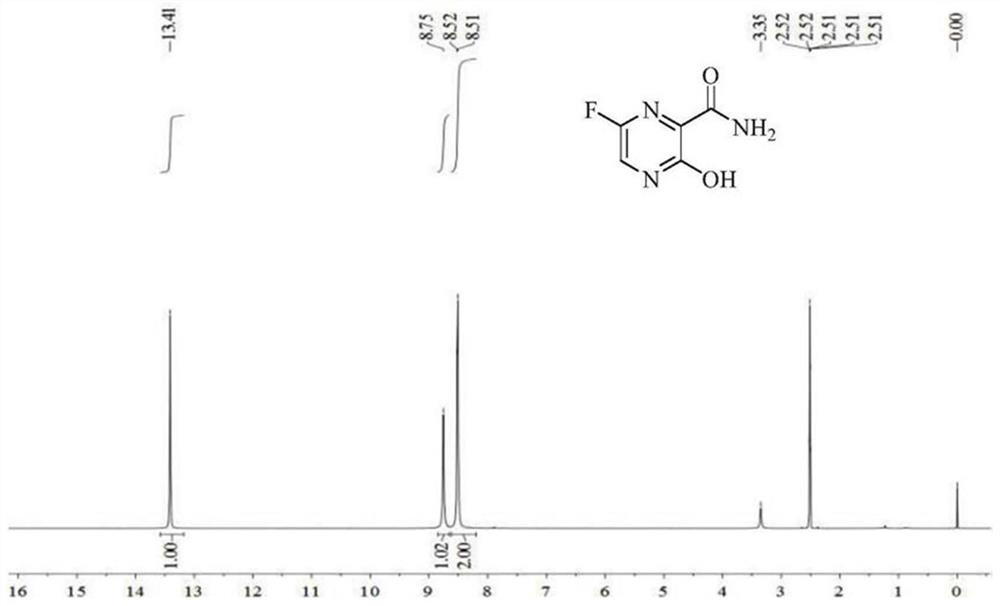
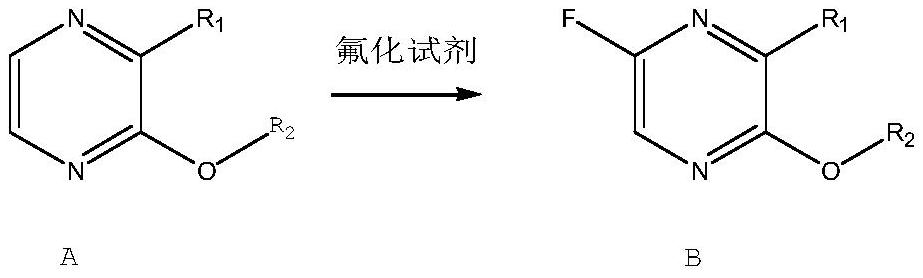
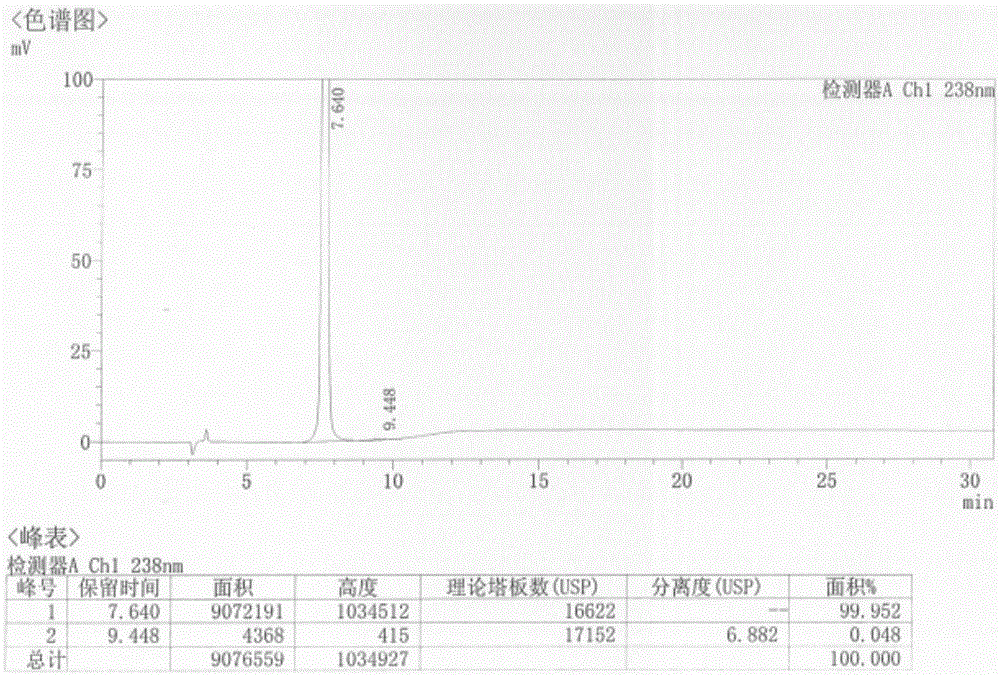
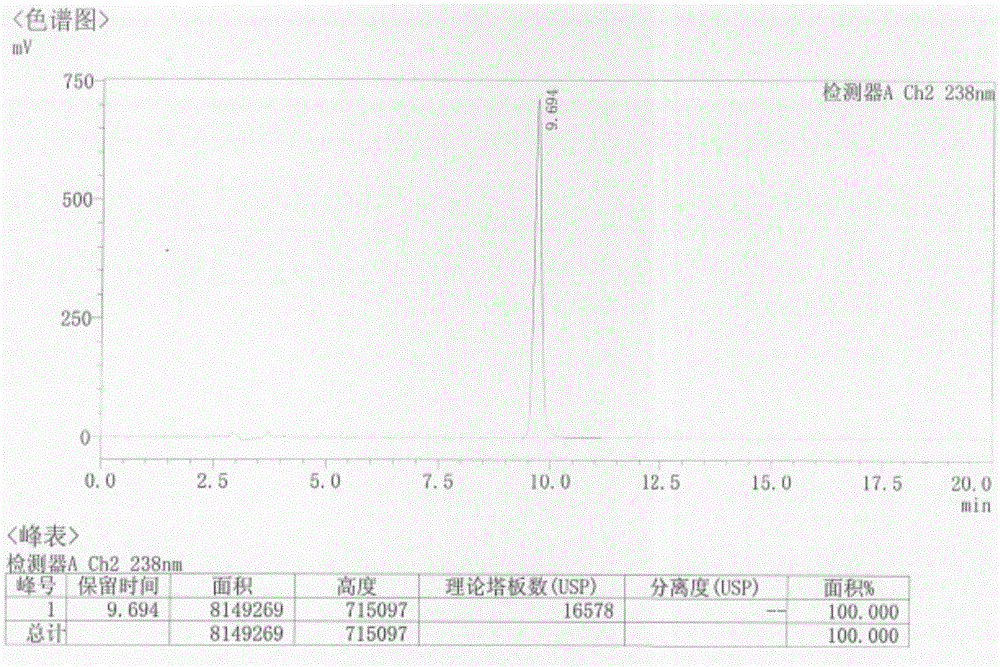
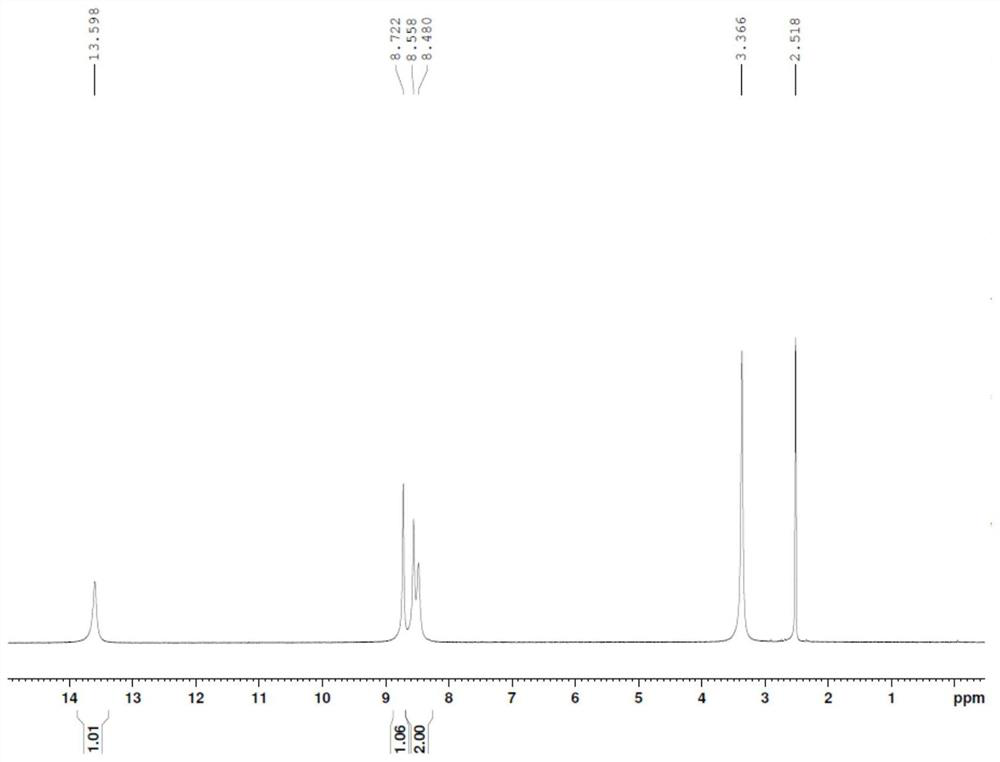
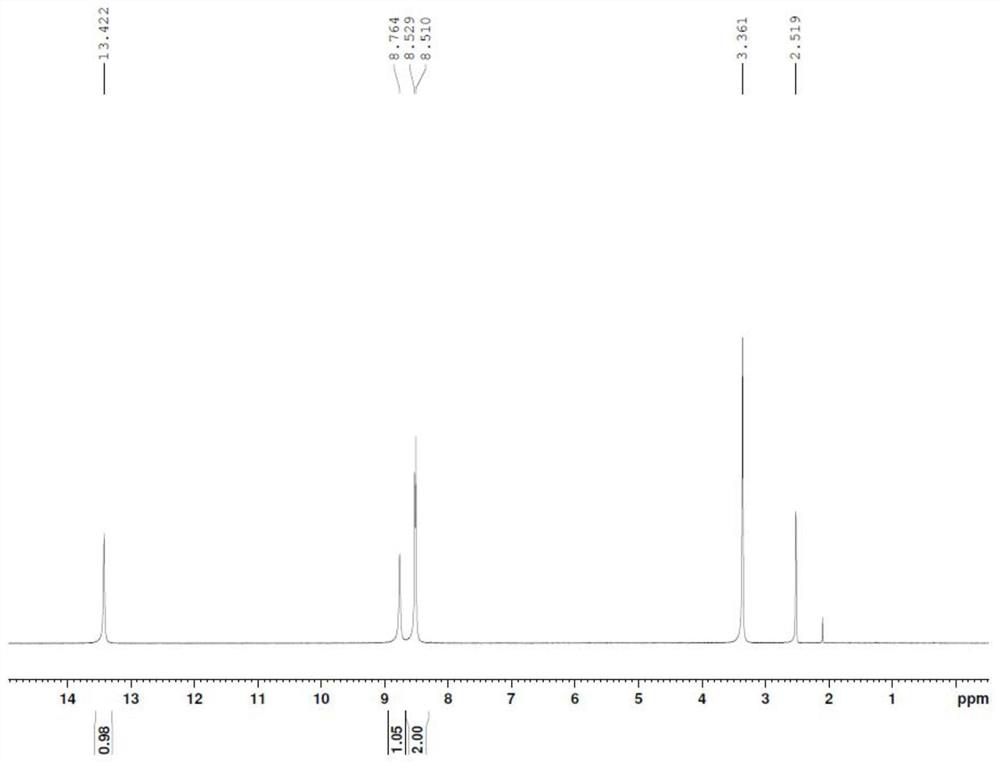
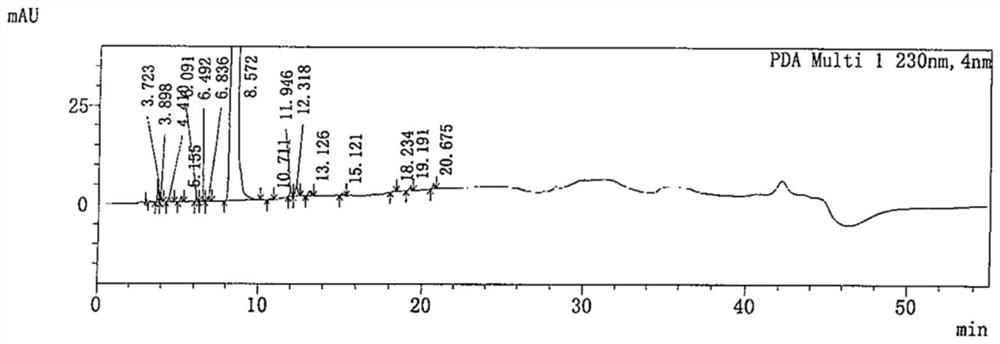
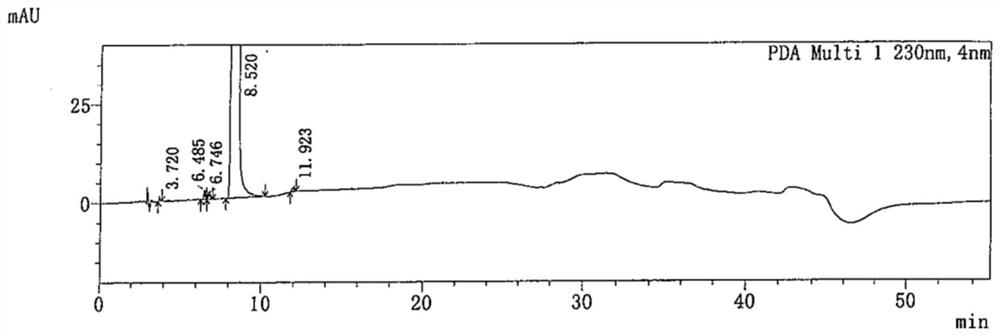
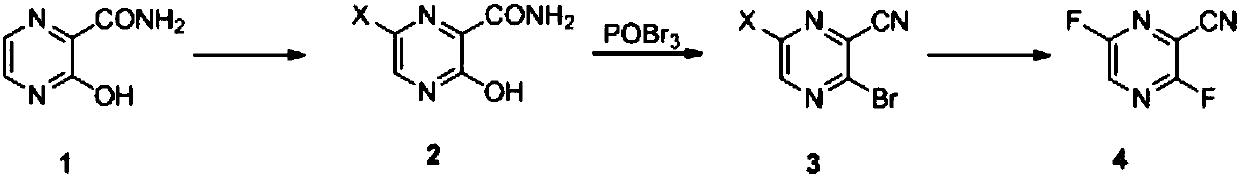Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76 results about "Favipiravir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
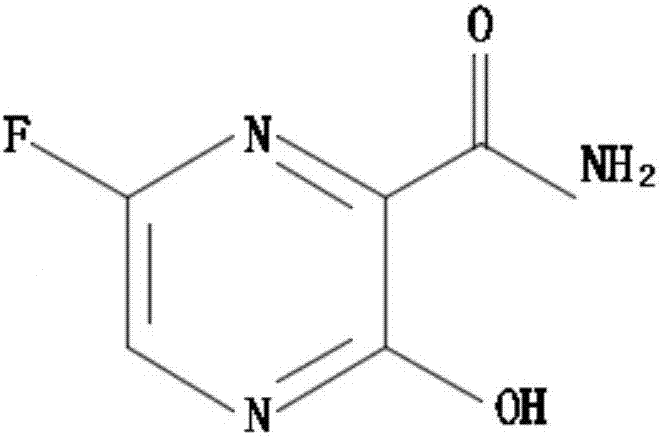
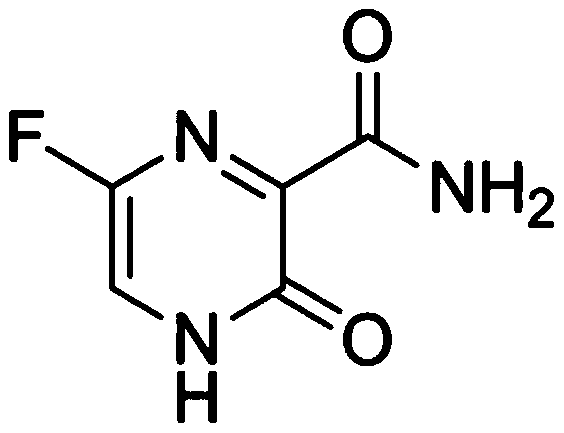
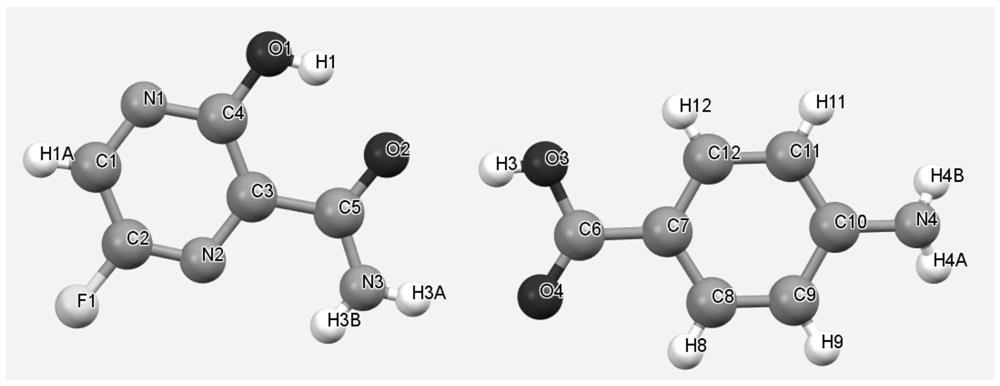
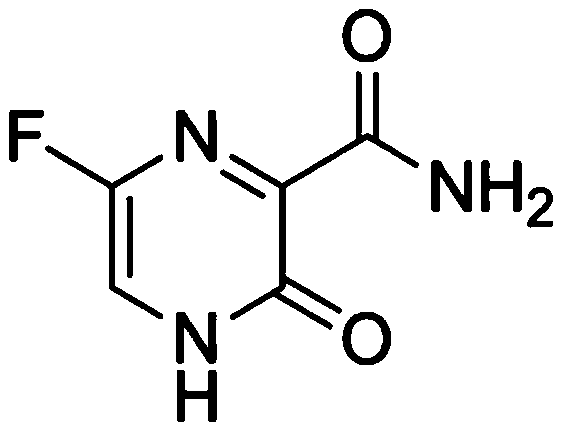
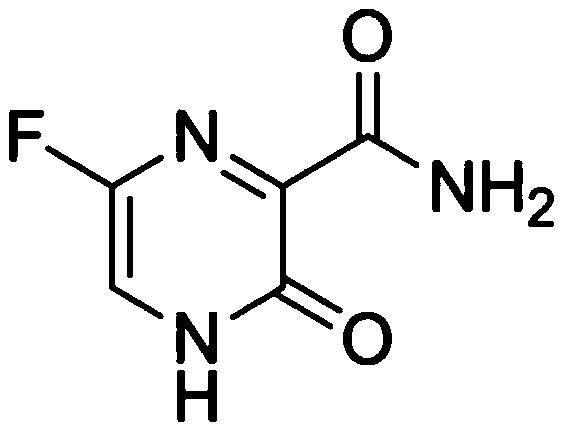
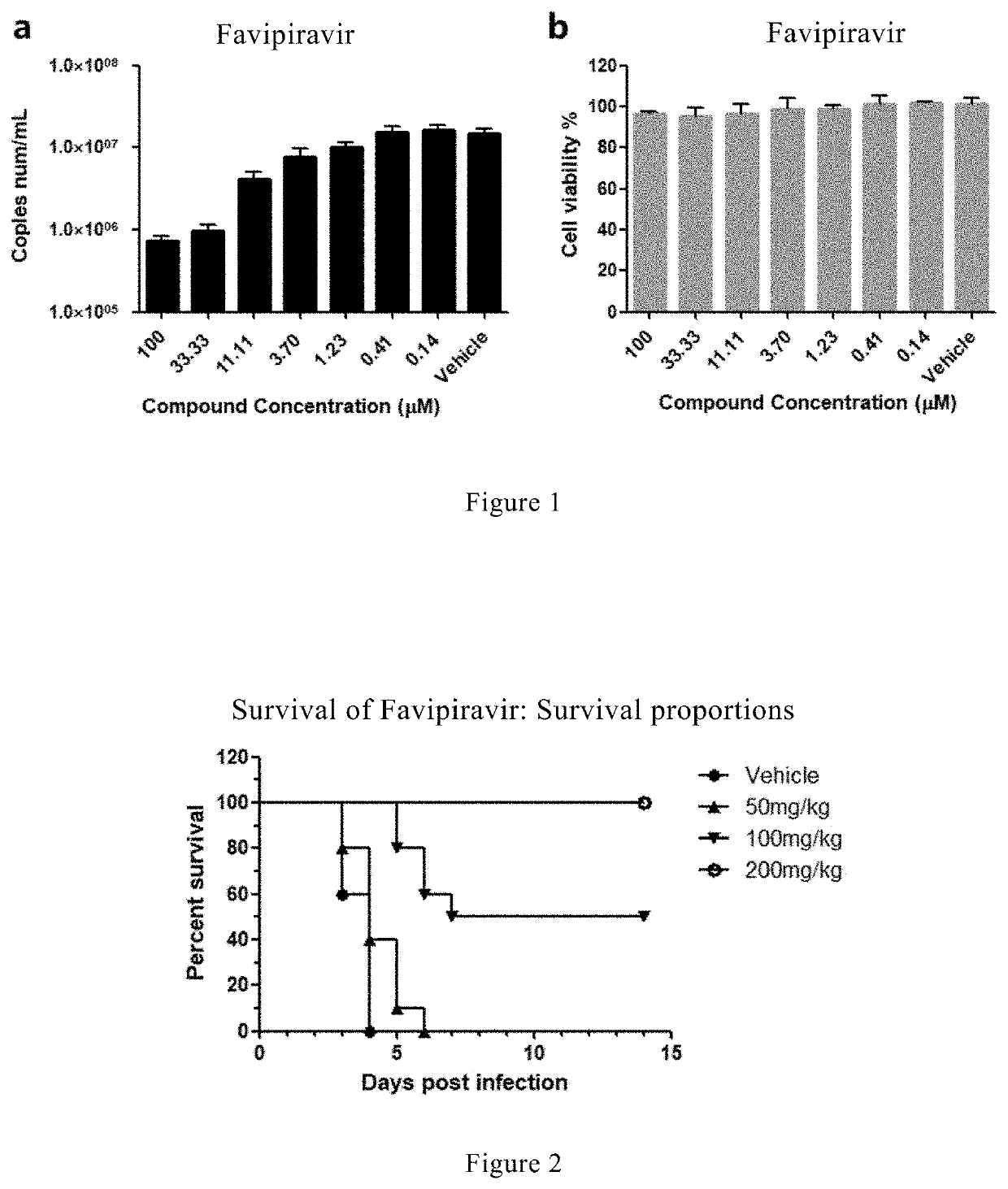
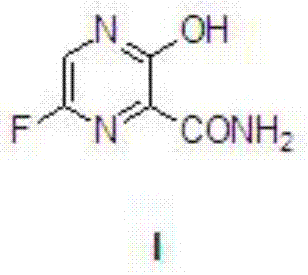
Favipiravir, also known as T-705 or Avigan, is an experimental antiviral drug being developed by Toyama Chemical of Japan with activity against many RNA viruses. Like some other experimental antiviral drugs (T-1105 and T-1106), it is a pyrazinecarboxamide derivative. Favipiravir is active against influenza viruses, West Nile virus, yellow fever virus, foot-and-mouth disease virus as well as other flaviviruses, arenaviruses, bunyaviruses and alphaviruses. Activity against enteroviruses and Rift Valley fever virus has also been demonstrated. Favipiravir showed limited efficacy against Zika virus in animal studies, but was less effective than other antivirals such as MK-608. The agent has also shown some efficacy against rabies, and has been used experimentally in some humans infected with the virus.
