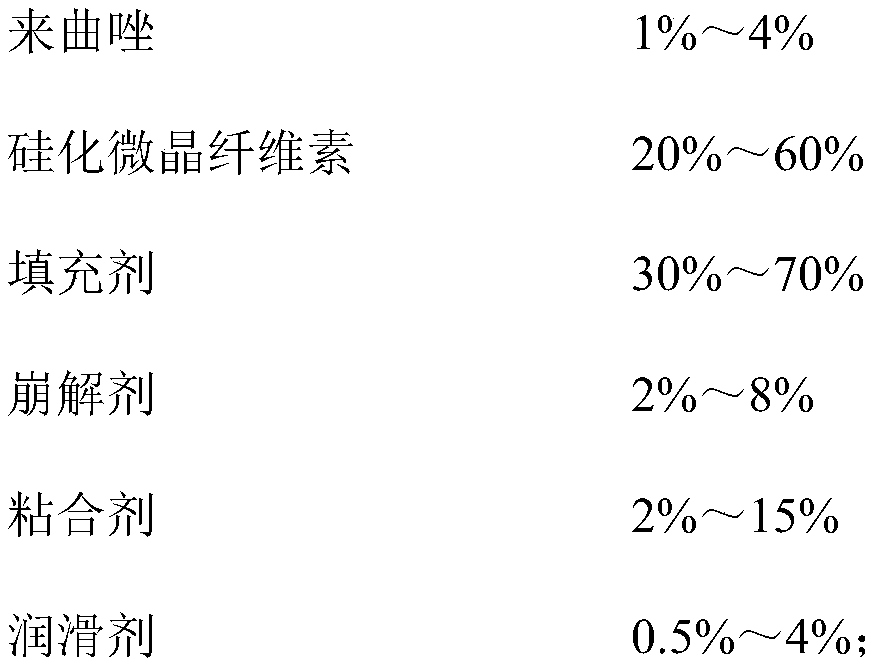
[0041] prescription:
[0042]
[0043] Preparation:
[0044] (1) Letrozole bulk drug and SMCC, lactose monohydrate, sodium carboxymethyl starch and pregelatinized starch are respectively passed through 80 mesh sieves, weighed and placed in a mixer, and mixed for 30 minutes;
[0045] (2) Add magnesium stearate, mix for 15min, and compress into tablets;
[0046] (3) Put the plain tablet on a high-efficiency coating machine, and control the coating weight gain to 2% to 6%, to obtain the letrozole tablet.
Embodiment 2
[0047] Embodiment 2, letrozole tablet
[0048] prescription:
[0049]
[0050]
[0051] Preparation method: with embodiment 1.
Embodiment 3
[0052] Embodiment 3, letrozole tablet
[0053] prescription:
[0054]
[0055] Preparation method: with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com