Preparation method of favipiravir
A compound and solvent technology, applied in the field of preparation of favipiravir, can solve problems such as being unfavorable to industrialized production, unsuitable for industrialized production, expensive environment for reagents, etc., and achieves a reduction in labor and raw material costs, a short and novel route, and an economical and effective method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of formula I compound (X is Br)
[0033]
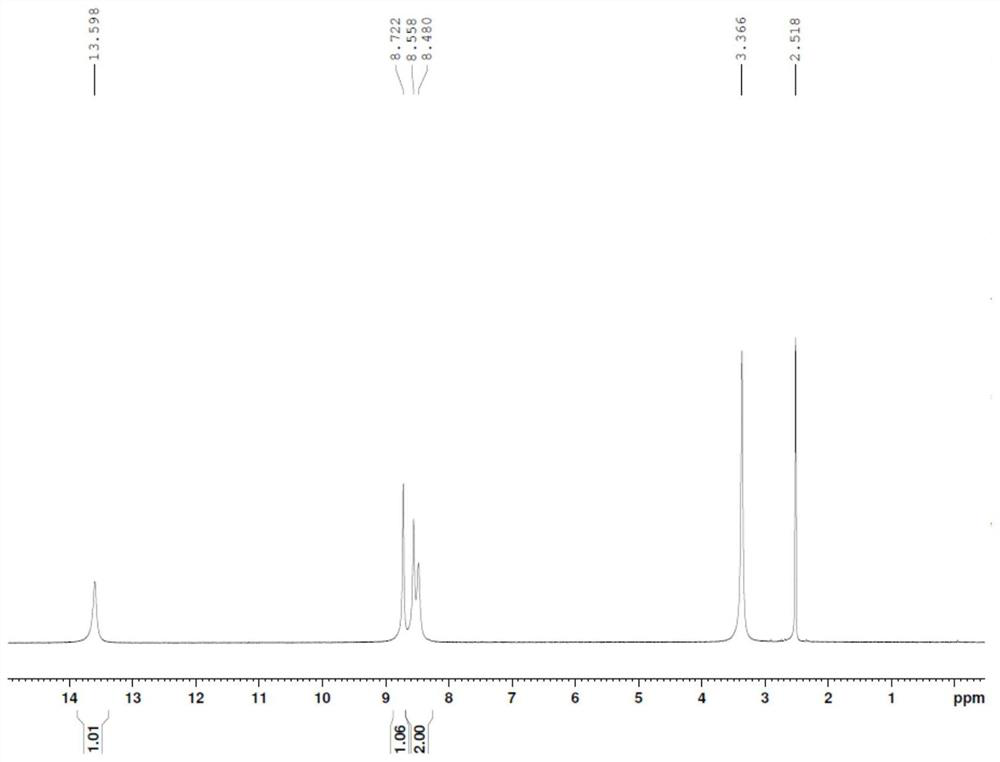
[0034] Add 14.7g of 3-hydroxypyrazine-2-carboxamide (molecular weight 139.1, 106mmol) into a three-necked flask, add 35mL of DMF and 14.2g of pyridine, heat the oil bath to 80°C, and dropwise add 21.1g of bromine (molecular weight: 159.8, 132mmol) , control the temperature at 80-100°C, and drop it in 60 minutes. After dropping, keep warm for 3h. After the reaction is complete, add 8 mL of toluene, add dropwise 60 mL of water, drop it for 15 minutes, drop it down to room temperature, filter it with suction, wash the filter cake with 10 mL methanol, and dry the filter cake at 50 ° C for 6 hours to obtain 15.0 g of a brown solid, which was determined by HPLC Purity: 95.1%, yield: 62.1%.
