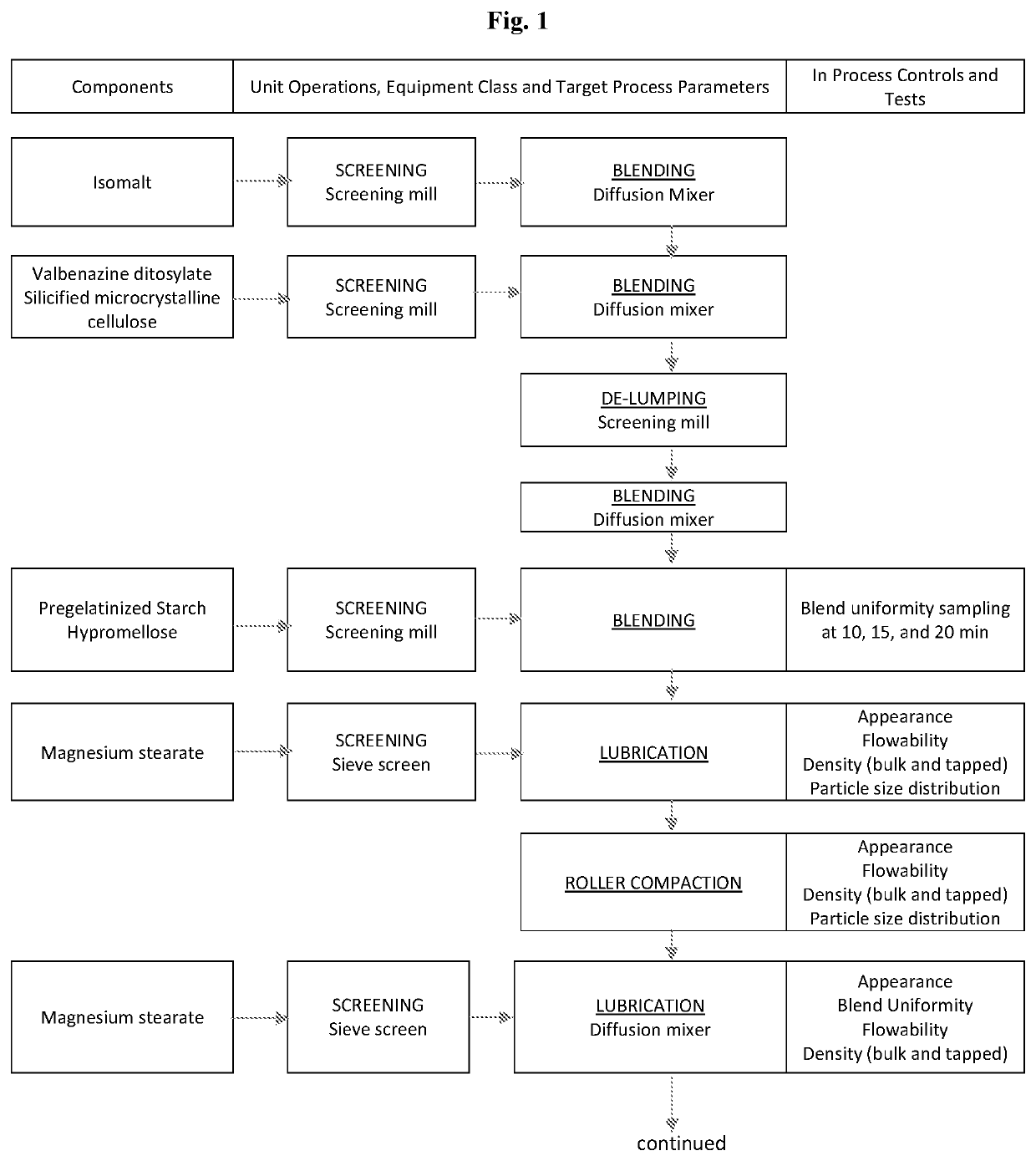
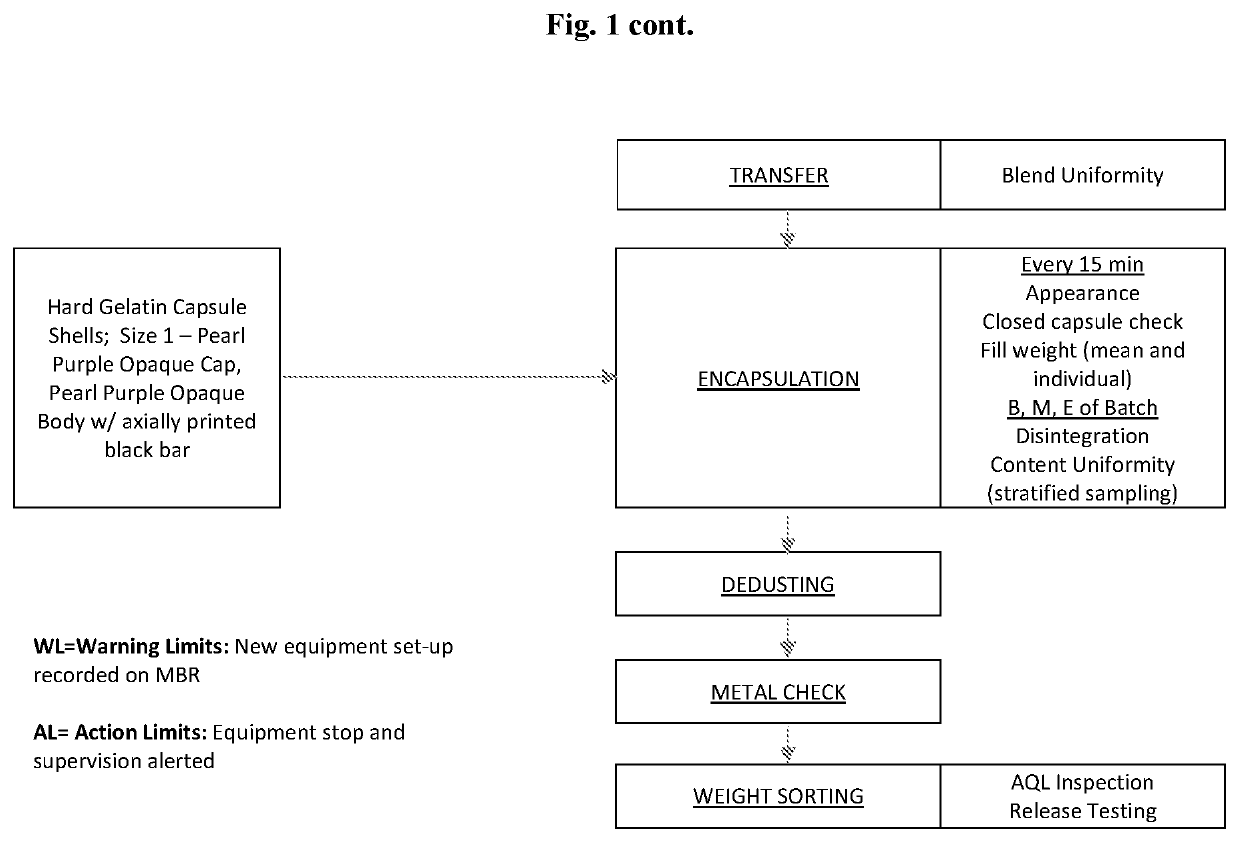
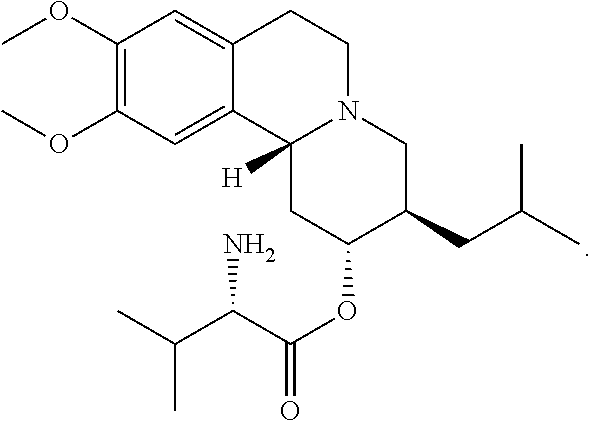
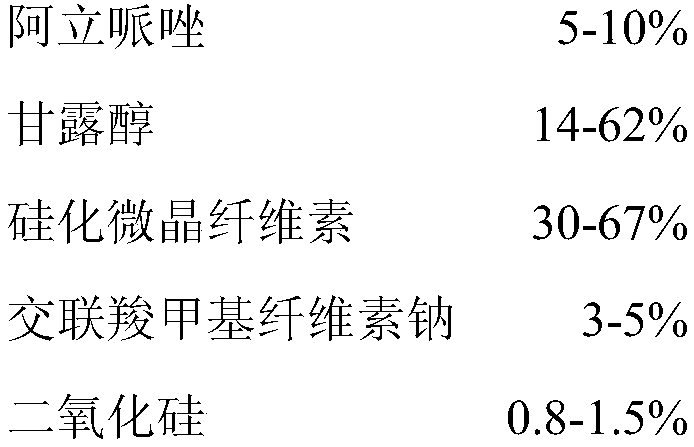
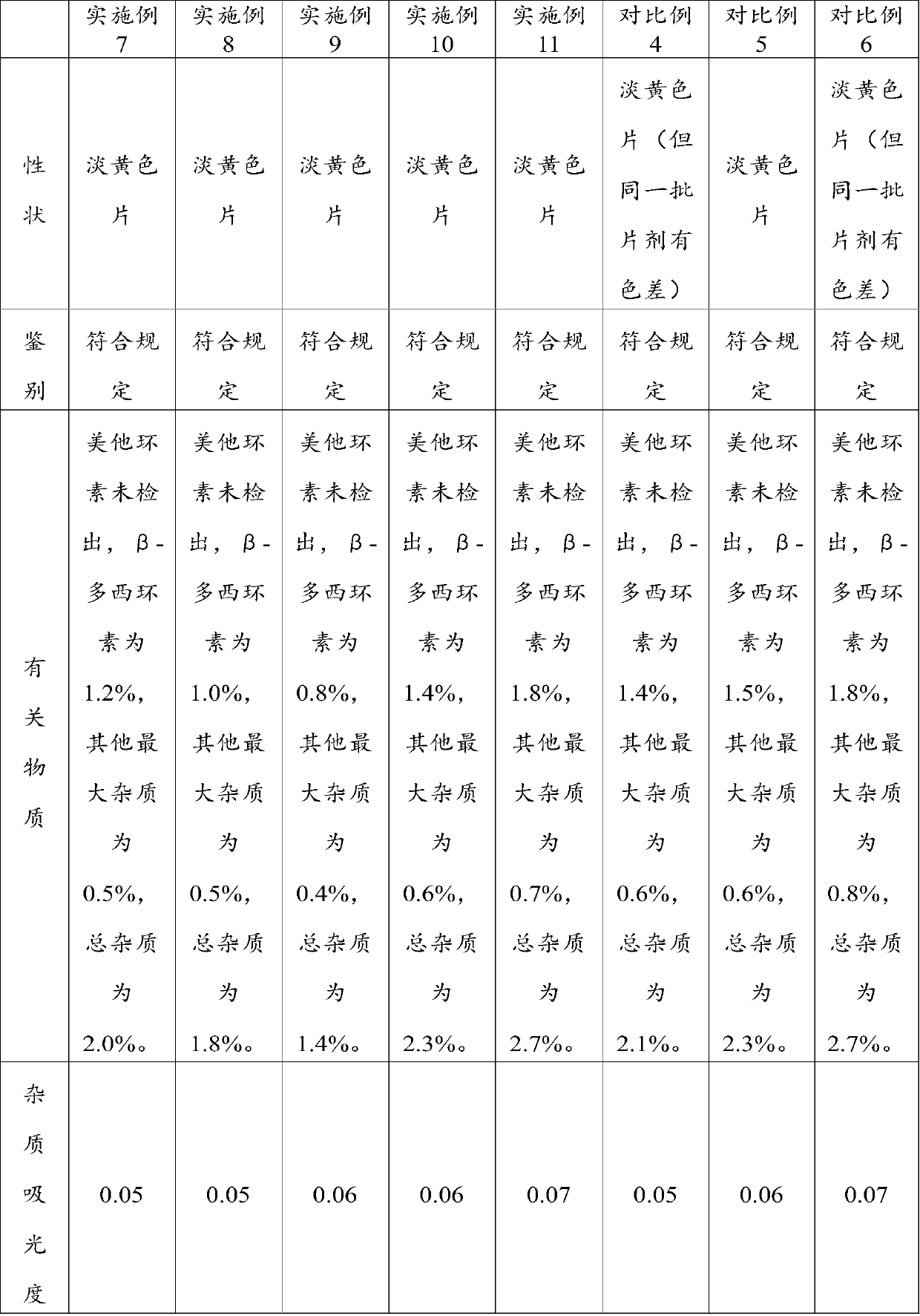
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Silicified microcrystalline cellulose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
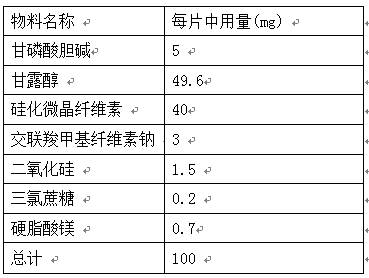
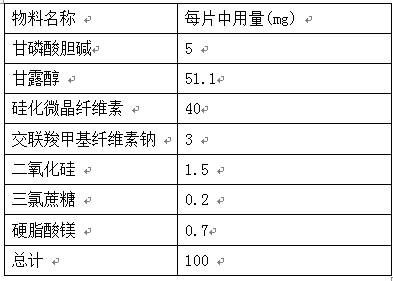
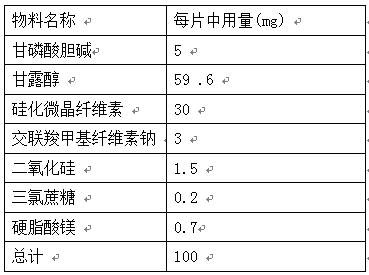
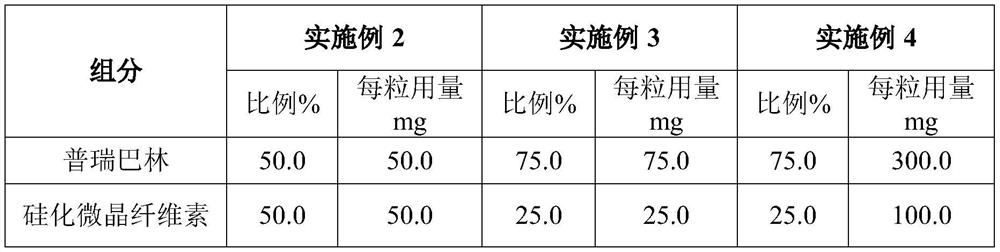
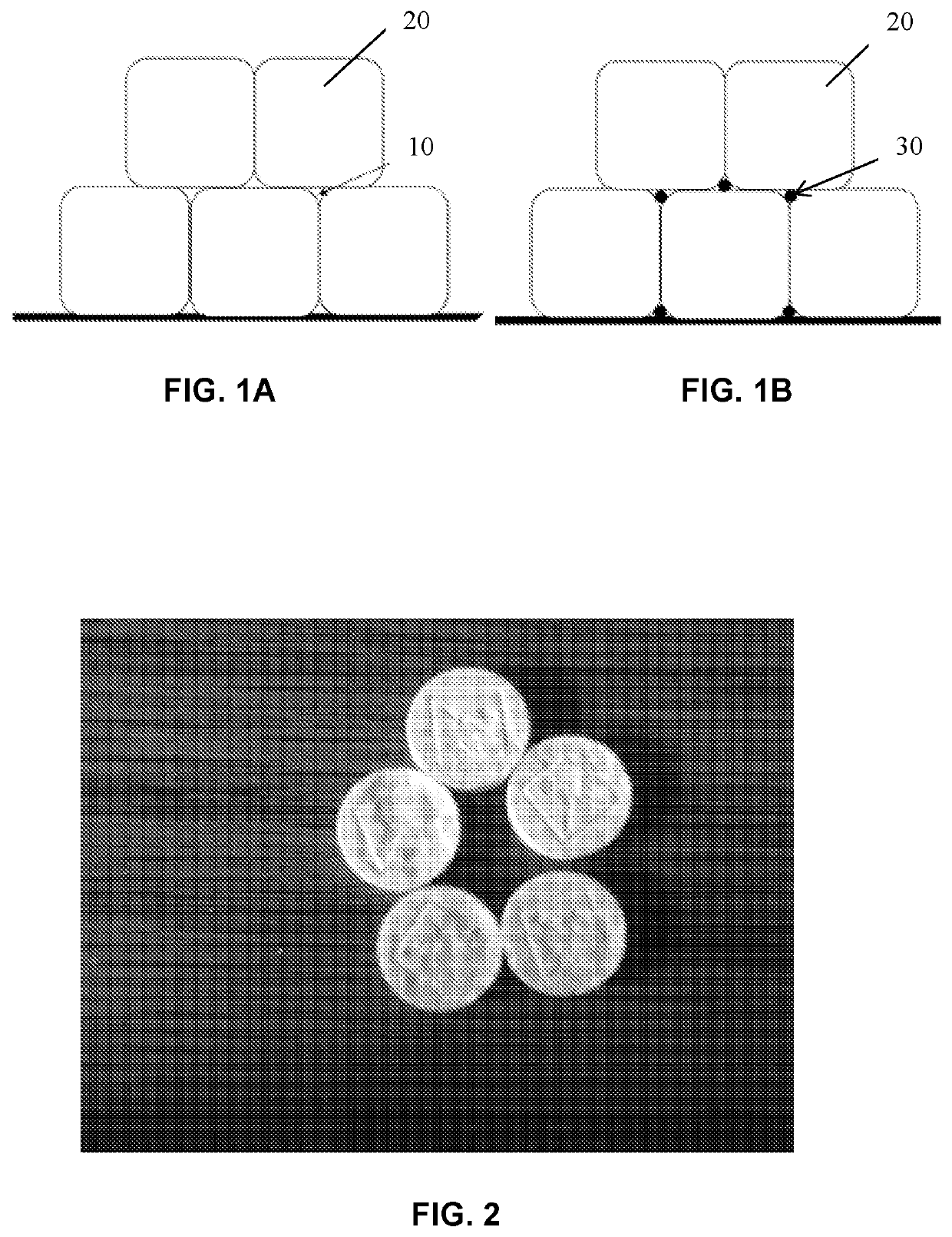
PROSOLV® SMCC, silicified microcrystalline cellulose, is a unique combination of microcrystalline cellulose (MCC) and colloidal silicon dioxide (CSD).
Pharmaceutical formulation containing ibuprofen and codeine
The invention consists of a new formulation of ibuprofen and codeine in the form of a tablet, which comprises L-leucine in a concentration ranging between 4%-15% as a lubricant, in order to prevent the formulation mixture from adhering to the punches and to other elements of the compression machine during the compression process. The new formulation additionally comprises talc (0.5%-5.0%) and silicified microcrystalline cellulose (30%-80%). The formulation is preferably arranged in the form of a core that comprises the active principles and, amongst others, the L-leucine, part of the talc and the silicified microcrystalline cellulose; this core is coated with a composition that contains a copolymer of methacrylic acid and ethyl acrylate. The tablets of the invention do not exhibit flaking problems, have an adequate hardness with a convenient attrition to allow for subsequent coating, offer disintegration values of less than 5 minutes, with dissolution values for both active principles in accordance with those specified for rapid-release tablets.
Owner:FARMASIERRA MFG
Corticosteroid containing orally disintegrating tablet compositions for eosinophilic esophagitis
ActiveUS20160206627A1High blend uniformity/homogeneityOrganic active ingredientsAntipyreticGastrointestinal inflammationOral medication
The present invention is directed to orally administered compositions of topically acting corticosteroids for the treatment of inflammation of the gastrointestinal tracts such as eosinophilic esophagitis. The present invention also provides a method for treating conditions associated with inflammation of the gastrointestinal tract in an individual. The method comprises administering to an individual in need thereof a phermaceutical compossition of the present invention as orally disintegrating tablets comprising a topically active corticosteroid adsorbed onto a pharmaceutically acceptable carrier such as silicified microcrystalline cellulose.