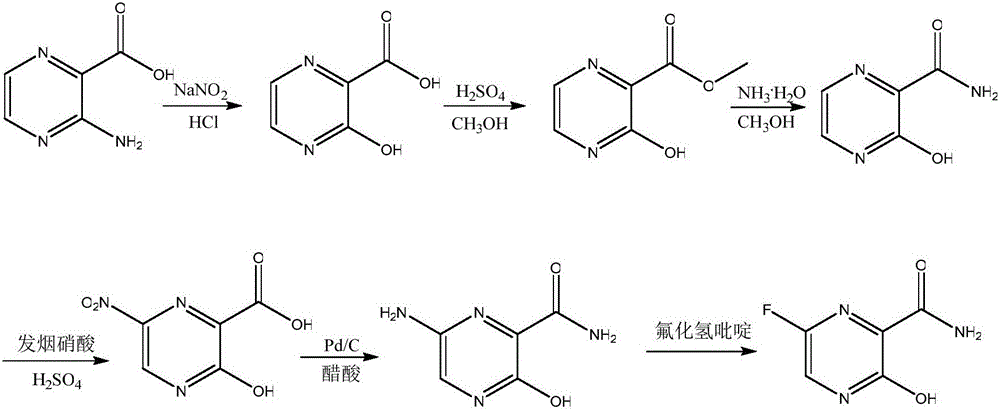
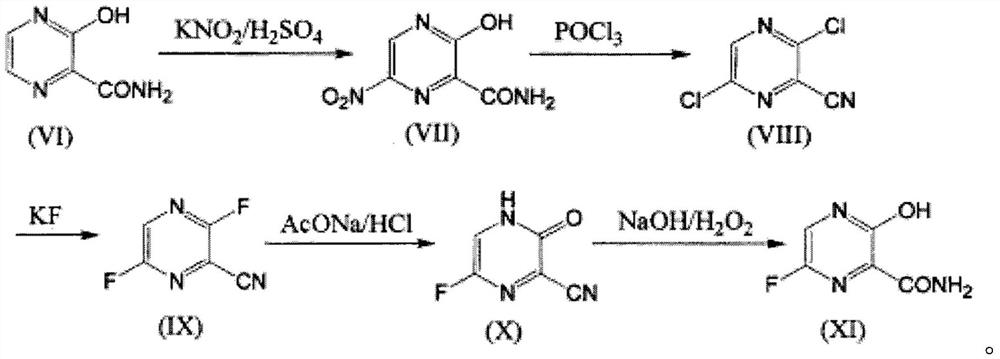
Synthesis method of Favipiravir
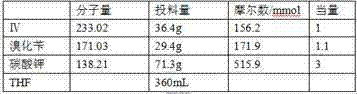
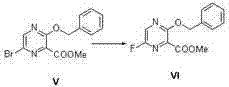
ActiveCN104496917ALow impurity contentShort reaction cycleOrganic chemistryPotassium fluorideSynthesis methods
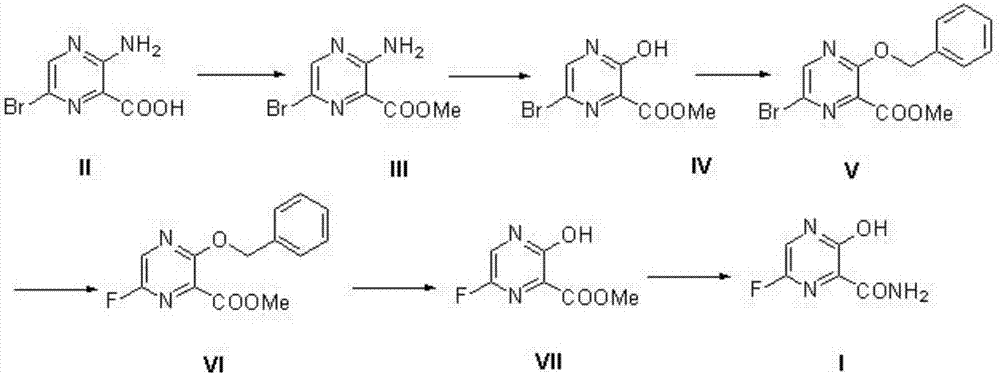
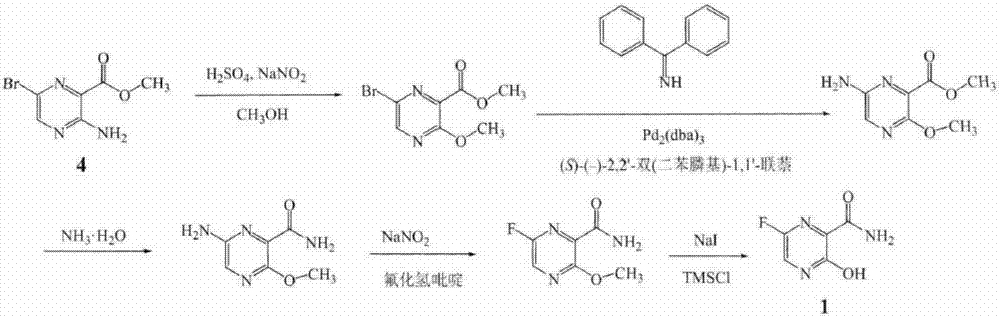
The invention belongs to the field of medicinal chemistry and particularly relates to a new synthesis method of Favipiravir. The method comprises the following steps: carrying out carboxyl protection on raw material shown in the formula (II) to generate a compound (III); carrying out diazotization hydrolysis reaction in the presence of concentrated sulfuric acid and sodium nitriteto generate a compound (IV); carrying out benzyl protection reaction to generate a compound (V), and then generating a compound (VI) in the presence of potassium fluoride and tetrabutylammonium bromide; removing a benzyl protection group to generate a compound (VII); and then adding an aminating agent to carry out amination to generate Favipiravir shown in the formula I. The method disclosed by the invention has the advantages that the reaction cycle is short, the operation is simple, the production cost is low, and the product is high in quality; therefore, the method is suitable for industrial production.
