Preparation method of favipiravir and derivatives thereof
A technology of derivatives and compounds, which is applied in the field of new preparation of Favipiravir and its derivatives, can solve the problems of cumbersome operation, unfriendly environment, and many reaction steps, and achieve simple operation, environmental friendliness, and high total yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
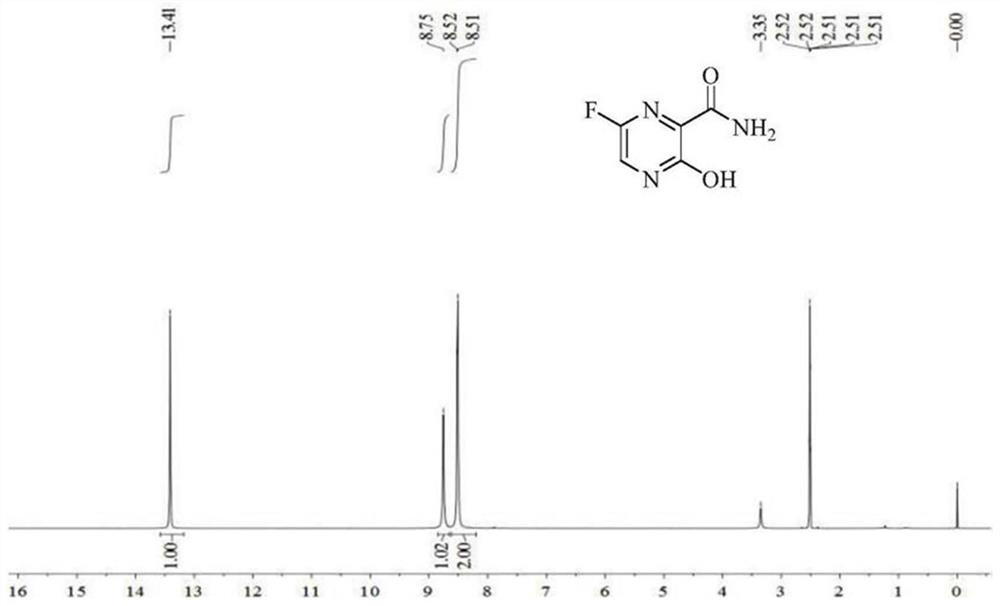
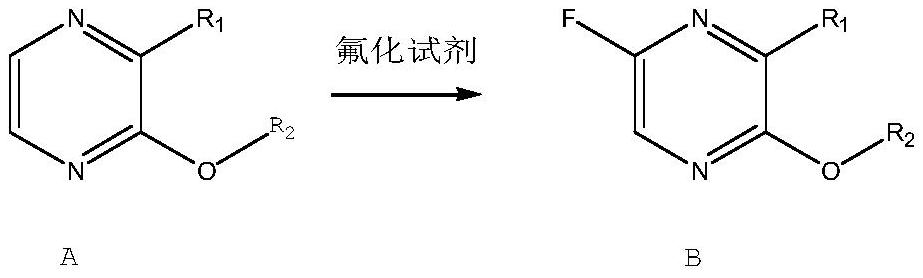
[0022] Add 13.9 g of 3-hydroxypyrazine-2-carboxamide to 100 ml of DMF, then add 60 g of Selectfluor, raise the temperature to 70-80°C, react for 12 hours, and cool down to 0-5°C. Filter out the unreacted raw material, distill off most of the solvent under reduced pressure, add 100 ml of water, 50 ml of methanol, stir for 1 hour, filter, collect the solid, obtain after drying under reduced pressure at 50-60 ° C, 11 g of light yellow solid (HPLC 99.2%, yield 70%), the nuclear magnetic spectrum of this light yellow solid is as figure 2 Shown, determine that it is Favipiravir, as shown in reaction formula (1) B:
[0023]
Embodiment 2
[0025] Add 13.9 g of 3-hydroxypyrazine-2-carboxamide to 100 ml of DMF, then add 63 g of NSFI, raise the temperature to 70-80°C, react for 12 hours, and cool down to 0-5°C. The unreacted raw material was filtered off, most of the solvent was distilled off under reduced pressure, 100 ml of water and 50 ml of methanol were added, stirred for 1 hour, filtered, and the solid was collected, dried under reduced pressure at 50-60°C to obtain 10.2 g of light yellow solid (HPLC99. 3%, yield 65%), the nuclear magnetic spectrum of this light yellow solid is the same as embodiment 1, confirms that it is Favipiravir, as shown in reaction formula (2) B:
[0026]
Embodiment 3
[0028] Add 13.9 g of 3-hydroxypyrazine-2-carboxamide to 100 ml of dichloroethane, then add 60 g of Selectfluor, heat up to 70-80°C, react for 24 hours, cool down to 0-5°C, add 100 ml of ethyl acetate ester. The unreacted raw materials were filtered off, most of the solvent was distilled off under reduced pressure, 100 ml of water and 50 ml of methanol were added, stirred for 1 hour, filtered, and the solid was collected, dried under reduced pressure at 50-60°C to obtain 10.67 g of light yellow solid (HPLC 99.4 %, yield 68%), the nuclear magnetic spectrum of this light yellow solid is the same as embodiment 1, confirms that it is Favipiravir, as shown in reaction formula (3) B:
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com