Owner:ADARE PHARMA US LP
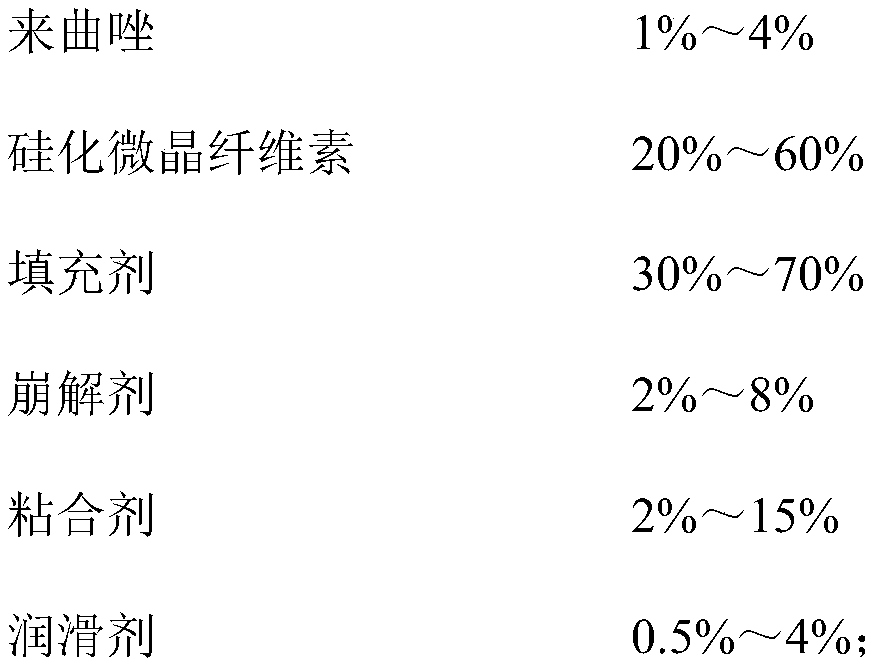
Lelrozol tablet and preparation method thereof
ActiveCN107737112AEvenly dispersedImprove liquidityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineSmallerThan
The invention belongs to the technical field of medicine and particularly relates to a lelrozol tablet and a preparation method thereof. The lelrozol tablet is prepared from lelrozol and silicified microcrystalline cellulose, wherein the content of the silicified microcrystalline cellulose accounts for 20 to 60 percent of total weight of the tablets. The particle size D(v, 0.9) of the lelrozol issmaller than or equal to 60mu m, preferably the particle size D(v, 0.9) of the lelrozol is smaller than or equal to 40mu m and more preferably, the particle size D (v, 0.9) of the lelrozol is smallerthan or equal to 8mu m. According to the preparation method of the lelrozol tablet, disclosed by the invention, the silica microcrystalline cellulose is used, so that the trazodone is uniformly dispersed in the mixed powder; in addition, the lelrozol tablet has good fluidity, and the powder can be directly pressured into tablets, so that the technique is simplified, the time is saved and the laborintensity is low; besides, after a crude drug is crushed, the particle size is obviously reduced; by controlling the content of a disintegrating agent, the dissolution rate of the prepared lelrozol tablet is significantly increased, 85 percent or above can be reached in 15 minutes and thereby the lelrozol tablet is rapidly dissolved out.
Owner:HAINAN JINRUI PHARMA CO LTD
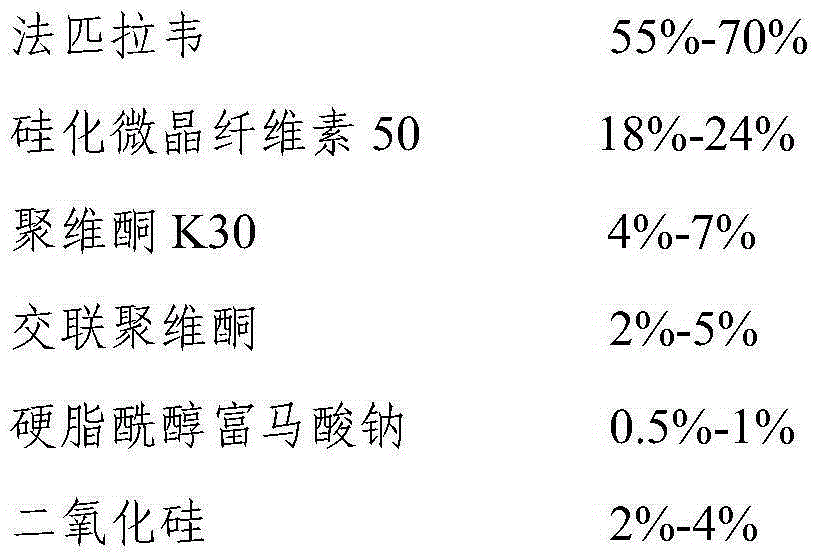
Favipiravir tablets and preparation method thereof
ActiveCN106667926APrescription costs are lowReduce sizeOrganic active ingredientsAntiviralsMedicineAdhesive
The invention relates to favipiravir tablets. The tablets comprise favipiravir, silicified microcrystalline cellulose and adhesive; the silicified microcrystalline cellulose is lower in price, proper in size and high in medicine compliance when being compared with low-substituted hydroxypropyl cellulose; the defective rate is lower and the dissolution rate is higher. The invention further relates to a preparation method of the tablets. The preparation method is simple in preparation process and suitable for industrialized large-scale production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Corticosteroid containing orally disintegrating tablet compositions for eosinophilic esophagitis
ActiveUS10471071B2High blend uniformity/homogeneityOrganic active ingredientsAntipyreticGastrointestinal inflammationOrally disintegrating tablet
The present invention is directed to orally administered compositions of topically acting corticosteroids for the treatment of inflammation of the gastrointestinal tracts such as eosinophilic esophagitis. The present invention also provides a method for treating conditions associated with inflammation of the gastrointestinal tract in an individual. The method comprises administering to an individual in need thereof a pharmaceutical composition of the present invention as orally disintegrating tablets comprising a topically active corticosteroid adsorbed onto a pharmaceutically acceptable carrier such as silicified microcrystalline cellulose.
Owner:ADARE PHARMA US LP
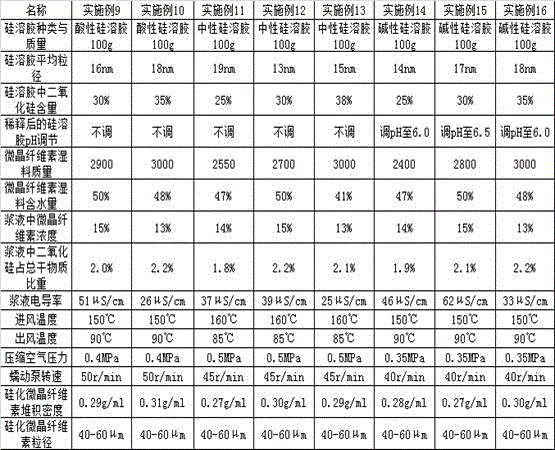
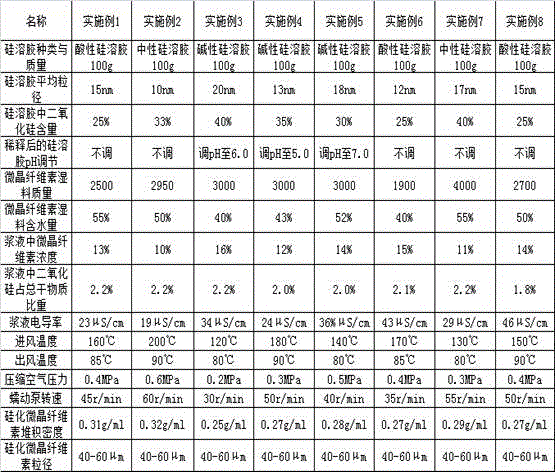
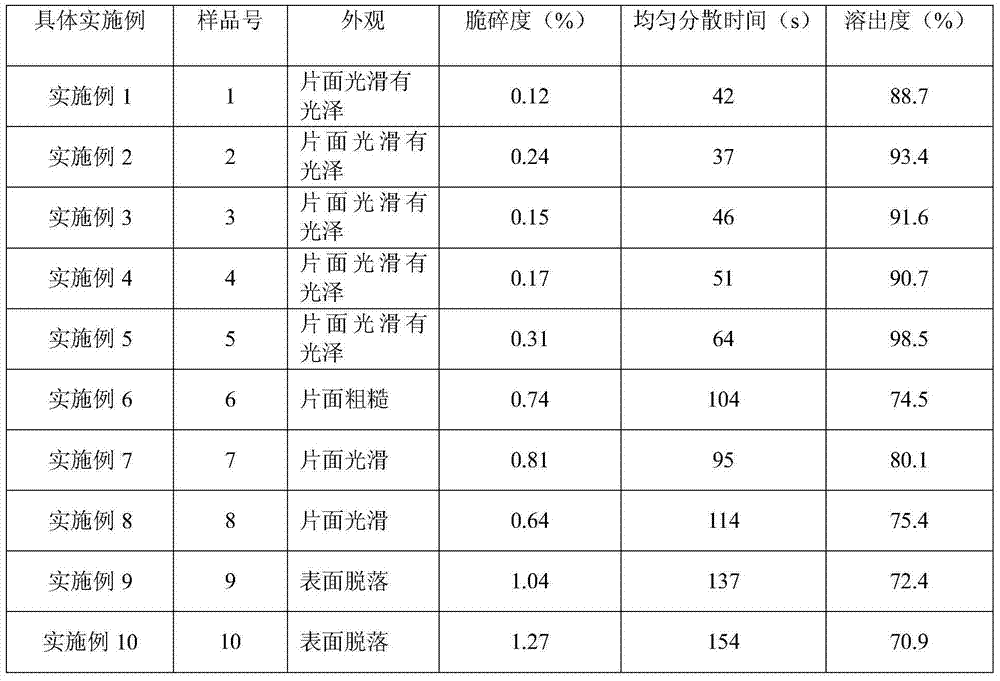
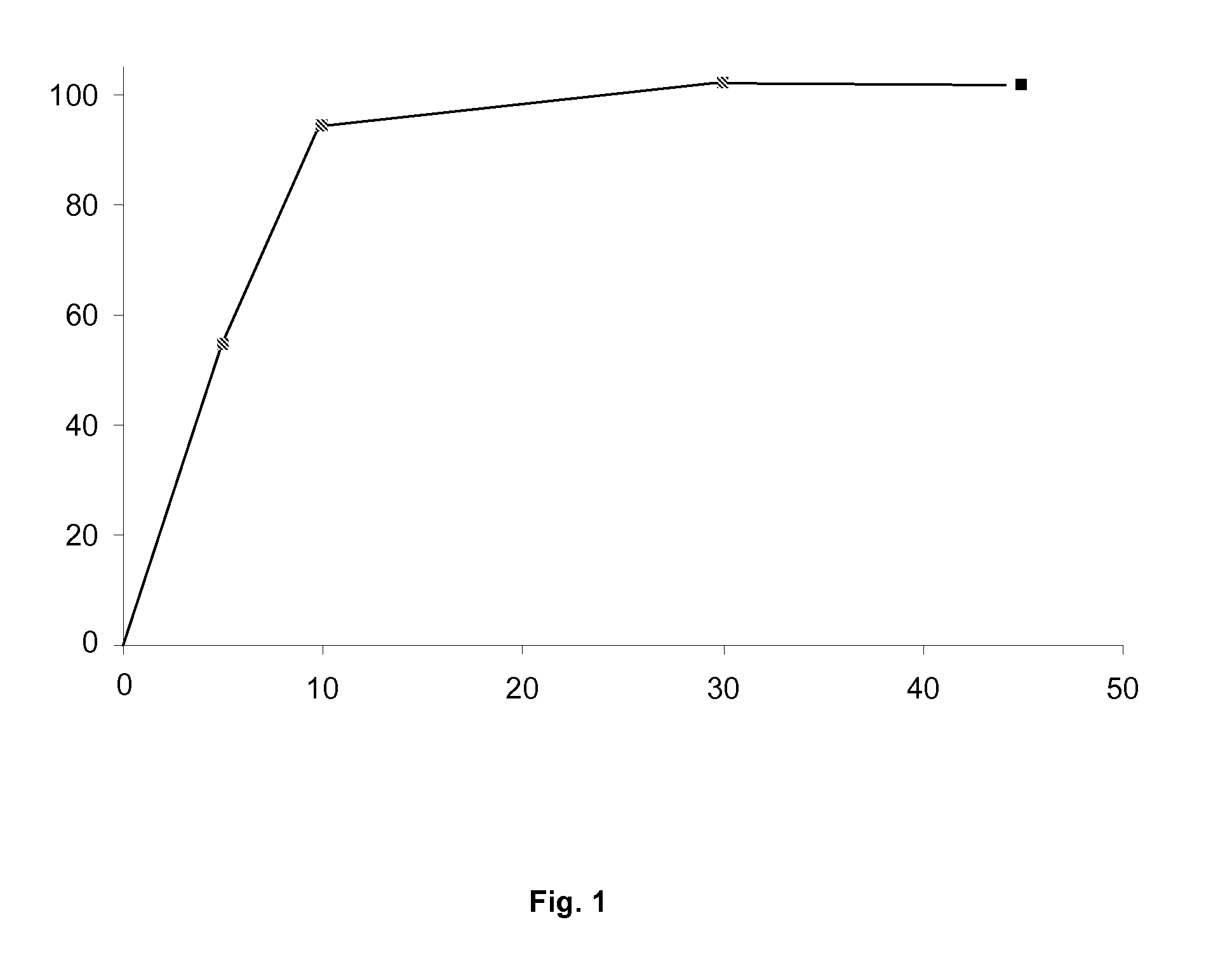
Silicified microcrystalline cellulose and preparation method thereof
ActiveCN106333928AMeet the requirementsUniform bulk densityPharmaceutical non-active ingredientsPill deliveryCompressibilitySlurry
The invention relates to silicified microcrystalline cellulose and a preparation method thereof. The preparation method comprises the following specific steps: diluting silica sol with pure water, adding a microcrystalline cellulose wet material into the diluted silica sol, performing homogenization, filtering to obtain slurry, finally performing spray drying on the slurry through air flow type double fluids, and screening to prepare the silicified microcrystalline cellulose. The bulk density and the particle size of the silicified microcrystalline cellulose are uniform, and the silicified microcrystalline cellulose is high in flowability and compressibility; as a medicinal auxiliary material, the silicified microcrystalline cellulose has the outstanding effect on direct tabletting. The preparation method of the silicified microcrystalline cellulose is simple in technology, short in process and low in cost, and the yield can meet the requirement on industrial production.