Owner:NANJING HUAWE MEDICINE TECH DEV
Favipiravir synthesis process
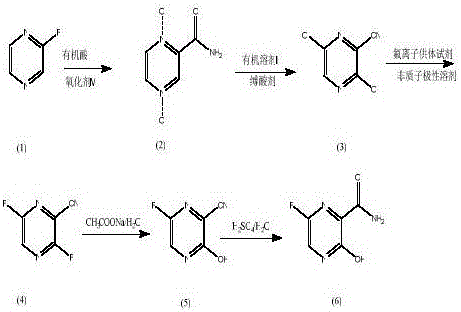
InactiveCN106478528AThe synthesis process is simpleRaw materials are easy to obtainOrganic chemistrySodium acetatePyrazine
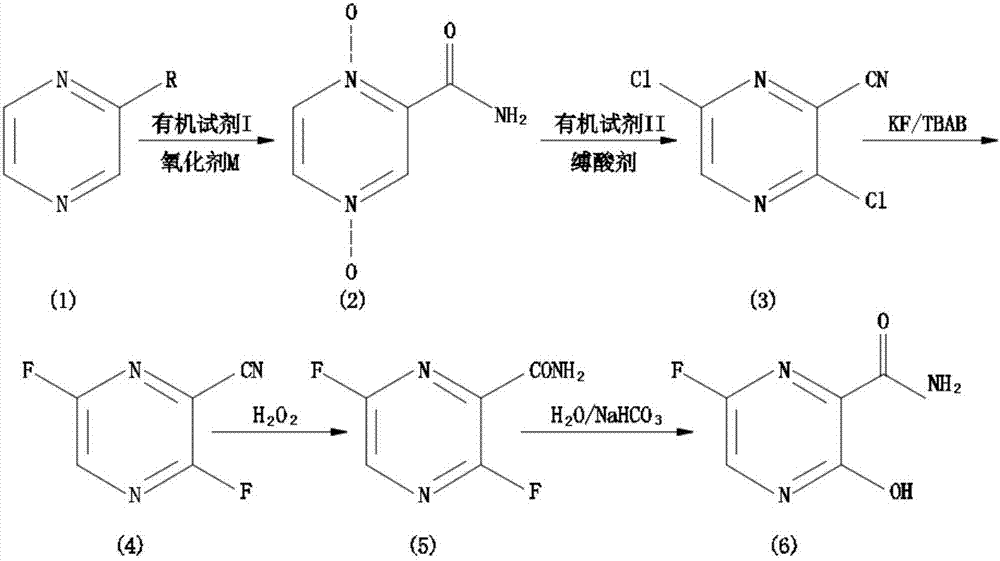
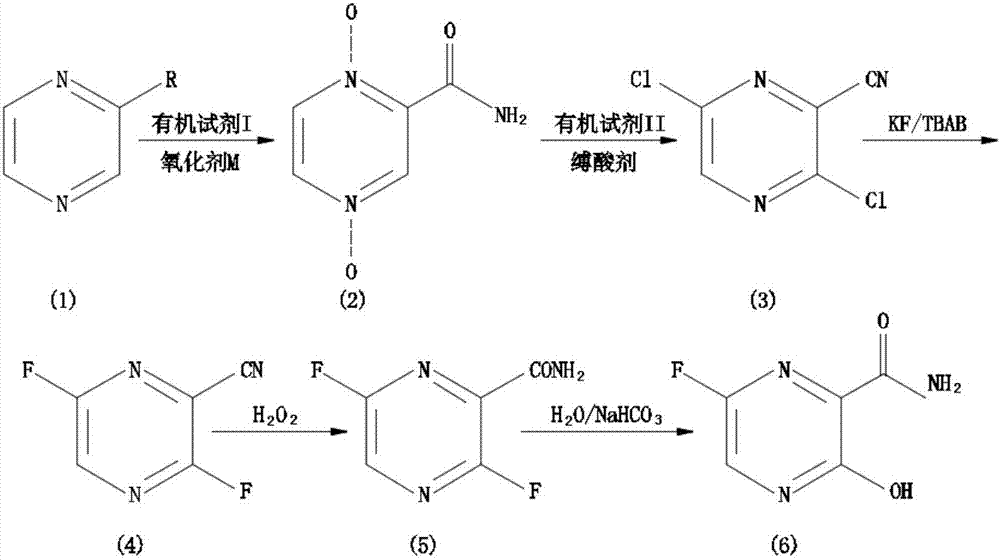
The invention relates to a favipiravir synthesis process, which comprises: 1) dissolving a pyrazine compound in an organic reagent I, adding an oxidizing agent M, and carrying out a nitrogen oxidation reaction to obtain a white solid; 2) adding the obtained white solid to an organic reagent II, and carrying out a chlorination reaction to obtain a pale yellow solid; 3) uniformly mixing the obtained pale yellow solid, a dried aprotic polar solvent, a dried fluorine ion donor reagent and tetrabutylammonium bromide, and carrying out a stirring reaction to obtain a pale yellow solid; 4) adding the obtained pale yellow solid to water, and carrying out a reaction with 1,4-dioxane and sodium acetate to obtain a yellow oily matter; and 5) uniformly mixing the obtained yellow oily matter and concentrated sulfuric acid to obtain the target product favipiravir. According to the present invention, the method has advantages of simple and easily available raw materials, simple synthesis process and mild conditions, nitrogen oxidation, chlorination, fluorization and hydrolysis are performed to finally prepare the 6-fluoro-3-hydroxypyrazine-2-formamide, and the good industrial value is provided.
Owner:WUHAN INSTITUTE OF TECHNOLOGY +1
Favipiravir intermediate and synthetic method for favipiravir
The invention relates to a favipiravir intermediate and a synthetic method for favipiravir, and specifically discloses a method for synthesizing 3,6-difluoro-2-cyanopyrazine with a formula 4 as shownin the specification. The method comprises the following steps: a) allowing a compound with a formula 1 as shown in the specification to react with a chlorinated reagent or a brominated reagent so asto obtain a compound with a formula 2 as shown in the specification; b) allowing the compound with the formula 2 as shown in the specification to react with phosphorus oxychloride in the presence of organic base so as to obtain a compound with a formula 3 as shown in the specification; and c) allowing the compound with the formula 3 as shown in the specification to react with a fluorinated reagentso as to obtain the compound with the formula 4 as shown in the specification. The invention also relates to a method for synthesizing the favipiravir by using the above-mentioned method.
Owner:富乐马鸿凯(大连)医药有限公司