[0035] 1 HNMR (DMSO-d6): 13.60 (1H, s), 8.72 (1H, s), 8.56 (1H, s), 8.48 (1H, s)
Embodiment 2
[0037] The preparation of formula I compound (X is NO 2 )
[0038]
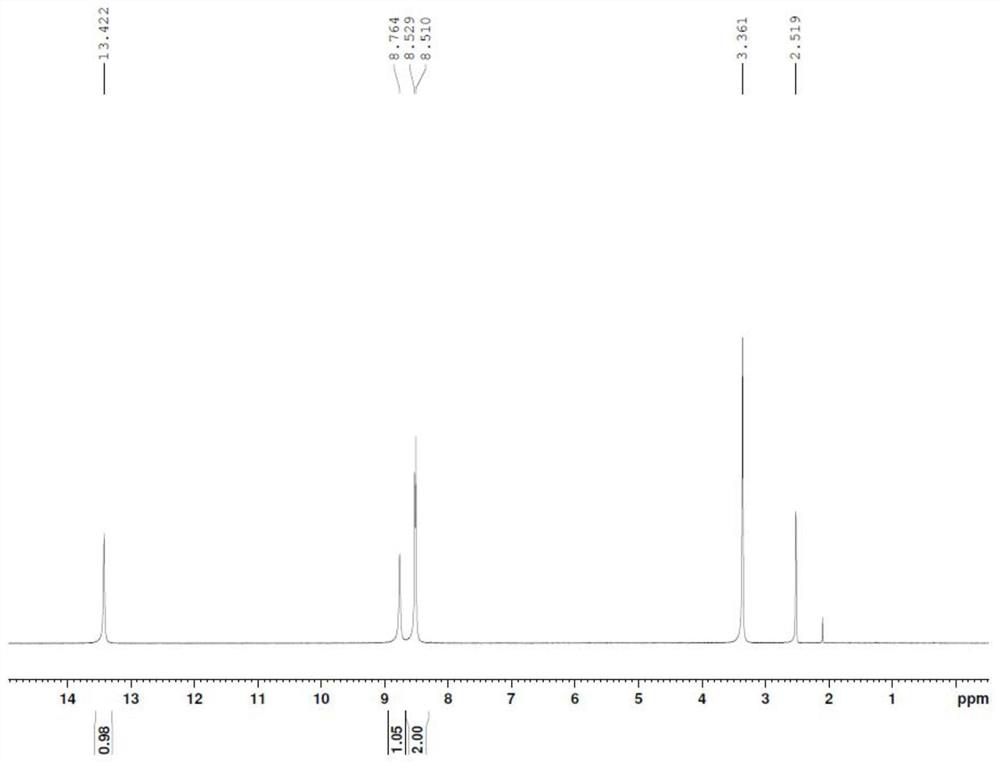
[0039] Add 2.0g of 3-hydroxypyrazine-2-carboxamide (molecular weight 139.1, 14.4mmol) into 20ml of concentrated sulfuric acid, cool to 0-5°C, and add KNO in batches 3 2.91g (molecular weight: 101.1, 28.8mol), after the addition, the temperature was raised to 40°C for 4h. The reaction solution was added to 100ml of ice water, precipitated, filtered, the filter cake was washed twice with 20ml of purified water, and the filter cake was air-dried at 50°C for 6h to obtain 1.93g of an off-white solid, the purity of which was determined by HPCL to be 96.5%. : 72.9%.
[0040] 1 HNMR (DMSO-d6): 8.98 (1H, s), 8.34 (1H, s), 8.07 (1H, s)
Embodiment 3
[0042] Preparation of formula II compound (X is Br, and fluorinating reagent is tetramethylammonium fluoride)
[0043]
[0044] In a 100mL three-neck flask, dissolve 0.61g of acetic acid (molecular weight 60.1, 10.2mmol) in 20mL of DMF, protect with nitrogen, cool down to 0-5°C, add 2.28g of DCC (molecular weight 206.3, 11.1mmol), raise to room temperature, stir for 1h, add 2.10 g the compound of formula I prepared in Example 1 (molecular weight 218, 9.17 mmol), stirred for 12 h, then added 1.72 g of anhydrous tetramethylammonium fluoride (molecular weight 93.1, 18.5 mmol), stirred at room temperature for 24 h, added 20 mL of 2N hydroxide Sodium solution, stir until the reaction is complete by TLC, extract impurities with 10mL ethyl acetate, separate layers, adjust the water layer to pH=3-4 with 2N HCl, extract with 20mL*3 ethyl acetate, combine the organic layers, and wash with saturated aqueous sodium bicarbonate solution , washed with water, washed with saturated sodium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com