Owner:HUZHOU ZHANWANG PHARMA
Novel pharmaceutical auxiliary material namely silicified microcrystalline cellulose and preparation method thereof
InactiveCN104771761ADisperse fastGood swelling propertiesPharmaceutical non-active ingredientsEmulsionDissolution
A novel pharmaceutical auxiliary material namely silicified microcrystalline cellulose and a preparation method thereof are disclosed. The preparation method comprises the following steps: preparing microcrystalline cellulose accounting for 98.15 to 99.45% of the total weight of raw materials and aerosil accounting for 0.55 to 1.85% of the total weight of raw materials, mixing microcrystalline cellulose with part of aerosil, processing the mixture by a high-speed shearing, dispersing, and homogenizing machine, adding the residual aerosil into the homogenized emulsion, evenly mixing, and carrying out spray-drying to prepare the silicified microcrystalline cellulose. The prepared silicified microcrystalline cellulose has good fluidity and compressibility, moreover the preparation properties such as disintegration property, dispersion uniformity, dissolution rate, and the like are effectively improved, and thus the silicified microcrystalline cellulose is especially suitable for the production of traditional Chinese medicine dispersible preparations. The silicified microcrystalline cellulose is used to prepare Fuyankang dispersible tablets, and the appearance, dispersion uniformity, and dissolution degree of the tablets are all effectively improved.
Owner:GUANGDONG GUOYUAN GUOYAO PHARMA CO LTD
Pharmaceutical formulation containing ibuprofen and codeine
The invention consists of a new formulation of ibuprofen and codeine in the form of a tablet, which comprises L-leucine in a concentration ranging between 4%-15% as a lubricant, in order to prevent the formulation mixture from adhering to the punches and to other elements of the compression machine during the compression process. The new formulation additionally comprises talc (0.5%-5.0%) and silicified microcrystalline cellulose (30%-80%). The formulation is preferably arranged in the form of a core that comprises the active principles and, amongst others, the L-leucine, part of the talc and the silicified microcrystalline cellulose; this core is coated with a composition that contains a copolymer of methacrylic acid and ethyl acrylate. The tablets of the invention do not exhibit flaking problems, have an adequate hardness with a convenient attrition to allow for subsequent coating, offer disintegration values of less than 5 minutes, with dissolution values for both active principles in accordance with those specified for rapid-release tablets.
Owner:FARMASIERRA MFG
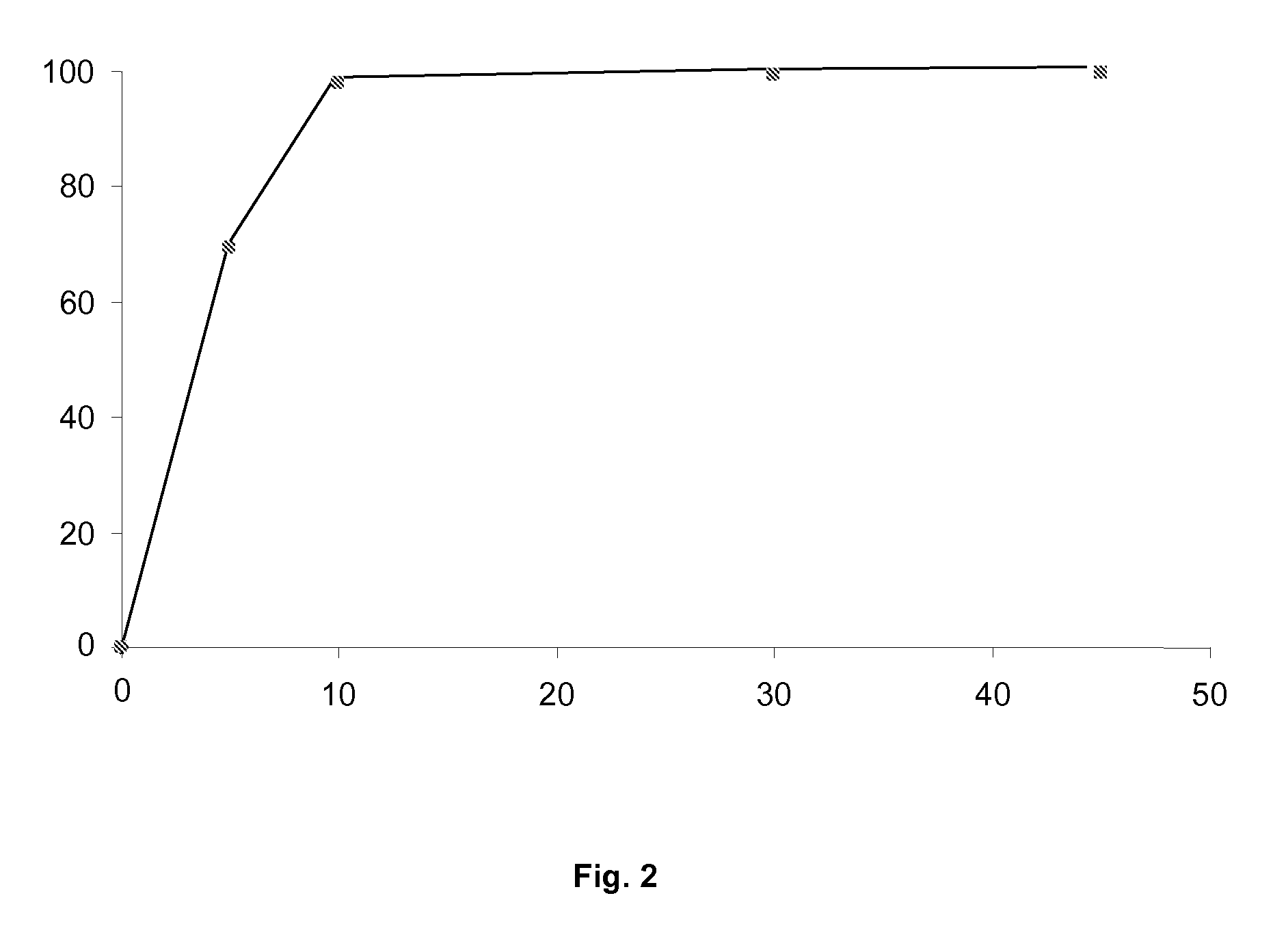
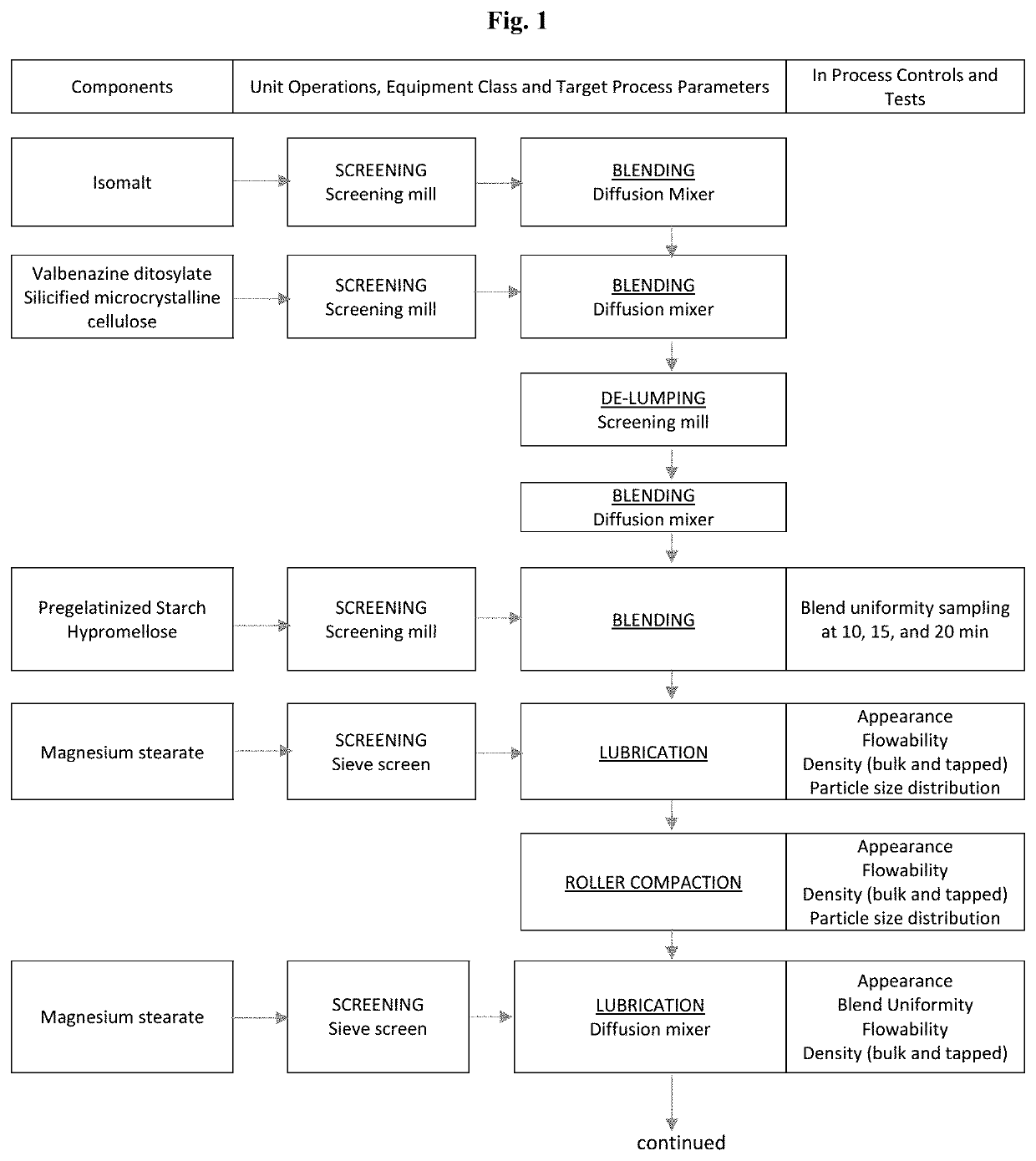
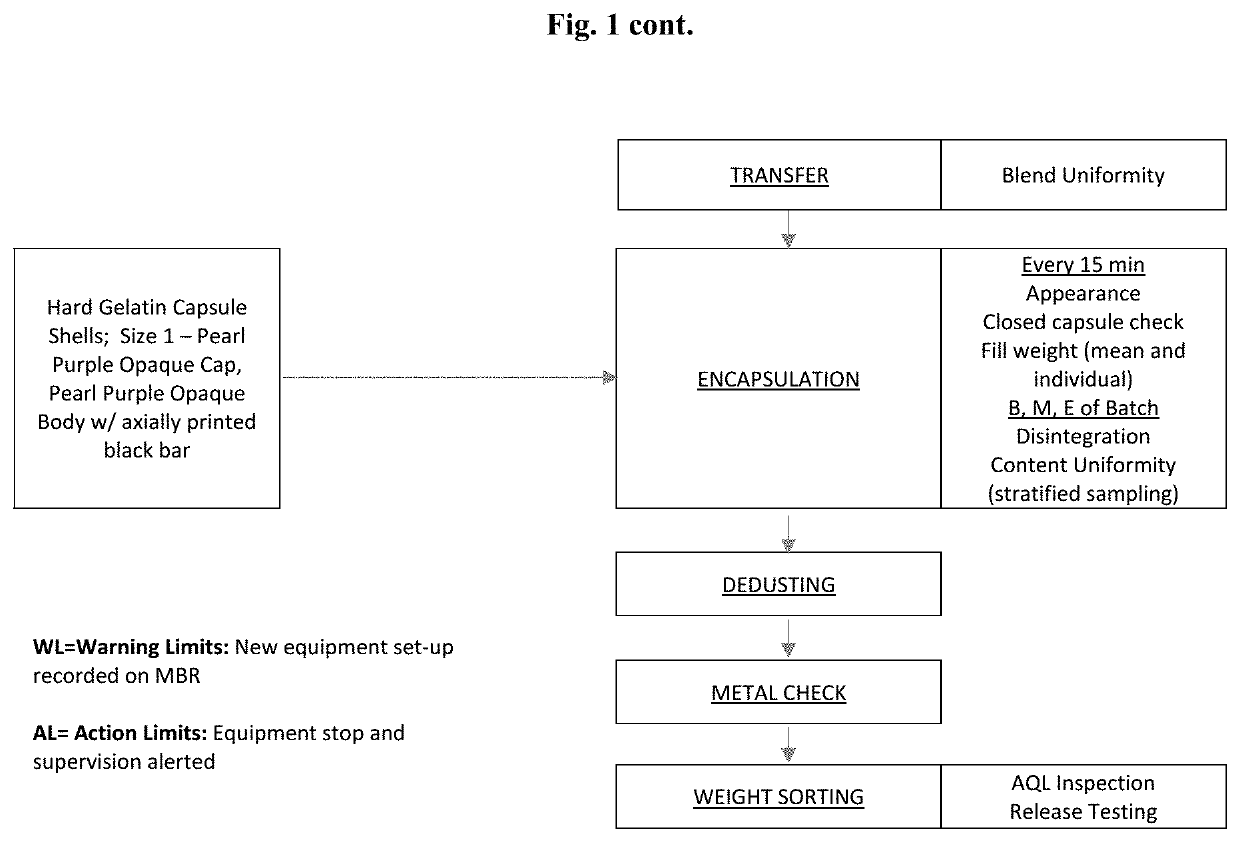
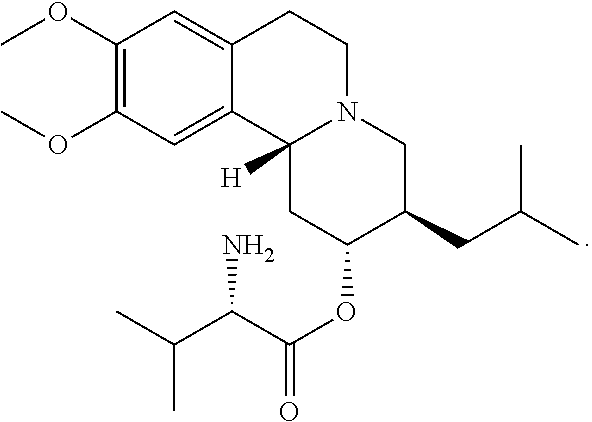
High Dosage Valbenazine Formulation and Compositions, Methods, and Kits Related Thereto
Solid pharmaceutical compositions with high drug loading are provided. A formulation useful for the solid pharmaceutical composition includes valbenazine, or a pharmaceutically acceptable salt thereof, silicified microcrystalline cellulose, isomalt, hydroxypropyl methylcellulose, partially pregelatinized maize starch, and magnesium stearate.
Owner:NEUROCRINE BIOSCI INC
High Dosage Valbenazine Formulation and Compositions, Methods, and Kits Related Thereto