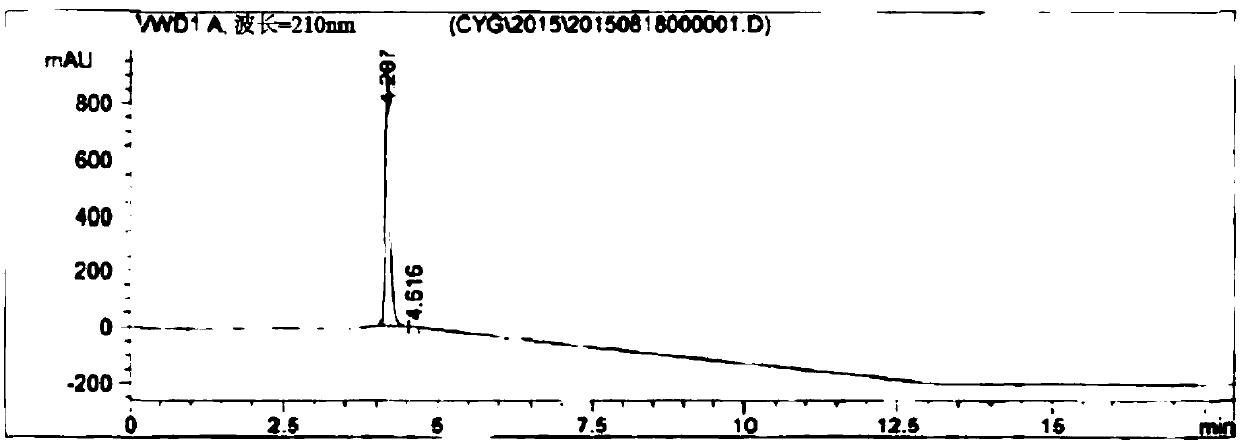
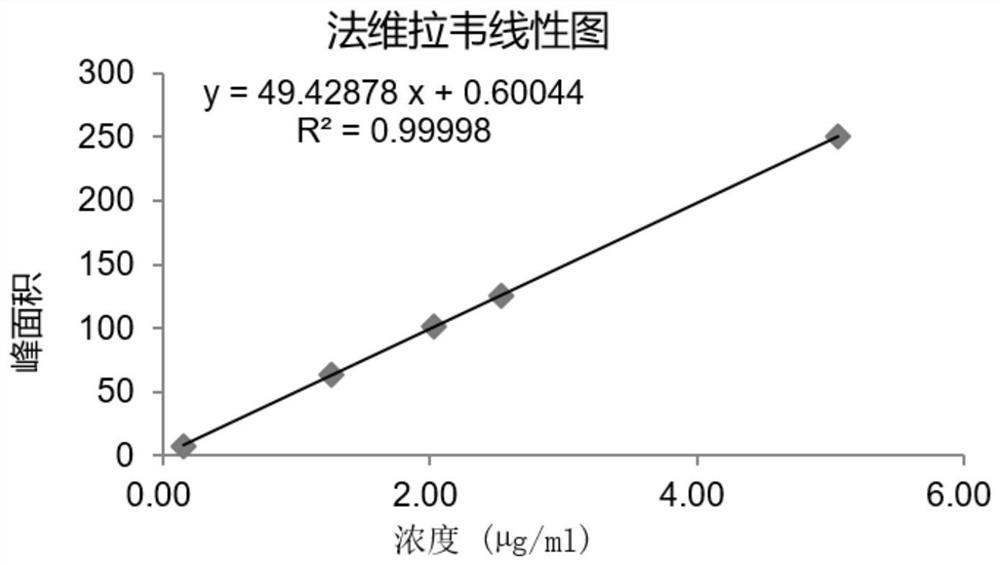
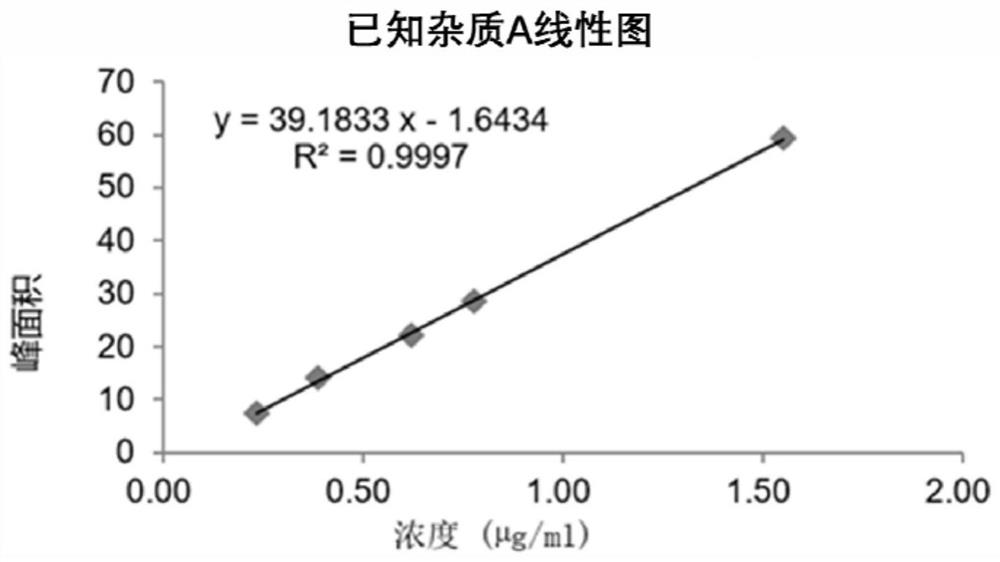
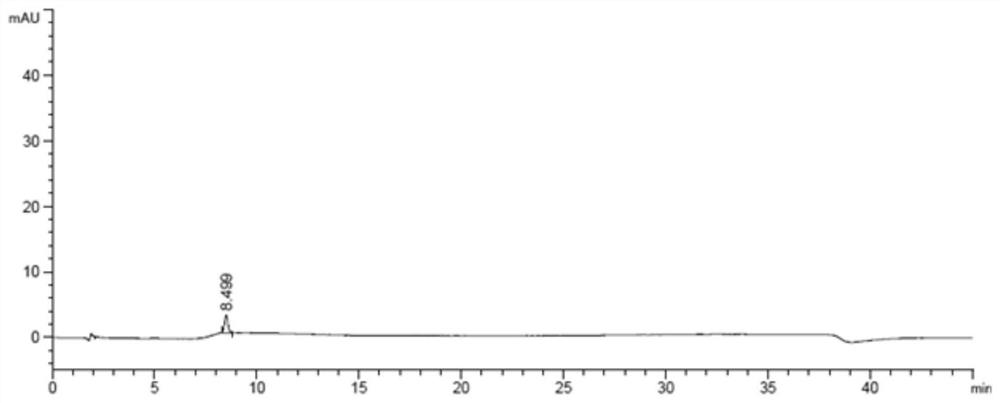
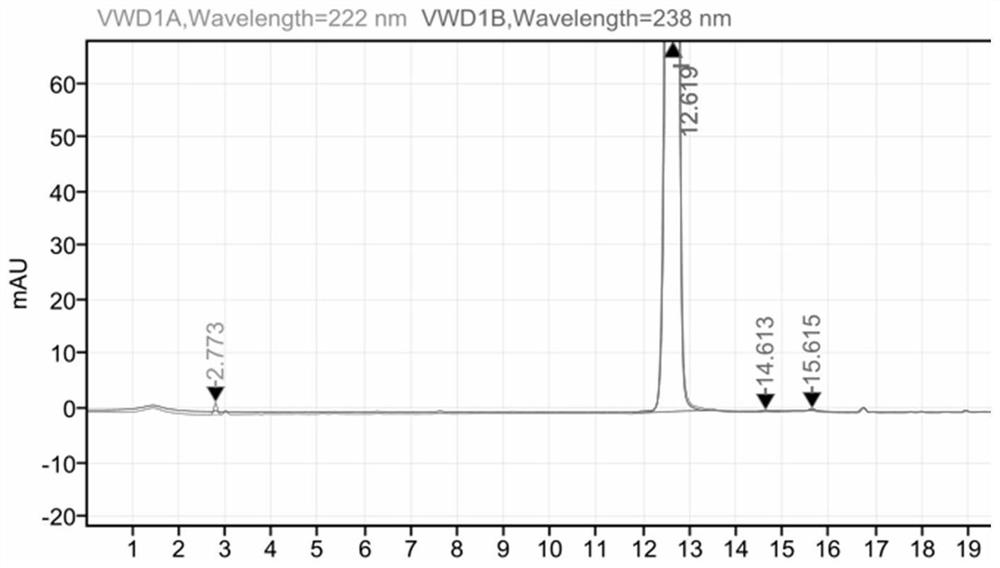
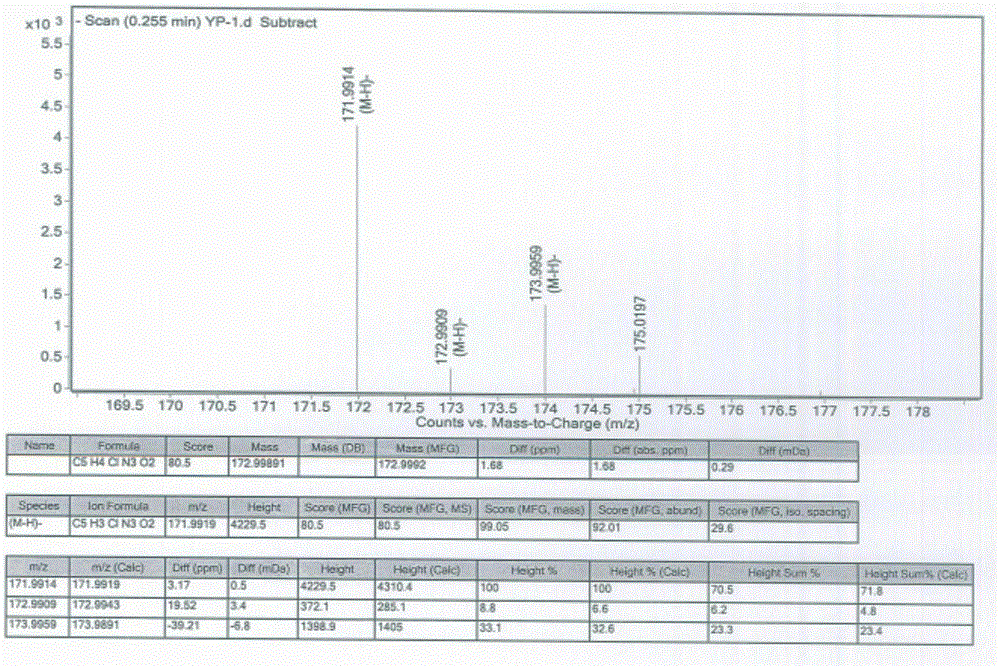
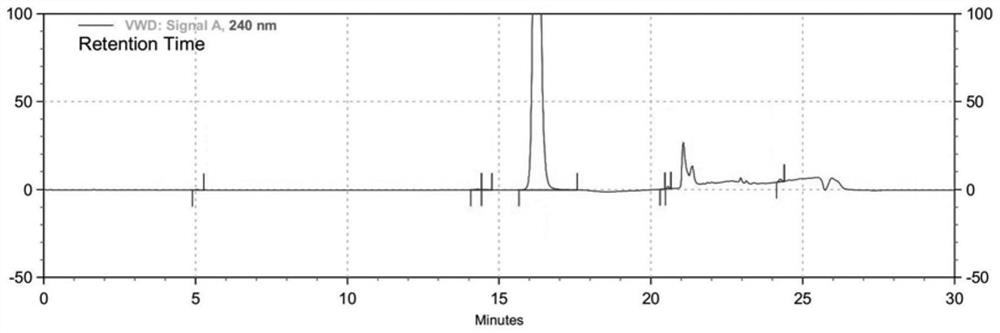
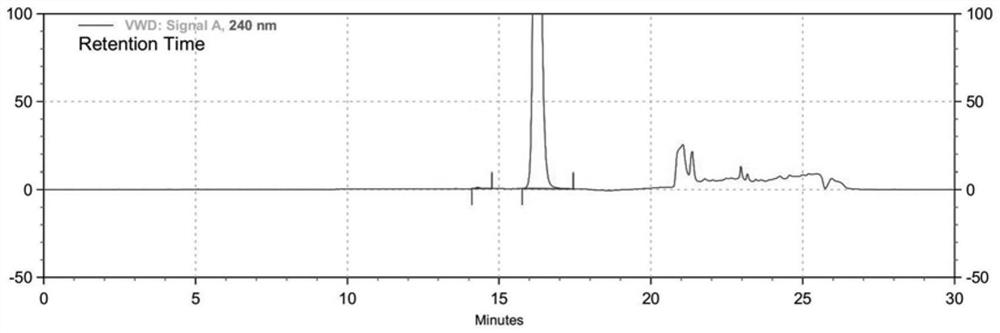
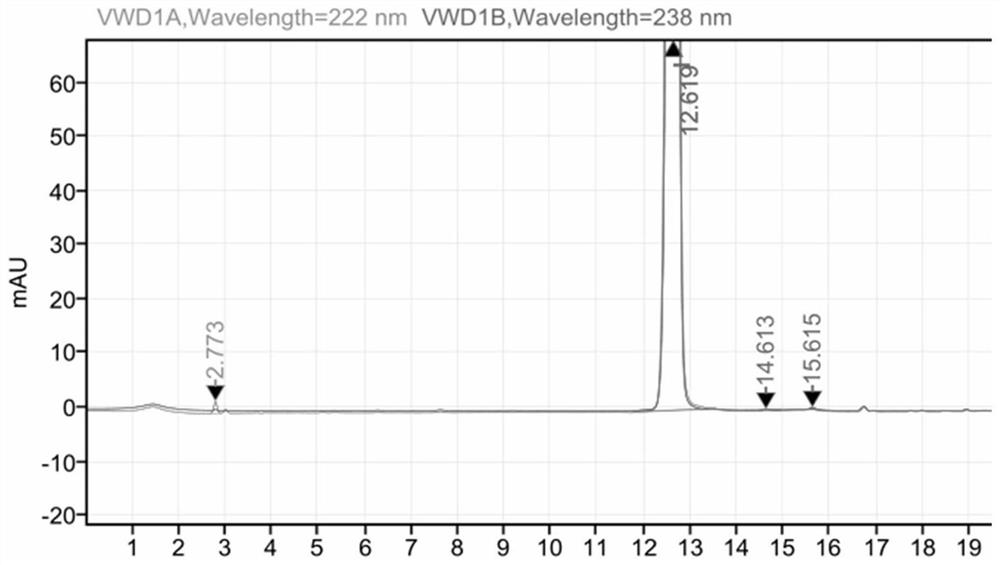
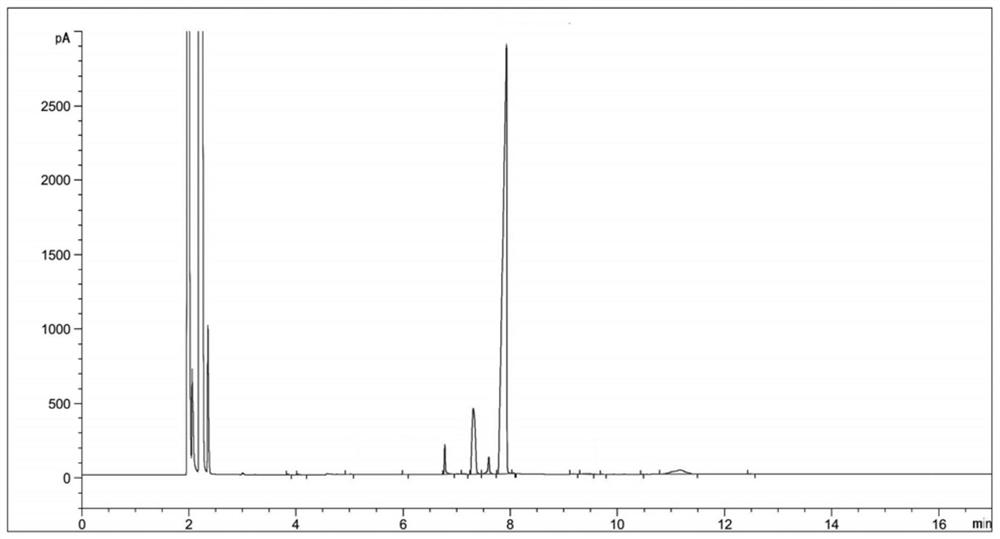
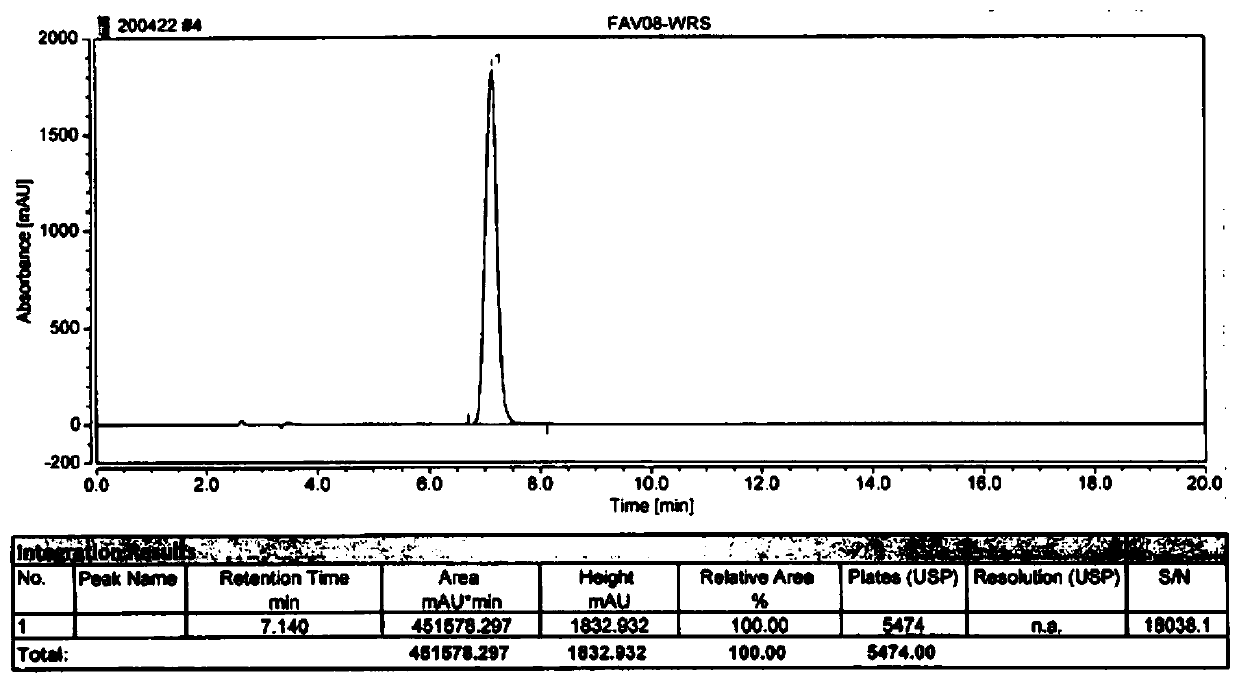
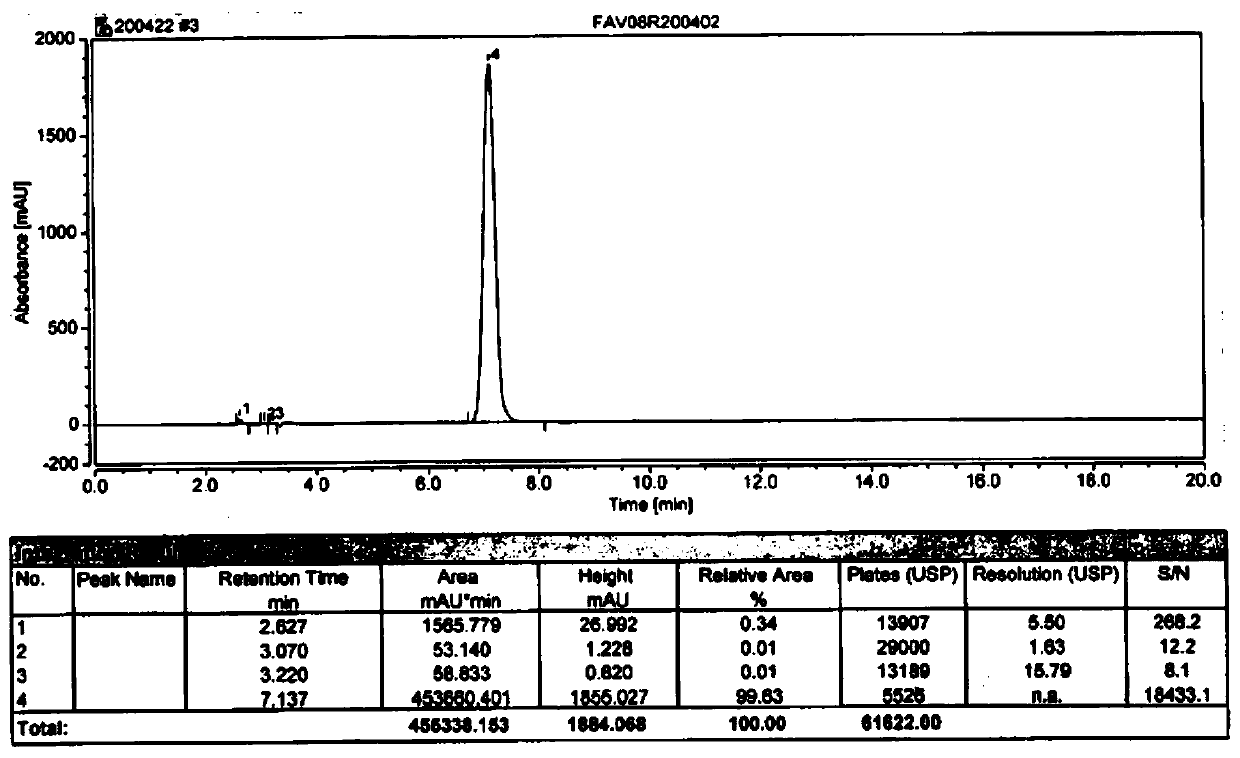
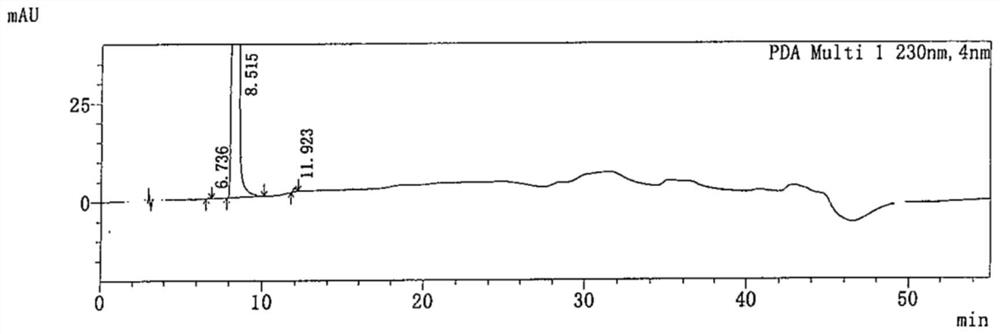
HPLC method for measuring related substances in Favipiravir
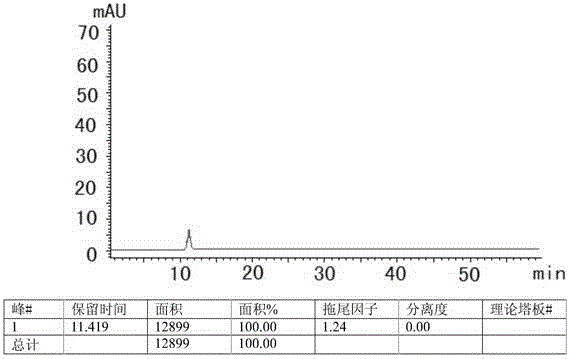
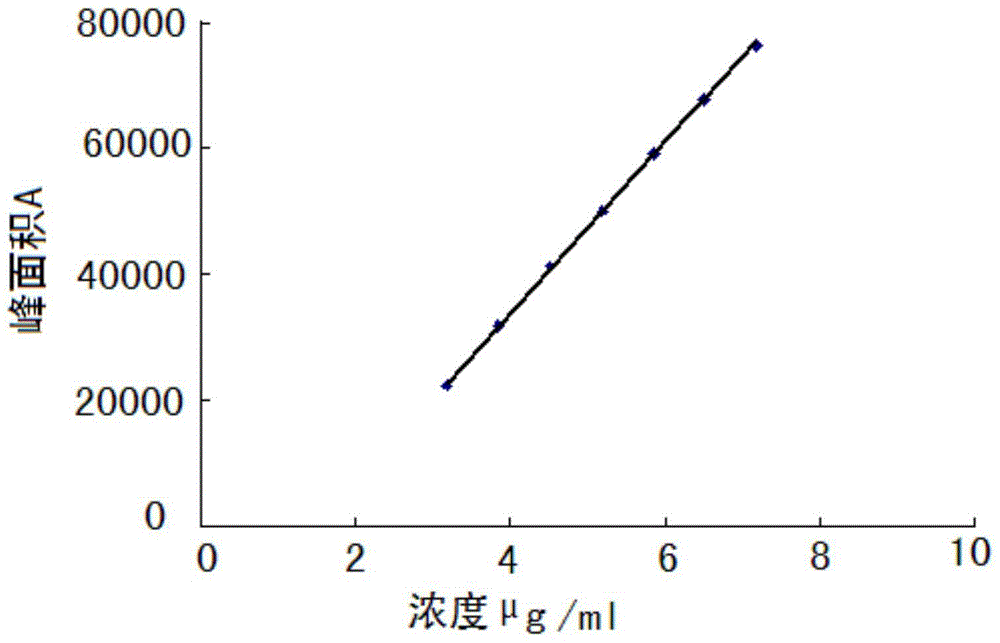
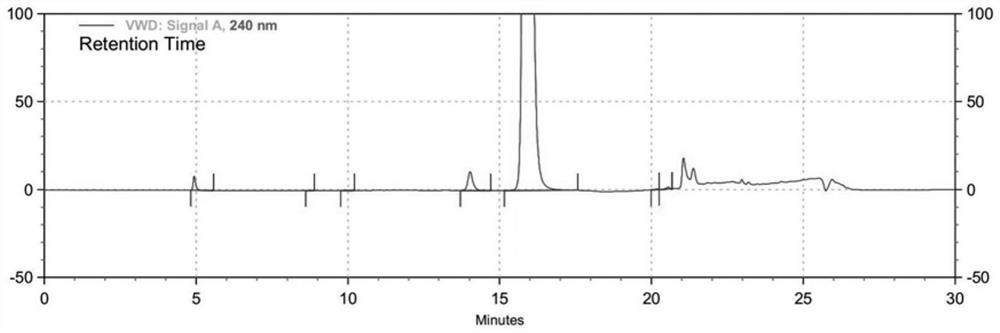
The invention discloses an HPLC method for measuring related substances in Favipiravir. According to the HPLC method for measuring related substances in Favipiravir, disclosed by the invention, specifically, a diode array detector is adopted, and acetonitrile (mobile phase A)-phosphate solution (mobile phase B) serves as a mobile phase. The method comprises the following steps: taking a proper amount of Favipiravir and related preparations containing Favipiravir, adding the substances into the mobile phase for preparing a solution of which every 1ml contains 0.2mg of Favipiravir, and taking the solution as a test solution; diluting into a solution of which every 1ml contains about 0.2mu g of Favipiravir by using the mobile phase, and taking the solution as a contrast solution; respectively performing sample introduction, wherein the sum of each impurity peak area in the chromatogram of the test solution is not more than the main peak area of the contrast solution. According to the method for detecting the related substances in Favipiravir and related preparations containing Favipiravir, disclosed by the invention, the conditions of the impurities and degradation products of Favipiravir can be rapidly and accurately detected. The operation is simple and convenient, the sensitivity is high, and the product quality can be well controlled.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
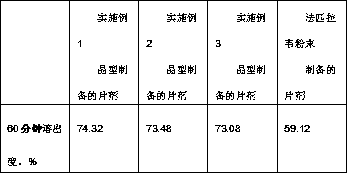
Favipiravir tablets and preparation method thereof
ActiveCN106667926APrescription costs are lowReduce sizeOrganic active ingredientsAntiviralsMedicineAdhesive
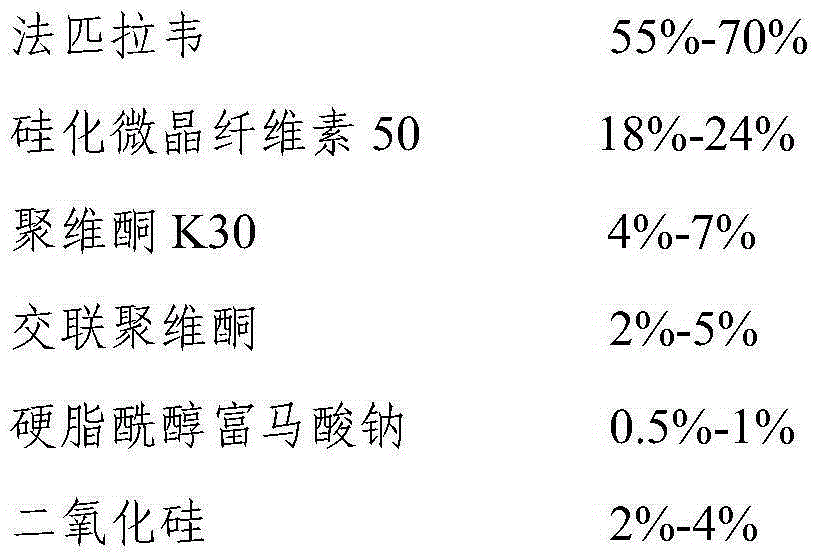
