Favipiravir medicinal conjugate and application thereof to preparation of antiviral medicinal preparation
A technology of favipiravir and conjugates, which is applied in the field of pharmaceutical conjugates, can solve the problems of limiting the bioavailability of favipiravir and the development and application of related dosage forms, the research blanks of drug combination and crystal form, etc., and achieve The effect of low production cost, strong operability and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
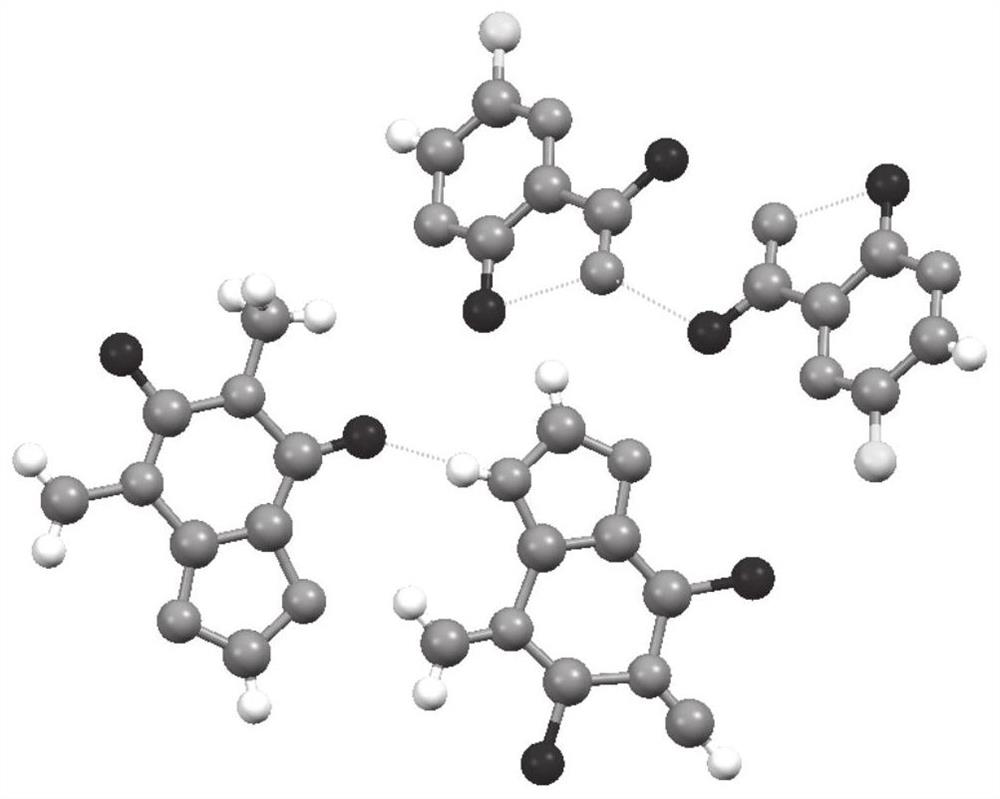
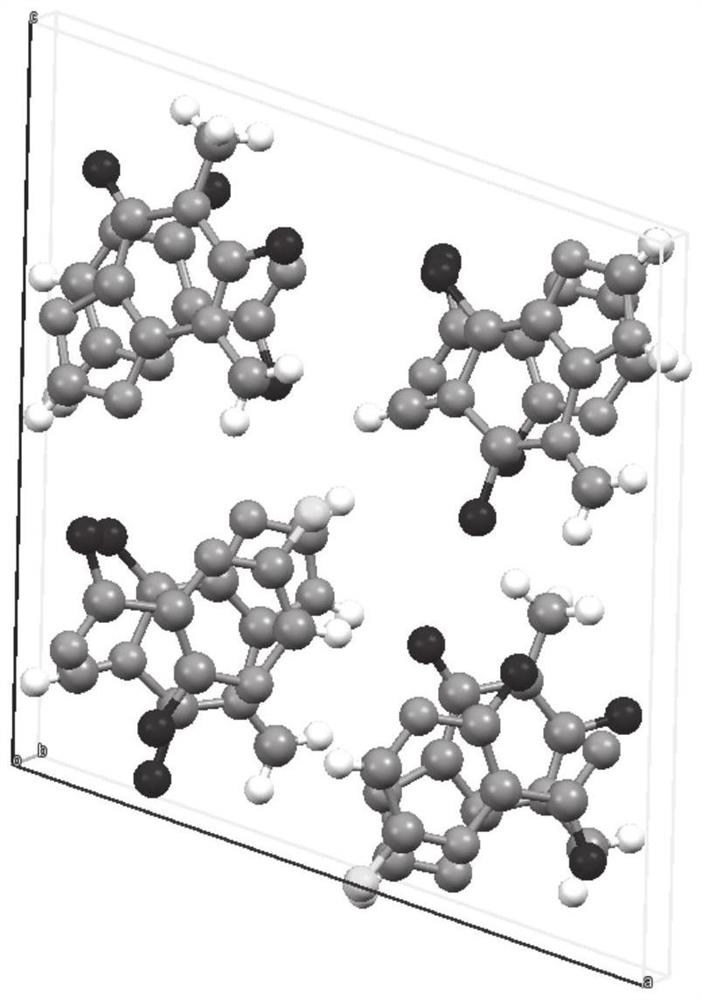
[0063] This embodiment provides a method for preparing favipiravir-theophylline drug co-crystal based on the solution method: accurately weigh 15.71 mg (0.1 mmol) of favipiravir raw material, 18.016 mg (0.1 mmol) of theophylline raw material, and Next, put the two weighed raw materials into a cleaned glass bottle (volume: 20ml), add an appropriate amount of ethanol until the drug is fully dissolved, the solution is clear, and the opening of the glass bottle is placed at room temperature and evaporated in a fume hood. Completely obtain the co-crystal drug of favipiravir and theophylline.
Embodiment 2
[0065] This embodiment provides another method for preparing favipiravir-theophylline drug co-crystal based on the solution method: Weigh 15.71mg (0.1mmol) of favipiravir raw material and 18.016mg (0.1mmol) of theophylline raw material, at room temperature Next, put the