Solid pharmaceutical compositions with high drug loading are provided. A formulation useful for the solid pharmaceutical composition includes valbenazine, or a pharmaceutically acceptable salt thereof, silicified microcrystalline cellulose, isomalt, hydroxypropyl methylcellulose, partially pregelatinized maize starch, and magnesium stearate.
Owner:NEUROCRINE BIOSCI INC
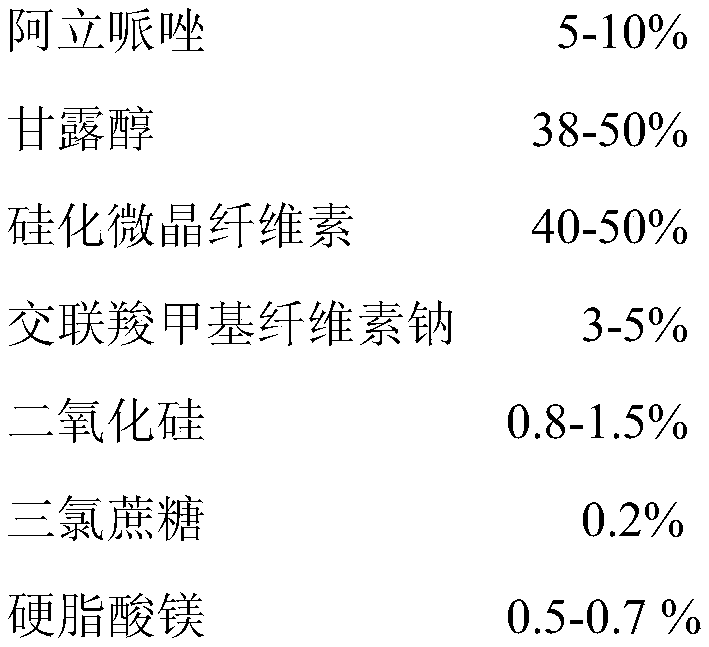
Orally disintegrating tablets containing aripiprazole and preparation method of orally disintegrating tablets
ActiveCN109864975AGreat tasteGood taste acceptanceOrganic active ingredientsNervous disorderActive componentOrally disintegrating tablet
The invention provides orally disintegrating tablets containing aripiprazole and a preparation method of the orally disintegrating tablets. The orally disintegrating tablets comprise the components ofaripiprazole as the active component, silicified microcrystalline cellulose, croscarmellose sodium, silicon dioxide, a stuffing agent, a corrigent and a lubricant. Based on a formula, the orally disintegrating tablets are prepared by a powder vertical compression technology. The orally disintegrating tablets have good mouth feel and good long-term stability, so that the validity and the safety ofdrugs are guaranteed.
Owner:CHENGDU KANGHONG PHARMA GRP
RAF kinase inhibitor preparation and preparation method thereof
ActiveCN111184693AImprove stabilitySolve the problems of easy aggregation, static electricity, and poor fluidityOrganic active ingredientsPharmaceutical non-active ingredientsEnzyme Inhibitor AgentPharmaceutical medicine
The invention belongs to the field of pharmaceutic preparations, and relates to an RAF kinase inhibitor particle composition. The composition comprises the following components: (1) 5-(((1R,1aS,6bR)-1-(6-(trifluoromethyl)-1H-benzo[d]imidazol-2-yl)-1a,6b-dihydro-1H-cyclopropal[b]benzofuran-5-yl)oxy)-3,4-dihydro-1,8-naphthyridin-2(1H)-one or a pharmaceutically acceptable salt as an active component,(2) silicified microcrystalline cellulose and (3) an optionally selected other pharmaceutically acceptable excipient. The particle composition is prepared by using a rolling granulation technology. The method adopts the rolling granulation technology to solve the problems of easy agglomeration and poor fluidity of materials, obtains the particle composition with better fluidity, stability and dissolution for preparing a corresponding formulation dosage form, and is suitable for commercial production.
Owner:百济神州(苏州)生物科技有限公司
Medicine composition containing colesevelam hydrochloride and preparation method thereof
InactiveCN103585128AGuaranteed hardnessGuaranteed friabilityOrganic active ingredientsMetabolism disorderPharmaceutical drugPharmaceutical Aids
The invention relates to a medicine composition containing colesevelam hydrochloride and a preparation method thereof. A prescription of the composition comprises a main medicine of colesevelam hydrochloride and two necessary auxiliaries of silicified microcrystalline cellulose and lubricant; the preparation method avoids the complex technique in the wet granulation or the dry granulation, and overcomes the defects of weak tablet fluidity, weak content uniformity, overgreat tablet weight and low compliance of patients of the large-dosage main medicine; the medicine composition can be prepared through the preparation technology of direct powder compression. The colesevelam hydrochloride tablet prescription of the invention has the advantages of rationality, simple and stable preparation technique, better effect on the selection of the auxiliaries and the ratio selection of the main material and the auxiliaries, lower production cost and convenience in industrial application.