The invention relates to favipiravir tablets. The tablets comprise favipiravir, silicified microcrystalline cellulose and adhesive; the silicified microcrystalline cellulose is lower in price, proper in size and high in medicine compliance when being compared with low-substituted hydroxypropyl cellulose; the defective rate is lower and the dissolution rate is higher. The invention further relates to a preparation method of the tablets. The preparation method is simple in preparation process and suitable for industrialized large-scale production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Favipiravir tablet composition
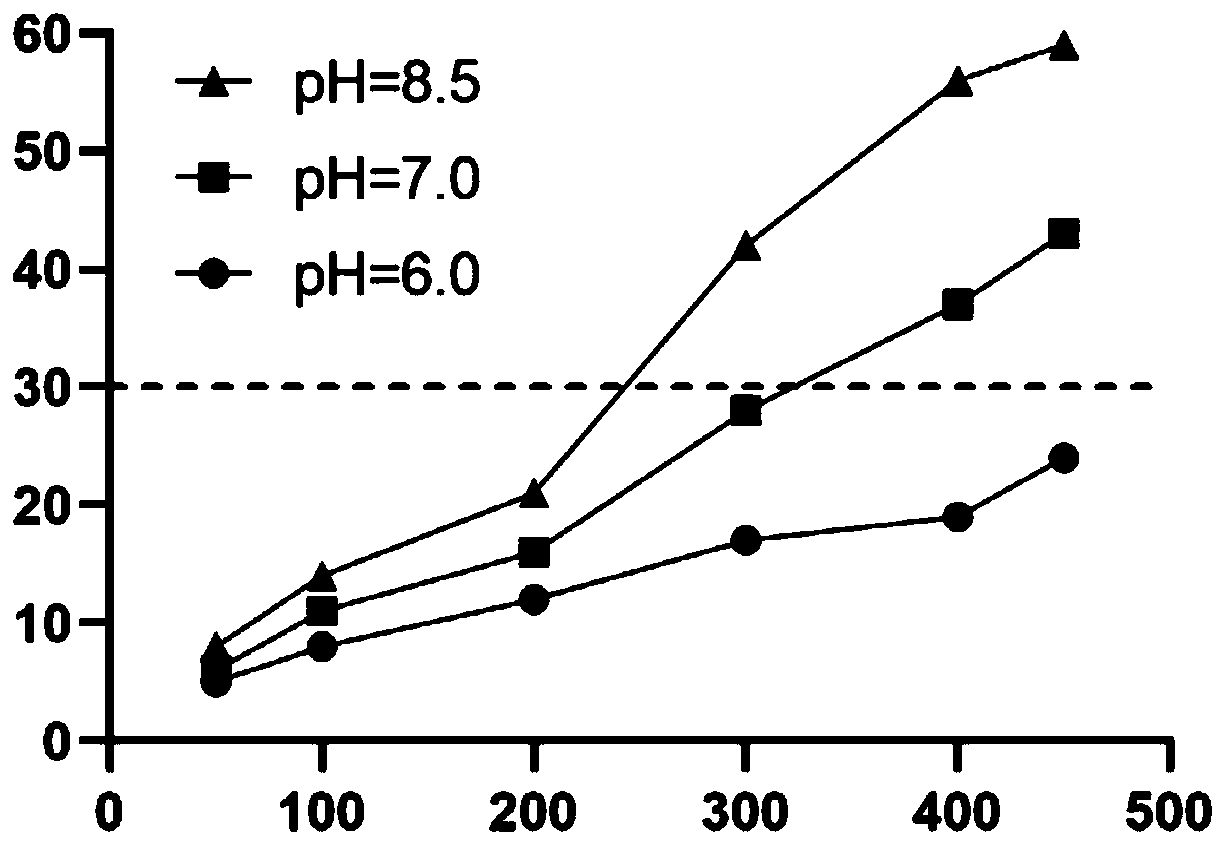
The invention relates to a favipiravir tablet composition, which belongs to the technical field of pharmaceutical preparations. The technical scheme of the present invention is: a kind of favipiravir tablet composition, in the composition of unit dose, containing D90 is less than 40 microns, and D50 is 200mg of favipiravir at 26-33 microns, microcrystalline cellulose 24‑45mg, lactose 10‑18mg, macrogol 6000 8‑16mg, sodium lauryl sulfate 0.8‑1.3mg, meteorological silica 2‑5mg, magnesium stearate 1‑2.5mg. The invention provides a qualified favipiravir tablet, which solves the defects that the tablet has pits and the dissolution rate decreases as the storage time prolongs.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Favipiravir synthesis method
InactiveCN107226794AControl impurity contentSignificant yield controlOrganic chemistryPyrazineSynthesis methods
The invention discloses a favipiravir synthesis method, which comprises the following steps that (1) a pyrazine compound is dissolved in an organic reagent I; nitrogen oxidation reaction is performed to obtain white solid matters; (2) the white solid matters of pyrazine dinitrogen oxides are added into an organic reagent II to perform chlorination reaction; light yellow solids are obtained; (3) the obtained light yellow solids, a dry aprotic polar solvent, a dry fluorion donor reagent and tetrabutylammonium bromide are uniformly mixed; stirring reaction is performed to obtain light yellow solids; (4) the prepared 3,6-dichloropyrazine-2-formonitrile and a fluorizating agent perform aromatic ring fluoronation; (5) hexafluoro reaction products are directly catalyzed through hydrogen peroxide; cyan-hydrolysis reaction is performed; (6) cyan-hydrolysis reaction products are directly catalyzed through an aqueous alkaline solution; aromatic ring hydroxyl substitution reaction is performed; then, through purification treatment, favipiravir is prepared. The method disclosed by the invention has the advantages that the raw materials are simple and are easily to obtain; the synthesis process is simple; good industrial value is realized; green and environment-friendly effects are achieved.
