Method for refining favipiravir and/or derivatives thereof
A refining method, the technology of favipiravir, is applied in the field of drug synthesis, which can solve the problems of substandard color, difficulty in removing solvents, and high cost, and achieve the effects of large decolorization, good purification effect, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] The refining of embodiment 1 Favipiravir
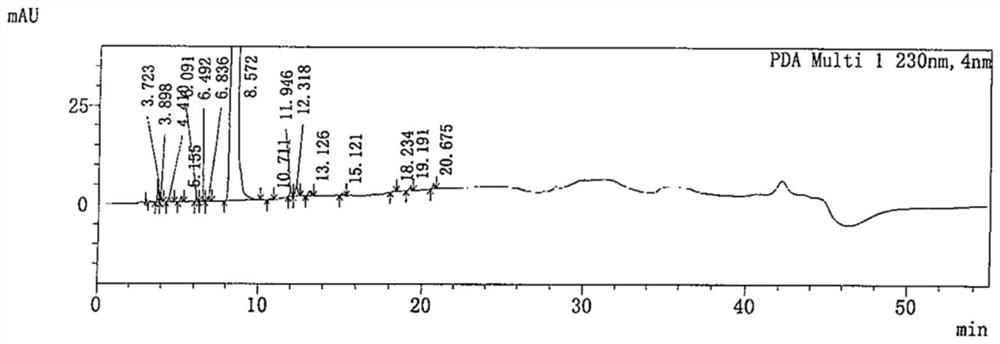
[0088] Get 5.00g of favipiravir crude product (light brown solid, purity: 99.427%, 15 impurities, impurity A: 0.048%, impurity B: 0.105%) and add in 1L single-necked bottle, add 330ml dichloromethane / ethyl acetate= 5:1 (w / w) mixed solvent was stirred and dissolved, and then 25.00g of 200-300 mesh silica gel was evenly spread in the Buchner funnel, filtered, and rinsed with 40ml of the corresponding mixed solvent, the filtrate was collected, concentrated and evaporated under reduced pressure. Dry, then add 45ml of ethyl acetate, heat and stir until the system reflux, add 0.50g of activated carbon, stir and heat for 1h, heat filter, rinse the filter cake with 5ml of ethyl acetate, cool the filtrate to 0-10°C, keep stirring and crystallize for 2h , the filter cake was rinsed with 5ml pre-cooled ethyl acetate, and the filter cake was vacuum-dried at 60° C., and the vacuum degree was greater than or equal to 0.08 MPa. After drying, ...
Embodiment 2
[0089] The refining of embodiment 2 Favipiravir
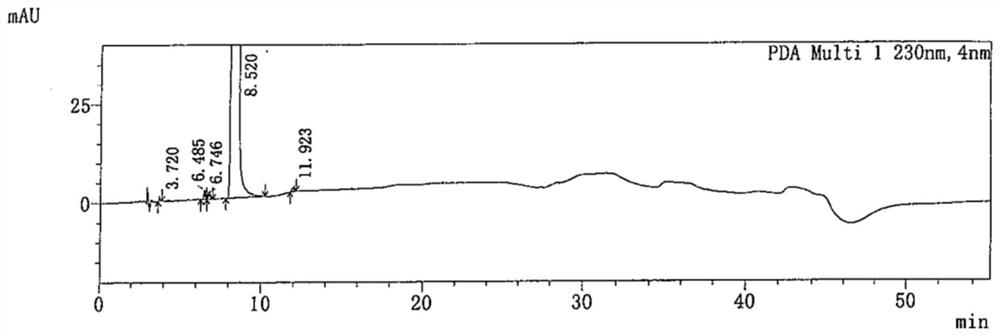
[0090] Take 5.00g of favipiravir crude product (light brown solid, purity: 99.427%, 15 impurities, impurity A: 0.048%, impurity B: 0.105%,) into a 1L single-necked bottle, add 600ml of dichloromethane / ethyl acetate =10:1 (w / w) mixed solvent was stirred and dissolved, then evenly spread 25.00g of 200-300 mesh silica gel in the Buchner funnel, filtered, and rinsed with 40ml of the corresponding mixed solvent, collected the filtrate, and concentrated under reduced pressure Evaporate to dryness, then add 45ml of ethyl acetate, heat and stir until the system returns to reflux, add 0.50g of activated carbon, stir and heat for 1h, heat filter, rinse the filter cake with 5ml of ethyl acetate, cool the filtrate to 0-10°C, keep stirring and crystallize 2h, the filter cake was rinsed with 5ml of pre-cooled ethyl acetate, and the filter cake was vacuum-dried at 60°C, with a vacuum degree ≥0.08MPa, and dried to obtain 3.40g of off-white pow...
Embodiment 3
[0091] The refining of embodiment 3 Favipiravir
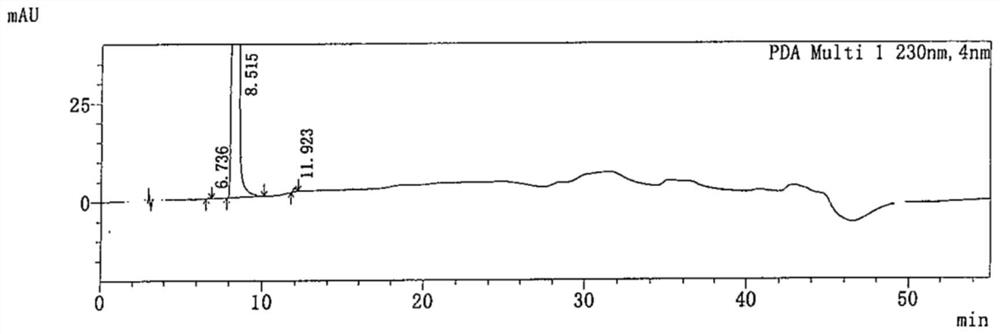
[0092] Take 5.00g of favipiravir crude product (light brown solid, purity: 99.427%, 15 impurities, impurity A: 0.048%, impurity B: 0.105%,) into a 3L single-necked bottle, add 2800ml of dichloromethane solvent and stir to dissolve, Then spread 25.00g of 200-300 mesh silica gel evenly in the Buchner funnel, filter and rinse with 40ml of the corresponding solvent, collect the filtrate, concentrate under reduced pressure and evaporate to dryness, then add 45ml of ethyl acetate, heat and stir until the system refluxes, Add 0.50g of activated carbon, stir and heat for 1h, heat filter, rinse the filter cake with 5ml of ethyl acetate, cool the filtrate to 0-10°C, keep stirring and crystallize for 2h, rinse the filter cake with 5ml of pre-cooled ethyl acetate, filter The cake was vacuum-dried at 60° C., vacuum degree ≥ 0.08 MPa, and dried to obtain 3.46 g of white powder with a yield of 69.2% and a purity of 99.936% (2 impurities, impu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com