Owner:BEIJING TIDE PHARMA
Metroprolol succinate sustained-release tablets and preparation method thereof
InactiveCN106692093AOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletFluidized bed
The invention relates to metroprolol succinate sustained-release tablets and a preparation method thereof. The sustained-release tablets comprise: a main medicine: metroprolol succinate; a sustained-release material: ethyecellulose; a pore-forming agent: hydroxyethyl cellulose; an adhesive: ethyecellulose aqueous dispersion; a filling agent: silicified microcrystalline cellulose; a flow aid: micropowder silica gel; a coating material: opadry II. The preparation method comprises the steps: mixing the main medicine and other auxiliary materials except for the flow aid and the coating material and performing one-step pelletization by a fluidized bed top-spray one-step pelletization process to obtain sustained-release granules; adding the flow aid into the sustained-release granules, mixing uniformly and tabletting; coating the compressed tablets with the opadry II to obtain the final sustained-release tablets.
Owner:YAOPHARMA CO LTD
Bilastine tablet and preparation method thereof
InactiveCN110787140AProduct quality is easy to controlSuitable for mass productionOrganic active ingredientsPharmaceutical non-active ingredientsPharmaceutical formulationTableting
The invention belongs to the technical field of medicinal preparations, and particularly relates to a bilastine tablet capable of realizing direct powder compression adopting silicified microcrystalline cellulose, and a preparation method thereof. The tablet is prepared from the following components by weight percent: 1 to 10 percent of bilastine, 10 to 90 percent of silicified microcrystalline cellulose, 3 to 20 percent of a disintegrating agent, 0.5 to 5 percent of a lubricating agent, and 0 to 5 percent of a flow aid. According to the bilastine tablet and the preparation method thereof provided by the invention, the defects of poor fluidity and compressibility of the bilastine are overcome, a recipe and a technology of direct bilastine compression are realized, the preparation technology is simpler, the stability of the product quality is ensured, and the method is suitable for mass production.
Owner:BEIJING VENTUREPHARM BIOTECH
Orally Disintegratable Simvastatin Tablets
InactiveUS20070087050A1Improve stabilityBiocideDispersion deliverySilicified microcrystalline celluloseOral cavity
An orally disintegratable tablet containing simvastatin and silicified microcrystalline cellulose is provided with a non-alkaline lubricant.
Owner:SYNTHON BV
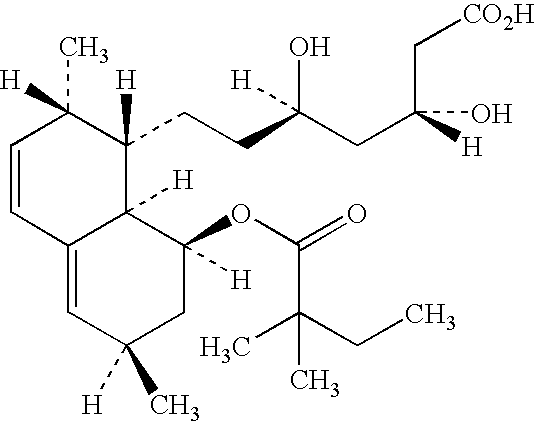
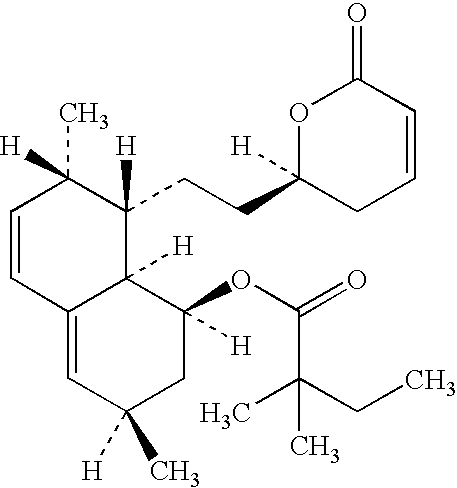
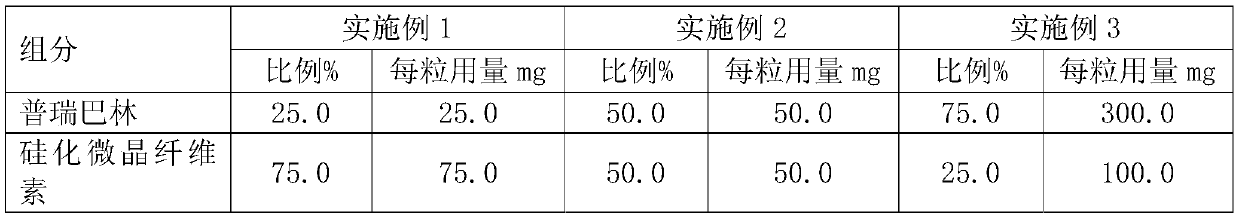
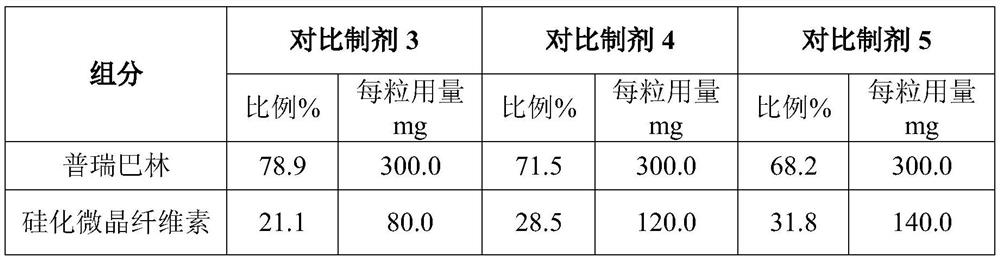
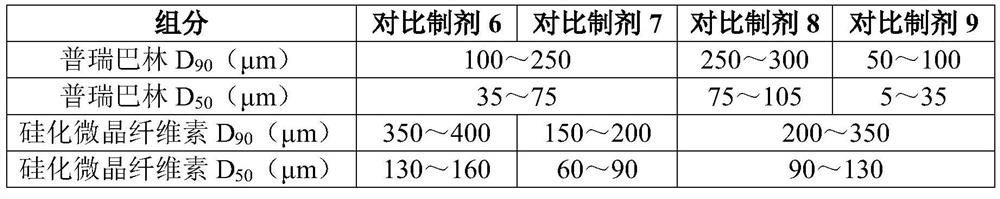
Pregabalin capsule and preparation method thereof
InactiveCN110613694AIncrease dissolution rateGuaranteed liquidityOrganic active ingredientsNervous disorderMedicineSilicified microcrystalline cellulose