Owner:ZHENGZHOU UNIV
Fapiravir and preparation method of intermediate thereof
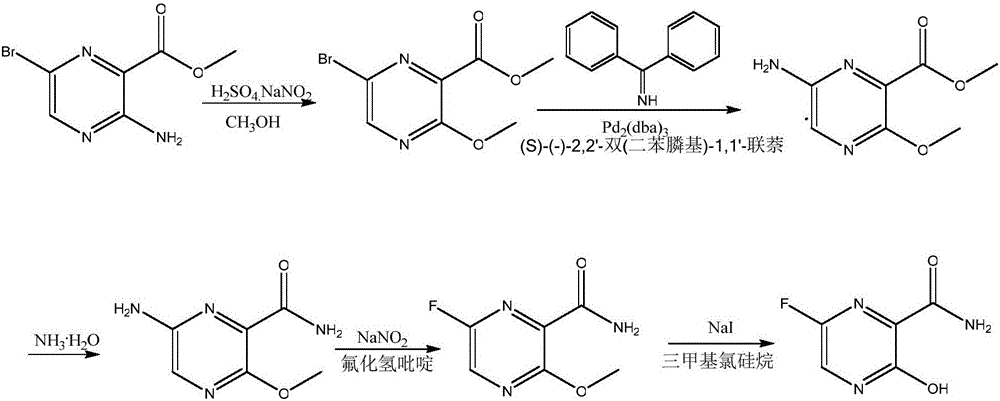
ActiveCN111978263ALow priceShort reaction stepsOrganic chemistryBulk chemical productionLithium chloridePyrazine
The invention relates to a fapiravir and a preparation method of an intermediate thereof, and belongs to the field of pharmaceutical chemicals. The invention provides a preparation method of a fapiravir intermediate 3-hydroxy sodium pyrazine-2-formamide, wherein the fapiravir intermediate 3-hydroxy sodium pyrazine-2-formamide is prepared by reacting aminopropanedioide with lithium chloride in thepresence of a NaOH solution and glyoxal. The invention also provides a preparation method of 6-bromo-3-hydroxy pyrazine-2-formamide, wherein the 6-bromo-3-hydroxy pyrazine-2-formamide is prepared froman acetonitrile solution of 3-hydroxy sodium pyrazine-2-formamide and an acetonitrile solution of liquid bromine in a microchannel reactor. The invention also provides a preparation method of 3, 6-difluoropyrazine-2-formamide, wherein the 3, 6-difluoropyrazine-2-formamide is prepared by reacting 6-bromo-3-hydroxy pyrazine-2-formamide with potassium bifluoride in the presence of PEG-400 and DMF. Finally, the invention also provides a total synthesis method for preparing fapiravir from the intermediate. The reactions avoid the use of highly dangerous diazotization reactions, and the methods have the advantages of high safety, low raw material price, short steps, low cost and simple post-treatment, and are suitable for industrial enlarged production.
Owner:长沙创新药物工业技术研究院有限公司
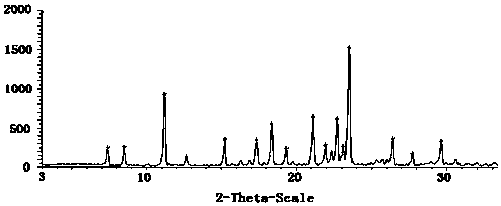
Novel favipiravir crystal form
The invention relates to a new crystal form of favipiravir, which belongs to the technical field of preparation of raw materials. The technical solution of the present invention is: a new crystal form of favipiravir. In the powder X-ray diffraction of the crystal form, the 2θ° is 7.4, 8.5, 11.1, 15.4, 17.8, 18.5, 19.2, 21.0, 21.8, 22.6, There are strong absorption peaks near 23.5 and 26.4. The technical solution of the present invention provides a favipiravir crystal form with good solubility, which plays a positive role in the improvement of the favipiravir preparation.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Stable favipiravir injection and preparation method thereof
ActiveCN111249229AGood feature propertiesSteady injectionOrganic active ingredientsPharmaceutical delivery mechanismCyclodextrinBiomedical engineering
The invention relates to a stable favipiravir injection and a preparation method thereof. The stable favipiravir injection comprises favipiravir, a solubilizer sulfobutyl ether-beta-cyclodextrin sodium and an acid-base regulator. The stable favipiravir injection can endure terminal sterilization and has good safety and stability.
Owner:FUKANGREN BIO PHARMA
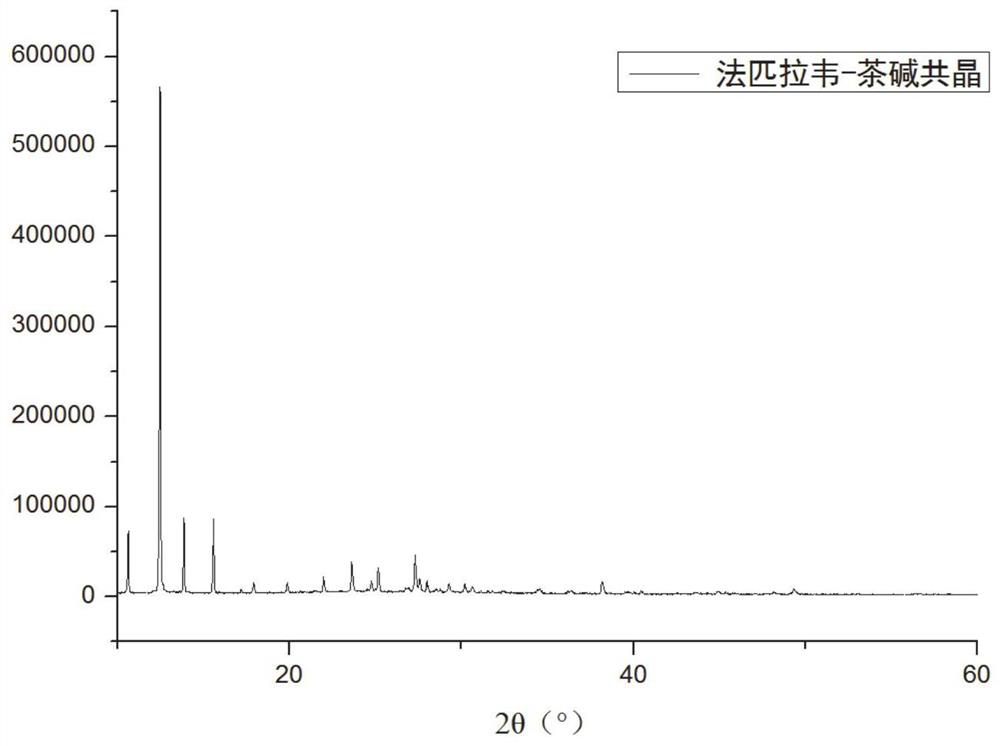
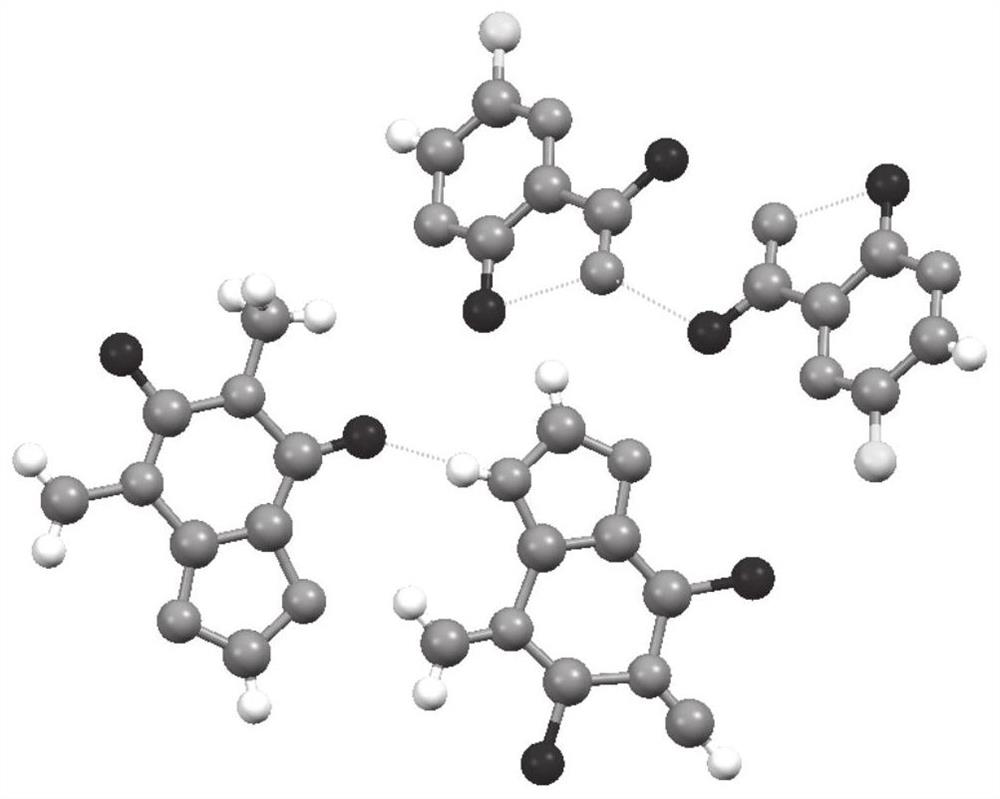
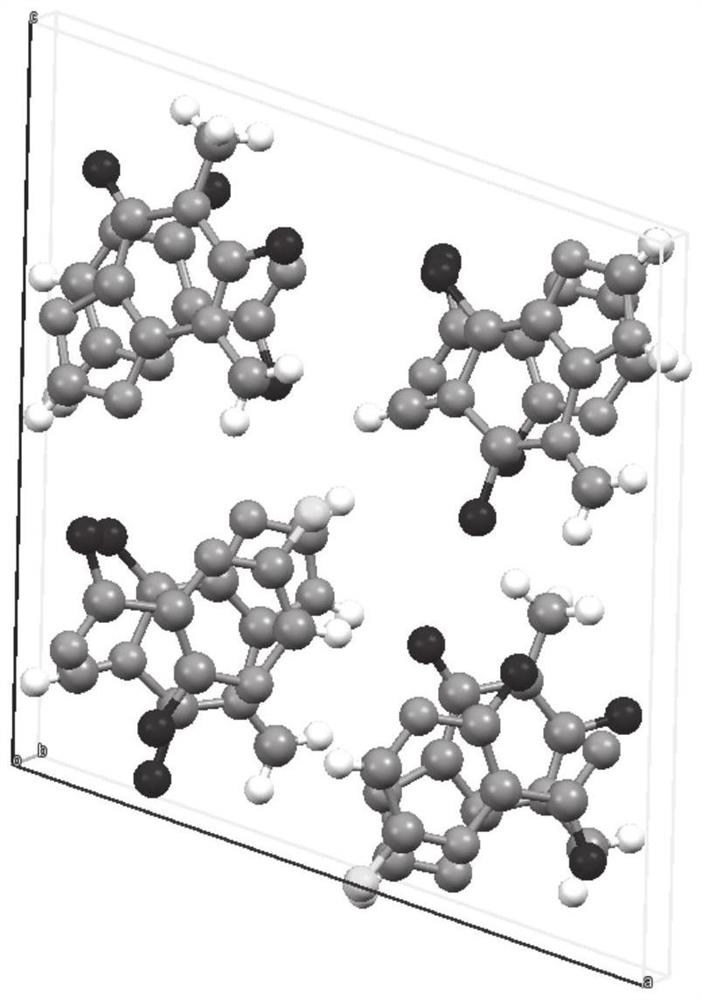
Compound crystal, preparation method and application
ActiveCN112624985AEquivalent Solubility DecreasedReduce dissolution rateOrganic active ingredientsOrganic compound preparationBenzoic acidPhysical chemistry
The invention discloses a compound crystal, and a preparation method and application thereof, belonging to the technical field of chemical medicines. The compound crystal is a eutectic crystal of favipiravir and p-aminobenzoic acid, the unit cell of the eutectic crystal belongs to a monoclinic system and a P21 / c space group, and the unit cell parameters are as follows: alpha = gamma = 90 degrees and beta = 92.578 (3) degrees. The equivalent solubility of the favipiravir in the compound crystal provided by the invention is reduced by about 50%, so that the dissolution rate of the favipiravir is favorably reduced, the onset time of the favipiravir is prolonged, and the toxic and side effects of the favipiravir are reduced.