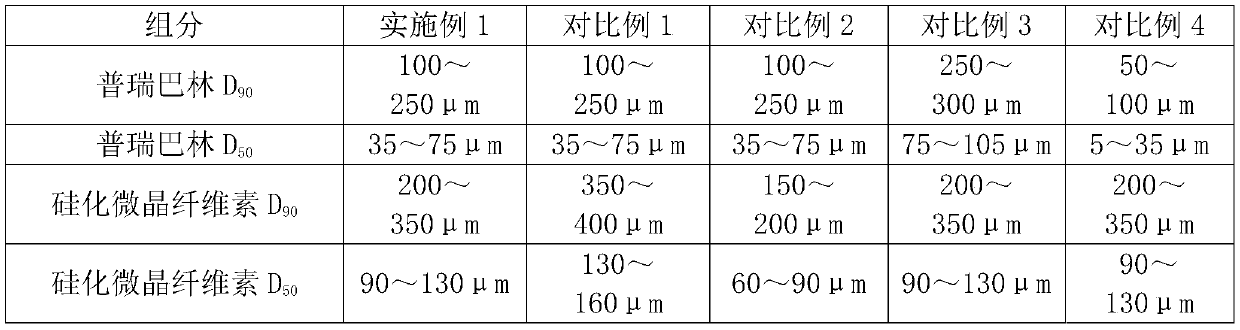
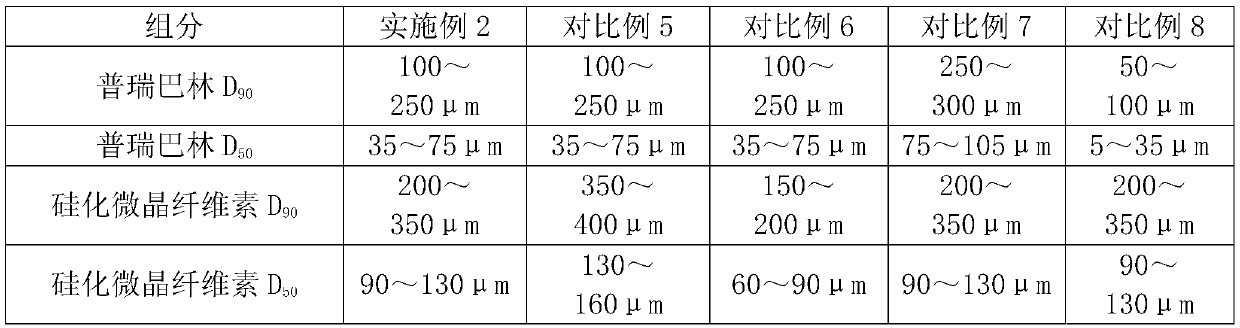
The invention discloses a pregabalin capsule and a preparation method thereof. The content is composed of pregabalin and silicified microcrystalline cellulose, wherein the silicified microcrystallinecellulose is prepared by blending microcrystalline cellulose and micro-powder silica gel in water and performing drying. In the pregabalin capsule, a weight percentage of pregabalin is 25-75%, D90 is100-250 [mu]m, and D50 is 35-75 [mu]m; and D90 of the excipient silicified microcrystalline cellulose is 200-350 [mu]m, and D50 is 90-130 [mu]m. The production process is simple, the product is stable, and the pregabalin capsule is suitable for large-scale industrial production.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Doxycycline hydrochloride tablet, preparation method thereof, application and antibacterial drug
ActiveCN110368367AImprove dispersion uniformityEasy to shapeAntibacterial agentsTetracycline active ingredientsFiller ExcipientDoxycycline hydrochloride
The invention relates to the technical field of medicines, and particularly provides a doxycycline hydrochloride tablet, a preparation method thereof, an application and an antibacterial drug. The doxycycline hydrochloride tablet is mainly prepared from, in weight percent, 10%-30% of doxycycline hydrochloride, 2%-5% of flow aids, 0.5%-4% of lubricants and the balance directly-pressable fillers. The fillers comprise silicified microcrystalline cellulose. All raw materials are scientifically matched with each other, and the doxycycline hydrochloride tablet can be directly formed by tableting andhas the advantages of high uniformity, high hardness, surface smoothness, good product quality stability, high safety and effectiveness, low raw material loss rare, high product yield and low cost.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
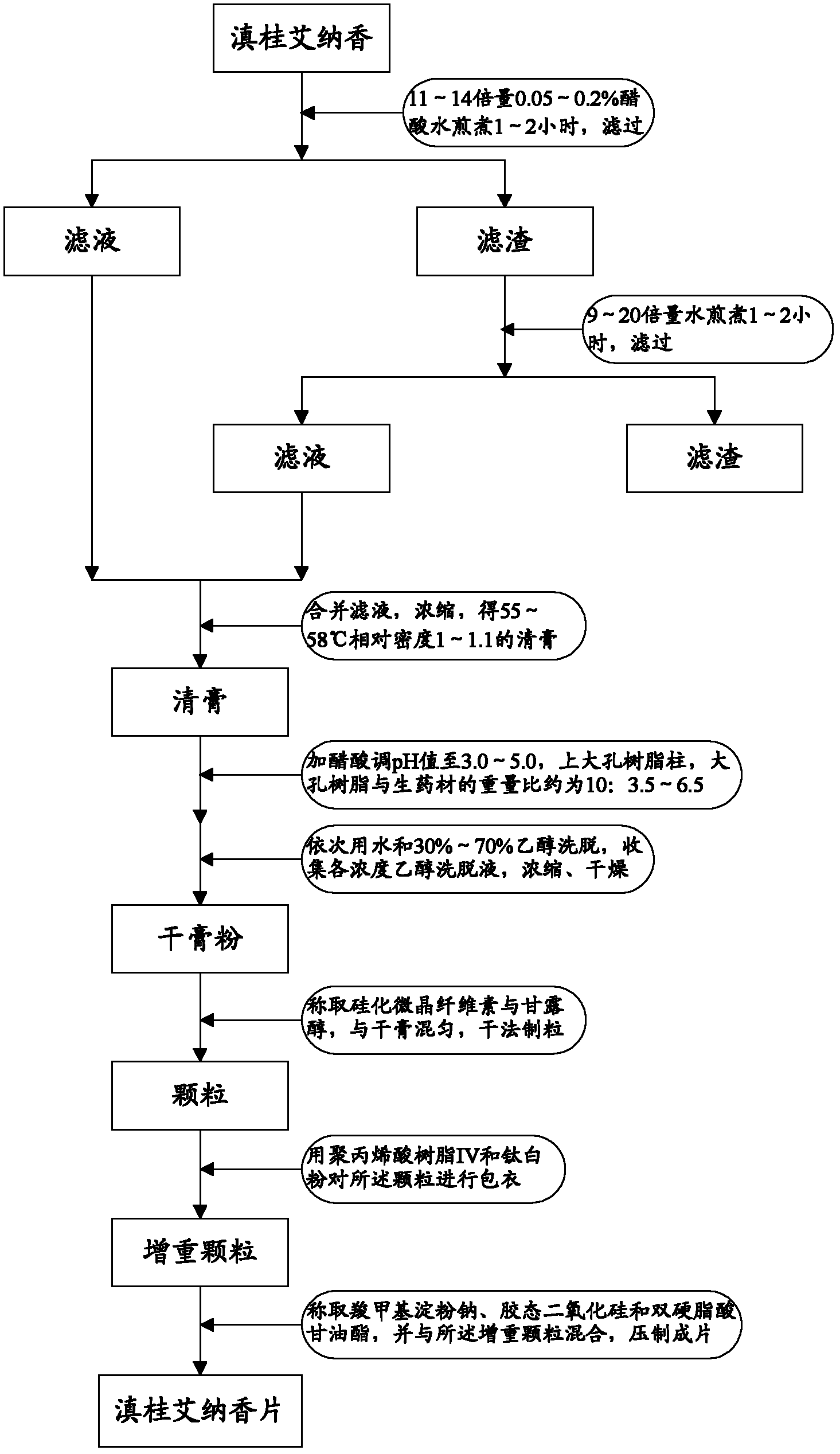
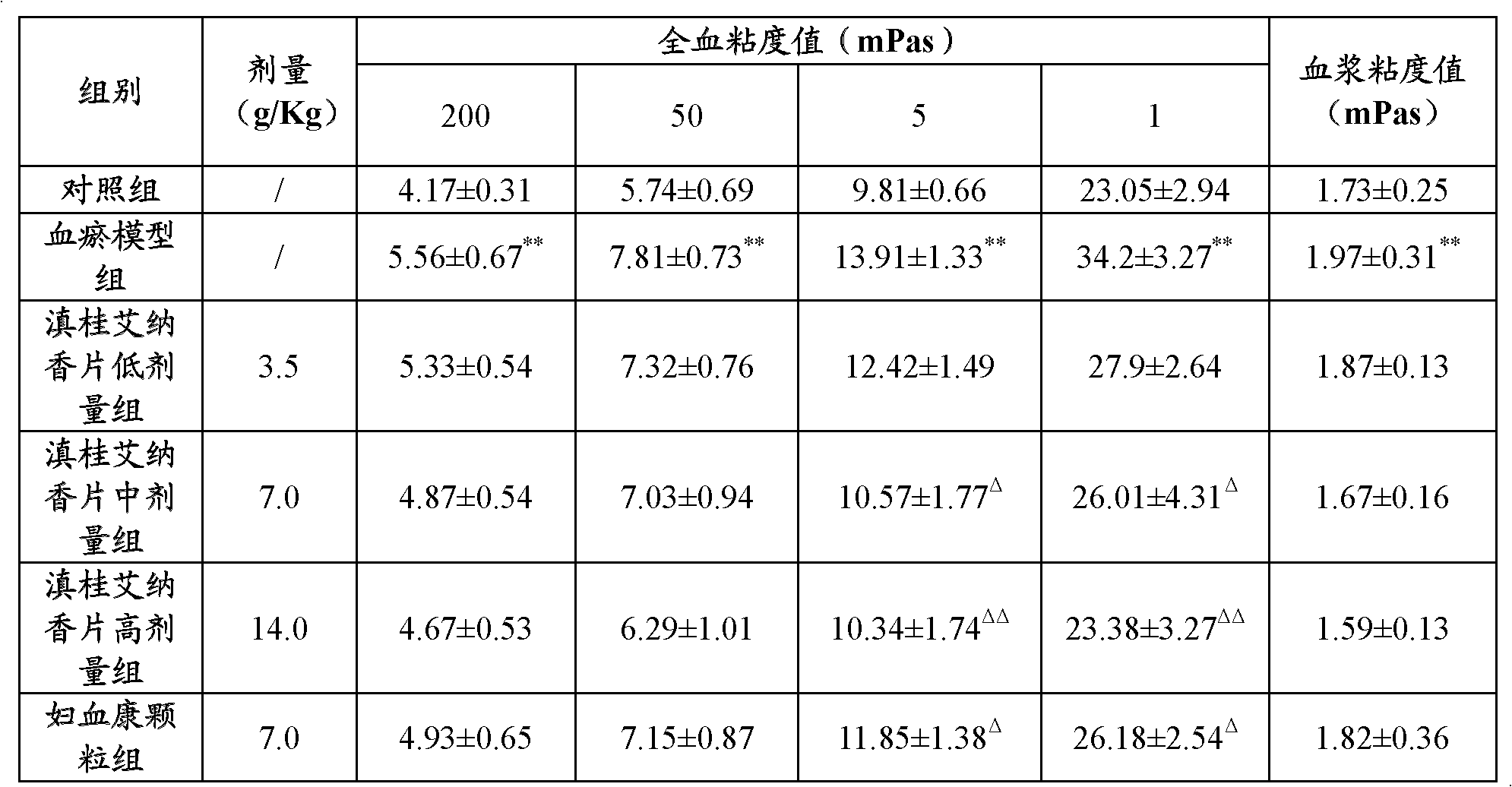
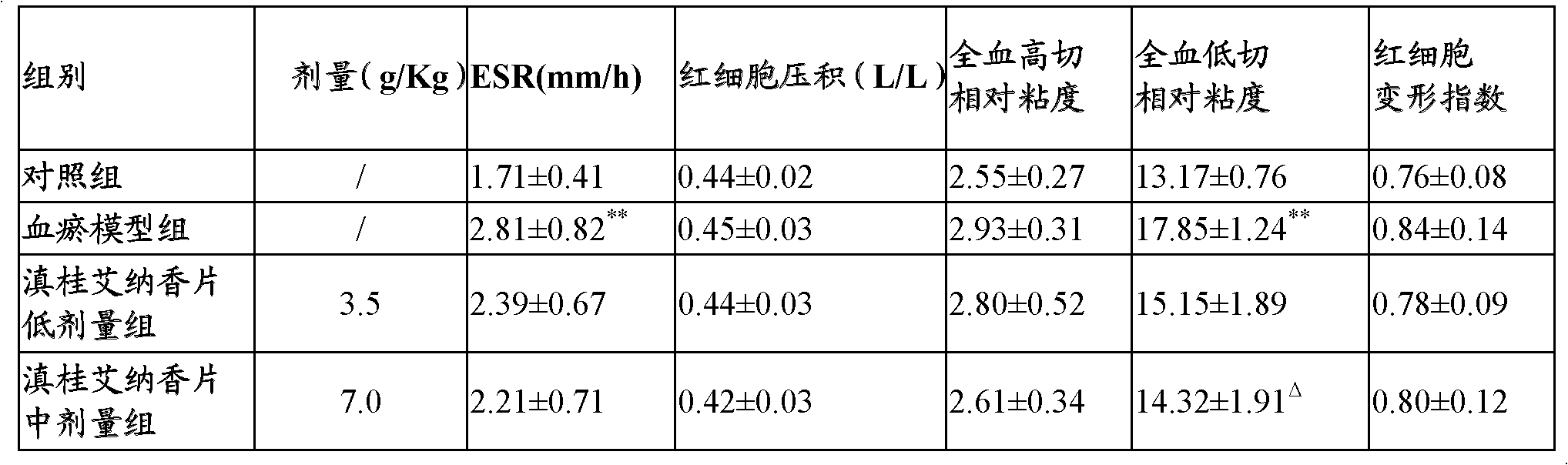
Blumea riparia tablet and preparation process thereof
ActiveCN102423330ASimple preparation processShort processAntipyreticAnalgesicsMANNITOL/SORBITOLALLYL SUCROSE
The invention provides a blumea riparia tablet, which comprises the following components: therapeutically effective amount of blumea riparia extractive, silicified microcrystalline cellulose, mannitol, polyacrylic acid resin IV, titanium dioxide, sodium carboxymethyl starch, colloidal silicon dioxide and glyceryl distearate. The invention also provides a preparation process of the blumea riparia tablet. With the same medicinal material content, the blumea riparia tablet can reduce the everyday doses of a patient. In the invention, the auxiliary materials are reasonably selected and prepared, so that the coordination role of components in the auxiliary materials can be fully played. The preparation process is simple, the process flow is short, and the production is easy.