Owner:BEIJING INSTITUTE OF PETROCHEMICAL TECHNOLOGY
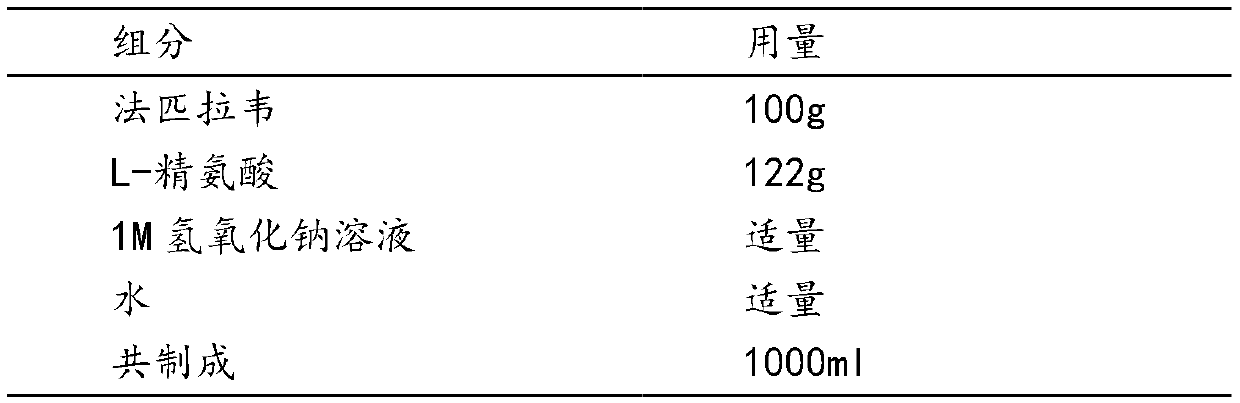
Favipiravir L-arginine frozen-dried preparation for injection
The invention relates to a favipiravir L-arginine frozen-dried preparation for injection. The frozen-dried preparation comprises favipiravir and L-arginine, wherein the molar ratio of the favipiravirto the L-arginine is (1 to 1.1) to (1 to 1.3) ( weight ratio is (1 to 22) to (1 to 1.44) ). The invention further discloses a preparation method of the favipiravir L-arginine frozen-dried preparationfor injection, the obtained frozen-dried preparation can be guaranteed to have favorable redissolution properties, and based on high unit load dosage, the favipiravir L-arginine frozen-dried preparation for injection has favorable stability.
Owner:REYOUNG PHARMA +1
Synthesis process of favipiravir and intermediate thereof
ActiveCN111349049AClear formExplicitly derivedOrganic chemistryBulk chemical productionPharmaceutical SubstancesPotassium carbonate
The invention relates to the technical field of medicine synthesis, and in particular, relates to a synthesis process of favipiravir and an intermediate thereof. The synthesis method of favipiravir comprises the following steps: 1) 2-aminopropanediamide and glyoxal serve as raw materials, and generating a compound III through a cyclization reaction; 2) performing benzyl protection on the compoundIII under the catalysis of potassium carbonate to generate a compound IV; 3) performing fluorine substitution on the compound IV under the action of fluorine gas, a solvent and a catalyst to generatea compound V; and 4) performing debenzylation protection on the compound V to generate favipiravir. Compared with other synthetic routes of favipiravir, the synthetic route provided by the invention has the advantages that the whole process route is shortened by virtue of high-selectivity fluorination reaction, the production cost is greatly reduced, and the synthesis process is suitable for industrial production.
Owner:JIANGSU ALPHA PHARM CO LTD
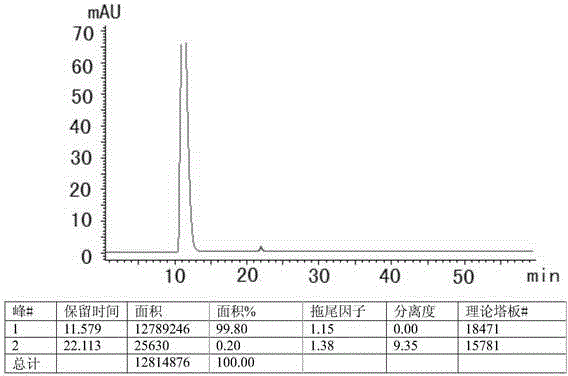
Determination method of related substances in favipiravir
InactiveCN112903838AReduce inspection costsShort detection timeComponent separationPhysical chemistryFavipiravir
The invention relates to the field of analytical chemistry, and discloses a method for detecting related substances of favipiravir, which takes a phosphate solution (mobile phase A)-acetonitrile (mobile phase B) as a mobile phase and a sodium carbonate solution as a diluent. taking a proper amount of favipiravir and a proper amount of a tablet containing the favipiravir, adding a diluent to prepare a sample solution containing 0.5 mg / mL of the favipiravir, and adding the diluent to dilute the favipiravir into a reference substance solution of 2.5 [mu] g / mL; quantitatively determining the contents of related substances in the favipiravir and the preparation by adopting a main component and correction factor method. According to the method for detecting the related substances of the favipiravir , the conditions of impurities and degradation products of the favipiravir can be rapidly and accurately detected. Operation is easy and convenient, accuracy is good, sensitivity is high, and product quality can be well controlled.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Preparation method of favipiravir and derivatives thereof
ActiveCN111704582AEasy to operateLow costOrganic chemistryBiochemical engineeringElectrophilic fluorination
The invention provides a novel preparation method of a favipiravir derivative. Specifically, a fluorinating reagent is directly used for an electrophilic fluorination reaction, fluorine atoms are selectively introduced to the 6-position, and favipiravir or the derivatives thereof are efficiently synthesized through one-step reaction. Compared with the existing synthesis method, the synthesis method adopting one-step reaction has the advantages of strong creativity, novelty, simplicity in operation, safety, low cost, less three wastes, environmental friendliness and high total yield, so that the synthesis method has extremely strong industrial advantages and significance.
Owner:HANGZHOU HUANGSEN BIOLOGICAL TECH CO LTD +1
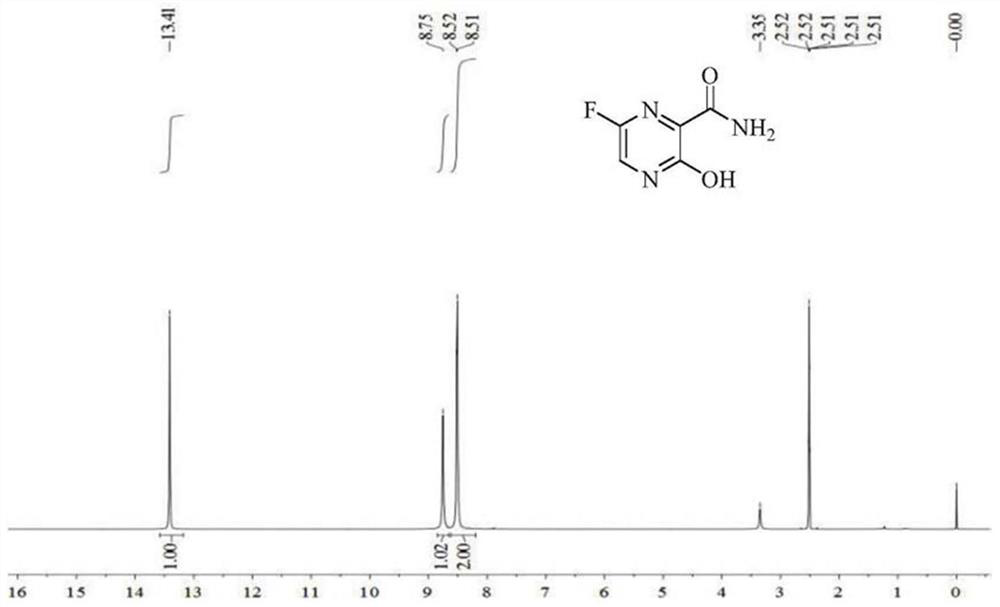
Preparation method of high purity favipiravir impurity
The invention relates to a preparation method of a favipiravir impurity 6-chloro-3-hydroxy-2-pyrazinecarboxamide shown as formula (1). By preparing the favipiravir impurity, and providing a referencesubstance for qualitative and quantitative analysis of favipiravir impurity, the invention improves the quality standard of favipiravir, and provides important guidance for safe medication of favipiravir.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Freeze-dried preparation of favipiravir for injection and preparation method thereof
ActiveCN111228226AAvoid sublimationAvoid quality defectsOrganic active ingredientsPowder deliveryMannitolExcipient
The invention relates to a freeze-dried preparation of favipiravir for injection. A formula of the freeze-dried preparation specifically comprises an active component favipiravir, a pH regulator sodium hydroxide, an excipient mannitol, a freeze-drying additive tert-butyl alcohol, and water for injection. The invention also discloses a method for preparing the freeze-dried preparation of favipiravir for injection. With the method, quality defects, such as clarity, turbidity and the like, of the freeze-dried preparation caused by sublimation of favipiravir can be effectively controlled; and theprepared freeze-dried preparation has good stability.
Owner:REYOUNG PHARMA +1
Method and composition for treating upper respiratory tract inflammatory and infectious diseases
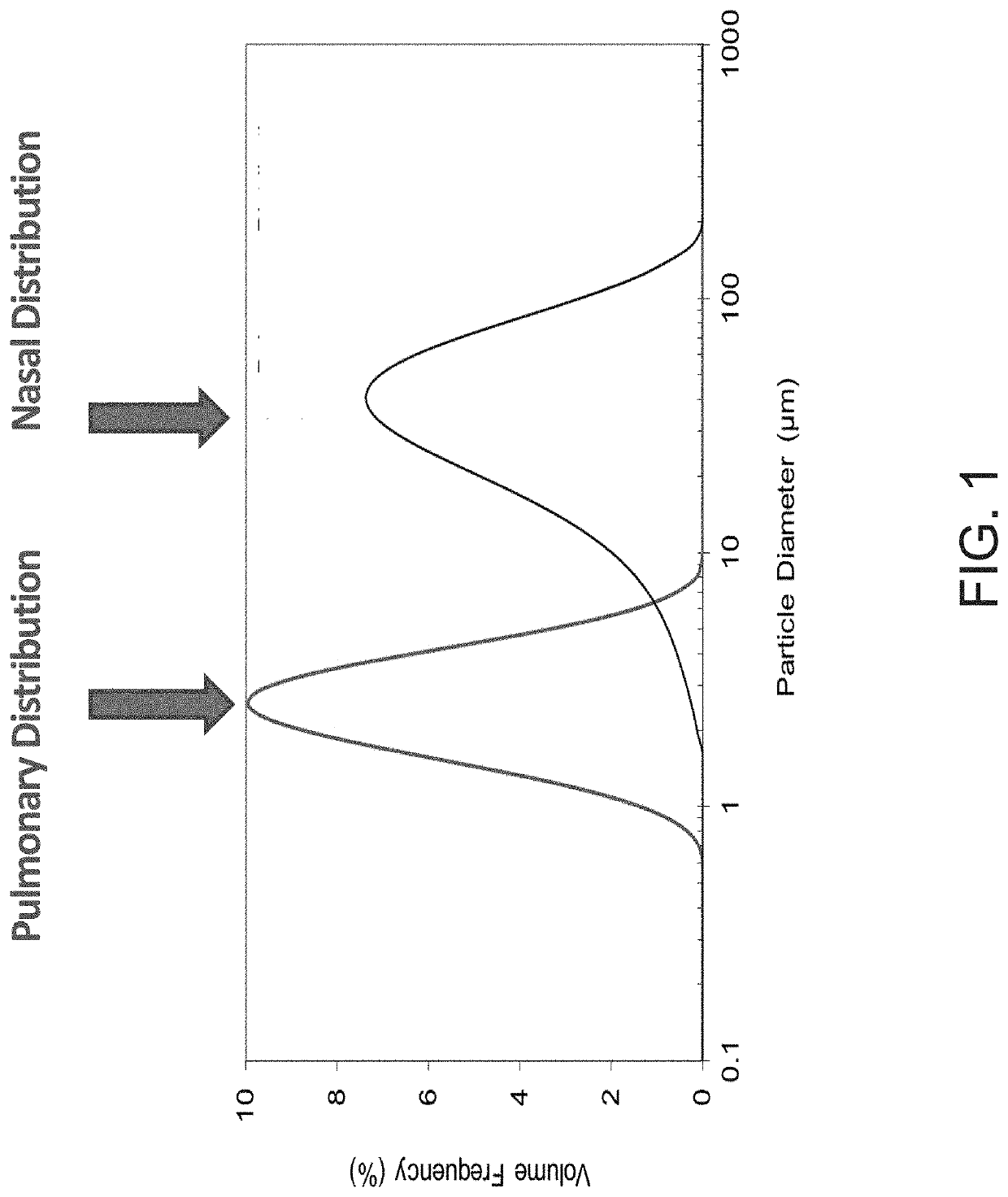
A composition and method for treating a mammal infected with a virus therein, and an inhalation delivery system for prevention and treatment of upper respiratory tract viruses, by administering a therapeutic dose of the composition to the mammal. The composition includes:microparticles and / or nanoparticles, wherein a pharmaceutically active agent, corticosteroid and / or hydroxychloroquine, niclosamide, and / or favipiravir, and a zinc salt are incorporated in the microparticles and / or nanoparticles. The pharmaceutically active agent is unfractionated heparin (UFH), Low Molecular Weight Heparin (LMWH), sulfated non-anticoagulant heparin (S-NACH), other glycosaminoglycans (GAGs), or combinations thereof.
Owner:VIROTHERA PHARM LLC
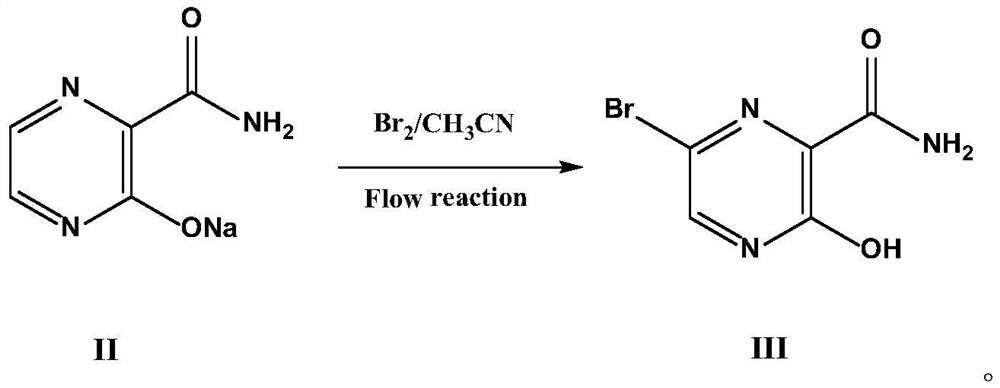
Preparation method of favipiravir intermediate 6-bromo-3-hydroxypyrazine-2-formamide
The invention relates to a preparation method of a favipiravir intermediate 6-bromo-3-hydroxypyrazine-2-formamide, and belongs to the technical field of organic synthesis. The 6-bromo-3-hydroxypyrazine-2-formamide is prepared by taking 3-hydroxypyrazine-2-amide as a raw material, controlling the pH value of a reaction solution and taking liquid bromine as a brominating agent. By adjusting the pH value of the reaction system to weak acidity, the bromination reaction can be ensured to be carried out under mild conditions, the high-temperature bromination method adopted in the existing synthesis method is avoided, the side reaction is reduced, and the purity and yield of the prepared product are obviously improved.
Owner:SHANDONG ZOUPING DAZHAN NEW MATERIALS
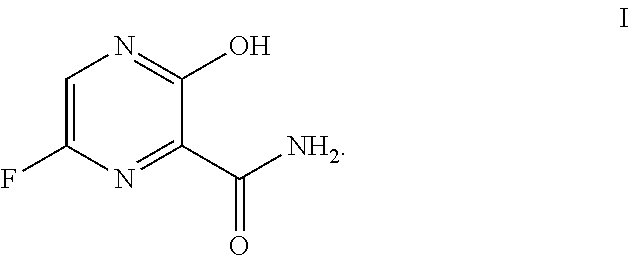
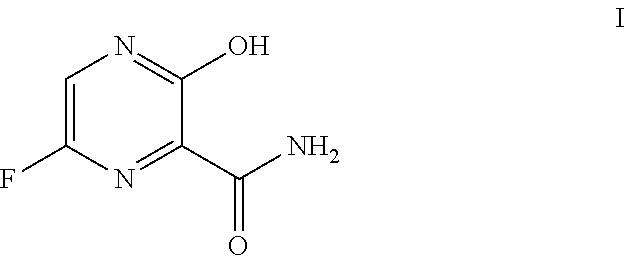
Use of favipiravir in treatment of coronavirus infection
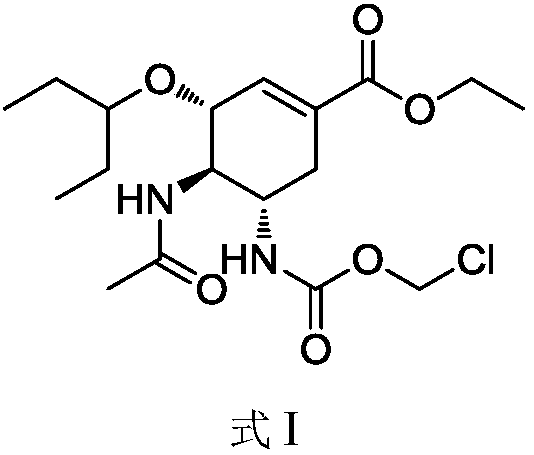
The present application relates to a Favipiravir compound represented by Formula I, a geometric isomer, a pharmaceutically acceptable salt, a solvate and / or a hydrate thereof, and a pharmaceutical composition comprising the compound for treating a coronavirus infection.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Preparation method of favipiravir
InactiveCN111675663AAvoid it happening againAvoid hydrolysisOrganic chemistryAntiviralsOrganosolvEnvironmental engineering
The invention provides a preparation method of favipiravir, which comprises the following step: by using one or two of 6-fluoro-3-hydroxyl-2-cyanopyrazine and 6-fluoro-3-hydroxyl-2-cyanopyrazine organic amine salt as raw materials, carrying out high-yield preparation in an anhydrous organic solvent under alkaline conditions to obtain favipiravir. Compared with the existing concentrated sulfuric acid hydrolysis process and hydrogen peroxide hydrolysis process, a large amount of acidic wastewater generated in the concentrated sulfuric acid hydrolysis process is avoided, explosion hazards possibly caused by use of hydrogen peroxide are also avoided, meanwhile, hydrolysis and oxygenolysis phenomena of products in the concentrated sulfuric acid hydrolysis process and the hydrogen peroxide hydrolysis process are avoided, and the preparation method is environmentally friendly, simple and safe to operate and relatively high in yield.
Owner:HANGZHOU HUANGSEN BIOLOGICAL TECH CO LTD
Favipiravir medicinal conjugate and application thereof to preparation of antiviral medicinal preparation
ActiveCN112546234ARelieves cramping symptomsImprove solubilityPowder deliveryOrganic chemistryPharmaceutical drugTheophylline
The invention particularly relates to a favipiravir medicinal conjugate and an application thereof to preparation of an antiviral medicinal preparation. Favipiravir is a broad-spectrum antiviral drugwith a good prospect, but the favipiravir is poor in water solubility and low in bioavailability. The invention provides a medicinal conjugate form of the favipiravir, and theophylline and piperazineare adopted as forming substances of eutectic crystals and salt, so that the solubility of the favipiravir in water is remarkably improved, and a new research thought is provided for development of afavipiravir drug combined application mode and dosage forms.
Owner:SHANDONG UNIV
Method for prevention and treatment of a viral-mediated infectious disease
A method for prevention or treatment of a viral-mediated infectious disease in a mammal. The mammal may be a human being. A therapeutic dose of a composition is administered, via an inhalation delivery apparatus, to the mammal. The composition includes microparticles and / or nanoparticles. The microparticles and / or nanoparticles include a first pharmaceutically active agent and a second pharmaceutically active agent. The first pharmaceutically active agent includes unfractionated heparin (UFH), Low Molecular Weight Heparin (LMWH), sulfated non-anticoagulant heparin (S-NACH) or combinations thereof. The second pharmaceutically active agent includes 10-30 mg of hydroxychloroquine in a form of hydroxychloroquine sulfate, 10-30 mg of favipiravir, or a combination thereof. The viral-mediated infectious disease is caused by one or more viruses in the mammal.
Owner:VIROTHERA PHARM LLC
Anti-influenza virus compound as well as preparation method and application thereof
ActiveCN108299316AOrganic active ingredientsOrganic chemistry methodsEthyl esterMethyl chloroformate
The invention relates to an anti-influenza virus compound as well as a preparation method and an application thereof, and belongs to the technical field of pharmaceutical synthesis. The compound contains oseltamivir and favipiravir structures and has the structure shown in the formula II in the description. The method comprises the following steps: oseltamivir and methyl chloroformate are subjected to condensation reaction, a compound (I) (3R,4R,5S)-4-acetamido-5-[chloromethoxycarbonyl]amino-3-(1-propoxyethyl)- -1-cyclohexane-1-carboxylic ethyl ester is obtained; the compound (I) and favipiravir are linked by nucleophilic substitution reaction, and a target compound (II) is obtained. The compound (II) has different degrees of inhibiting effects on H5N2, H5N6 and H5N8, and has better antiviral activity on H5N2 in vitro. Therefore, the invention also provides the application of the compound shown in the formula II in preparation of anti-influenza virus medicines.
Owner:SHANDONG UNIV
Preparation method of favipiravir intermediate 2-aminomalonamide
PendingCN112939797ALow priceAvoid prone to deactivation problemsOrganic compound preparationCarboxylic acid amides preparationNitrosoPtru catalyst
The invention belongs to the technical field of organic synthesis, and particularly relates to a preparation method of a favipiravir intermediate 2-aminomalonamide. According to the method, diethyl 2-nitrosomalonate is used as a starting raw material, Raney nickel is used as a catalyst for hydrogenation reduction to obtain dimethyl 2-aminomalonate, and then aminolysis is performed to obtain 2-aminomalonamide. Raney nickel is used as a catalyst, so that the problem that palladium carbon is easy to inactivate can be effectively avoided. Raney nickel has good tolerance to the raw materials, the inactivation phenomenon is not prone to occurring, and the defect that organic impurities such as phosphorus and sulfur exist in the raw materials can be well overcome. Compared with palladium carbon, Raney nickel is cheaper, and the production cost is reduced to a certain extent through the preparation method.
Owner:SHANDONG ZOUPING DAZHAN NEW MATERIALS
Preparation method of favipiravir
The invention relates to a preparation method of favipiravir. The preparation method comprises the following steps: dissolving 6-bromine-3-hydroxypyrazine-2-formamide in a first solvent, adding a first fluorinating agent, and carrying out a stirring reaction so as to prepare 6-bromine-3-fluoropyrazine-2-formamide; dissolving the 6-bromo-3-fluoropyrazine-2-formamide, a second fluorinating agent anda catalyst in a second solvent, and carrying out a heating reaction to prepare 6-fluoro-3-fluoropyrazine-2-formamide; and carrying out hydroxyl substitution on the 6-fluoro-3-fluoropyrazine-2-formamide to prepare the favipiravir. The invention further discloses a preparation method of the favipiravir. The preparation method of favipiravir has the advantages of simple and safe reaction operation,less three wastes, green and environment-friendly synthetic route and environmental friendliness; the favipiravir has the advantages of higher yield, stability and low production cost, and is suitablefor industrial production.
Owner:ENANTIOTECH CORP +1
Preparation method of favipiravir
The invention relates to the technical field of biological medicines, in particular to a preparation method of favipiravir, which comprises the following steps of: stirring 3-hydroxy pyrazine-2-formamide and a fluorinating reagent in a solvent, and carrying out one-step reaction to obtain favipiravir. The preparation method of favipiravir provided by the invention is short and novel in route, mildin reaction condition, economical and effective, higher in yield than the existing preparation method, and suitable for large-scale industrial production.
Owner:CHANGZHOU PHARMA FACTORY
Method for refining favipiravir and/or derivatives thereof
PendingCN113200928ASolve the problem of difficult to decolorizeHigh degree of decolorizationOrganic active ingredientsOrganic chemistryBiochemical engineeringOrganosolv
The invention relates to a method for refining favipiravir and / or derivatives thereof, which comprises the following steps of dissolving favipiravir and / or derivatives thereof to be refined in an organic solvent A, filtering with silica gel, drying and purifying to obtain the favipiravir. The purification is selected from any one or a combination of recrystallization and pulping. According to the refining method, the problem that favipiravir is difficult to decolor is solved, and the obtained product is a white or off-white powdery product and meets related requirements of drug quality standards. In addition, the method is suitable for purification and decoloration of other products and intermediates, and is high in decoloration degree, good in purification effect, simple and convenient to operate, low in cost and suitable for industrial production.
Owner:BEIJING SIHUAN PHARMA +2
A kind of synthetic method of favipiravir
ActiveCN104496917BLow impurity contentShort reaction cycleOrganic chemistryPotassium fluorideHydrolysis
The invention belongs to the field of medicinal chemistry and particularly relates to a new synthesis method of Favipiravir. The method comprises the following steps: carrying out carboxyl protection on raw material shown in the formula (II) to generate a compound (III); carrying out diazotization hydrolysis reaction in the presence of concentrated sulfuric acid and sodium nitriteto generate a compound (IV); carrying out benzyl protection reaction to generate a compound (V), and then generating a compound (VI) in the presence of potassium fluoride and tetrabutylammonium bromide; removing a benzyl protection group to generate a compound (VII); and then adding an aminating agent to carry out amination to generate Favipiravir shown in the formula I. The method disclosed by the invention has the advantages that the reaction cycle is short, the operation is simple, the production cost is low, and the product is high in quality; therefore, the method is suitable for industrial production.
Owner:NANJING HUAWE MEDICINE TECH DEV
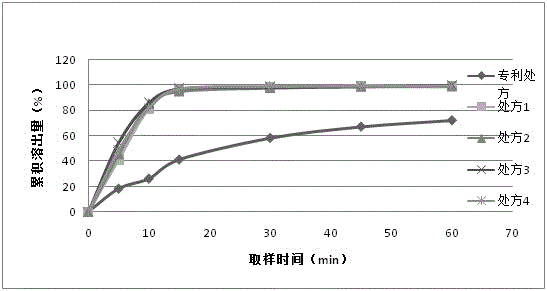
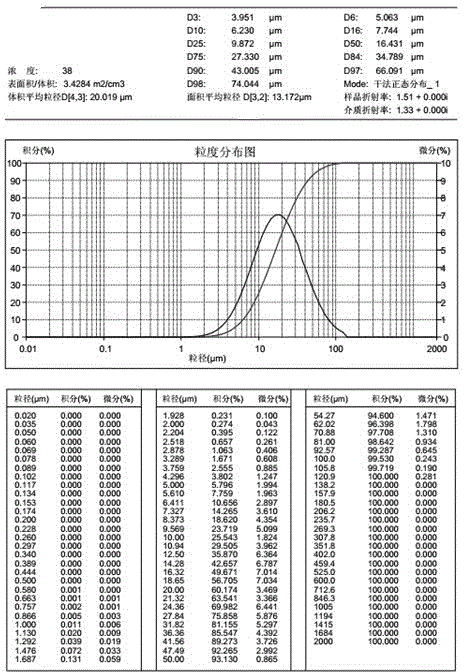
A kind of favipiravir pharmaceutical composition containing different particle size ranges
ActiveCN104288154BSolve the problem of poor dissolutionQuick releaseOrganic active ingredientsAntiviralsIn vivo absorptionDissolution
Owner:CHENGDU SINO STRONG PHARMA +1
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com