Owner:GUANGDONG GUOYUAN GUOYAO PHARMA CO LTD
Method for preparing pregabalin orally disintegrating tablet
InactiveCN111096953AOrganic active ingredientsNervous disorderOrally disintegrating tabletPregabalin
The invention provides a pregabalin orally disintegrating tablet by adopting direct powder compression on silicified microcrystalline cellulose and a preparation method thereof. According to the method, pregabalin and silicified microcrystalline cellulose are premixed, the premix is uniformly mixed with other auxiliaries, and the orally disintegrating tablet is prepared by adopting the direct powder compression process. The method can remarkably improve the stability and compressibility of pregabalin and can improve the medication compliance of a patient.
Owner:BEIJING VENTUREPHARM BIOTECH
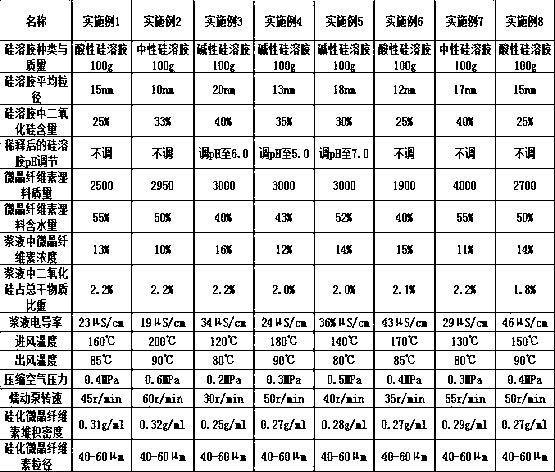
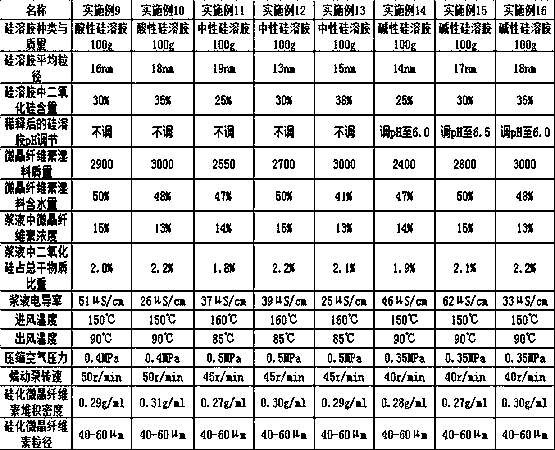
Method for preparing pharmaceutic silicified microcrystalline cellulose by silica hydrosol method
InactiveCN106377771APharmaceutical qualityControls are responsiveInorganic non-active ingredientsPharmaceutical industrySilica gel
The invention relates to a preparation method of a silicified microcrystalline cellulose pharmaceutic adjuvant and belongs to the technical field of pharmaceuticals industry. The method comprises the following steps: mixing microcrystalline cellulose with silica hydrosol, filtering, washing with water, filtering and drying to obtain silicified microcrystalline cellulose. According to the preparation method, silica gel is prepared in situ by a chemical method and then is synthesized with microcrystalline cellulose to obtain the silicified microcrystalline cellulose pharmaceutic adjuvant; a reaction end point can be effectively controlled, a reaction process is simplified, and the silicified microcrystalline cellulose according with the pharmaceutic quality is obtained.
Owner:安徽安生生物化工科技有限责任公司
Cetirizine hydrochloride tablet and preparation method thereof
ActiveCN111214449AAvoid corrosionSolve the sticking problemOrganic active ingredientsSenses disorderCelluloseCetirizine Hydrochloride
The invention discloses a cetirizine hydrochloride tablet, which is prepared by the method that cetirizine hydrochloride and polyoxyl stearate are dissolved in ethyl alcohol, diatomite is added into asolution to be evenly stirred, drying is carried out to obtain a mixture, and then, the mixture is mixed with silicified microcrystalline celluloses, disintegrating agents and lubricating agents to carry out tableting. The cetirizine hydrochloride tablet prepared by the method can be quickly dissolved out, is free from sticking and does not corrode a trimming die.
Owner:广东彼迪药业有限公司
Orally disintegrating tablet of choline glycerophosphate and preparation method thereof
PendingCN112137971AGreat tasteGood taste acceptanceOrganic active ingredientsNervous disorderCross-linkCarboxymethyl cellulose
The invention provides an orally disintegrating tablet of choline glycerophosphate and preparation method thereof, which includes components of aripiprazole, silicified microcrystalline cellulose, cross linked sodium carboxymethyl cellulose, silica, fillers, flavoring agents and lubricating agents, and the aripiprazole is used as active component. On the basis of the formula, the product is prepared by powder direct pressing process. The product has better taste and more excellent long-term stability, so that the effectiveness and safety of the medicine are guaranteed.
Owner:BEIJING VENTUREPHARM BIOTECH
Pregabalin capsule and preparation method thereof
ActiveCN112076176AImprove liquidityQualifiedOrganic active ingredientsNervous disorderPregabalinGelatin
The invention discloses a pregabalin capsule. The pregabalin capsule is composed of pregabalin and silicified microcrystalline cellulose, and in the pregabalin capsule, the weight percentage of the pregabalin is 25-75%. According to the pregabalin capsule and the preparation method thereof, a proper amount of the silicified microcrystalline cellulose is added as an auxiliary material to be mixed with the pregabalin, and the pregabalin and the silicified microcrystalline cellulose with proper particle size distribution are selected, so that the prepared pregabalin capsule is good in flowability, qualified in loading capacity, capable of avoiding the risk of gelatin crosslinking and good in stability; the dissolution behavior is stable and unchanged in a storage process, so that the long-term stability of the pregabalin capsule is ensured; and the method is suitable for industrial production (especially industrialization of 300 mg specification).
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
A kind of silicified microcrystalline cellulose and preparation method thereof
ActiveCN106333928BMeet the requirementsUniform bulk densityPill deliveryPharmaceutical non-active ingredientsCompressibilitySlurry
Owner:HUZHOU ZHANWANG PHARMA
Tablets, formulations and methods for low melting point active ingredients
PendingUS20210393573A1Formed easily and efficientlyLow melting pointHydroxy compound active ingredientsPill deliveryCannabinoidAlcohol sugars
A tablet comprises a granulate of an active pharmaceutical ingredient comprising at least one cannabinoid having a melting point less than about 80° C.; sugar, sugar alcohol, or a combination thereof; microcrystalline cellulose having an average particle size less than about 25 μm; silica, silicified microcrystalline cellulose, or a combination thereof; and lubricant comprising sodium stearyl fumarate and lecithin. Methods of forming such a tablet using direct compression of a tablet formulation can be conducted on a large manufacturing scale.
Owner:KELSIE BIOTECH LLC
Dapagliflozin tablet and preparation method thereof
ActiveCN114028356ALess types of prescriptionLow costOrganic active ingredientsMetabolism disorderDapagliflozin propanediolLactose
The invention provides a dapagliflozin tablet and a preparation method thereof. The dapagliflozin tablet is composed of a tablet core and a coating, wherein the tablet core is composed of the following raw and auxiliary materials in percentage by weight: 2.5-9.8% of dapagliflozin propylene glycol monohydrate; 80.2%-95.2% of an auxiliary material compound; 1.0%-5.0% of a disintegrating agent; 0.5%-2% of a lubricant; 0%-3% of a flow aid; wherein the auxiliary material compound is selected from a lactose microcrystalline cellulose compound or a silicified microcrystalline cellulose compound. The preparation method of the dapagliflozin tablet, provided by the invention, adopts a direct powder compression method, and the dapagliflozin tablet is obtained by uniformly mixing the dapagliflozin propylene glycol monohydrate raw material medicine with a controlled particle size with the auxiliary materials and then directly tabletting and coating. The dapagliflozin tablet provided by the invention is excellent in comprehensive performance, simple in preparation method, low in production cost and easy for industrial production.
Owner:ZHEJIANG HISOAR PHARMA +1
Bifendate oral disintegration tablet and its preparing process
InactiveCN1250217CQuality improvementEasy to takeOrganic active ingredientsDigestive systemDiseaseFiller Excipient
The present invention relates to one kind of orally disintegrated bifendate tablet for treating chronic unresolved hepatitis accompanied by abnormal or chemically elevated ALT and other diseases, and its preparation. The orally disintegrated bifendate tablet is prepared with bifendate as main material, and through adding stuffing, disintegrating agent, corrective, flow assistant and other supplementary material, pressing into tablet and other steps. The present invention has the features of low production cost, fast disintegration, good taste, etc.
Owner:COSCI MED TECH CO LTD
Olmesartan medoxomil amlodipine compound tablet and preparation method thereof
PendingCN114344298AGuaranteed qualified dissolution profileImprove product qualityOrganic active ingredientsDrageesOlmesartanAmlodipine besilate
The invention discloses a compound tablet of olmesartan medoxomil and amlodipine besylate and a preparation method of the compound tablet. The compound tablet is prepared from the following raw materials in parts by mass: 20 to 40 parts of olmesartan medoxomil, 5 to 10 parts of amlodipine besylate, 15 to 25 parts of pregelatinized starch, 50 to 60 parts of silicified microcrystalline cellulose, 5 to 10 parts of croscarmellose sodium, 1 to 2 parts of magnesium stearate and 2 to 5 parts of film coating premix. The preparation method comprises the following steps: 1) controlling the particle size D90 of olmesartan medoxomil to be less than or equal to 40 microns, and controlling the particle size D90 of amlodipine besylate to be less than or equal to 70 microns; (2) mixing olmesartan medoxomil, amlodipine besylate, pregelatinized starch, silicified microcrystalline cellulose and croscarmellose sodium in a mixing machine, then sieving with a 1.5 mm sieve, and continuously mixing; (3) preparing particles from the premixed materials by adopting a dry granulation method; 4) adding magnesium stearate and mixing; (5) tabletting; and (6) coating.
Owner:北京阳光诺和药物研究股份有限公司
A Blumea balsamifera tablet, and a preparation method thereof
InactiveCN109200075AReduce daily dosagePromote dissolutionPharmaceutical non-active ingredientsPill deliveryColloidal silicaFiber
A Blumea balsamifera tablet is disclosed and is prepared from, by weight, 20-40 parts of microcrystalline fiber, 3-5 parts of mannitol, 2-4 parts of titanium dioxide, 1-3 parts of sodium carboxymethylstarch, 2-4 parts of colloidal silica and 0.8-1 part of glycerol distearate. The tablet can reduce the daily dosage of the patient under the same crude drug content through the proportion of the Blumea balsamifera. Silicified microcrystalline cellulose, mannitol, and other adjuvants are reasonably prepared to give full play to the synergistic effect of each component. The silicified microcrystalline cellulose in the tablet and the added disintegrating agent carboxymethyl starch sodium can rapidly disintegrate the tablet, greatly improving the dissolution and absorption of the active ingredient, and improving the bioavailability of the drug. The preparation method is simple, the process is short and the production is easy.
Owner:贵州省罗甸县全兴药业开发有限责任公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com