two weighed raw materials into a clean glass bottle (volume: 15ml), add 5mL of ethanol (other solvents include water, methanol, ethanol, acetone, acetic acid, ethyl acetate, acetonitrile, trifluoroethanol, isopropanol, n-butanol, chloroform, dichloromethane, chloroform, carbon tetrachloride, hexane tetrahydrofuran, dimethyl sulfoxide, N,N-dimethylformamide, or combinations thereof), the Put the glass bottle with the solution in a 65°C oil bath, add the cleaned magnets, and dissolve the drug fully at a speed of 360r / m, and it will appear as a clear solution, then place it at room temperature, cool down and crystallize after a period of time Afterwards, the co-crystal drug of Favipiravir and theophylline will be ...
Embodiment 3
[0067] In this embodiment, a method for preparing favipiravir-theophylline drug co-crystal based on grinding method is provided: accurately weigh 47.13 mg (0.3 mmol) of favipiravir raw material and 54.048 mg (0.3 mmol) of theophylline raw material, The sample is placed in a grinding bowl, Retsch MM200 grinding instrument, the frequency is 25Hz, grinding for 30 minutes, grinding without adding solvent or adding solvent (including water, methanol, ethanol, acetone, acetic acid, ethyl acetate, acetonitrile, trifluoroethanol, iso Propanol, n-butanol, chloroform, dichloromethane, chloroform, carbon tetrachloride, hexane tetrahydrofuran, dimethyl sulfoxide, N,N-dimethylformamide, or a combination of grinding aids can be obtained eutectic product.
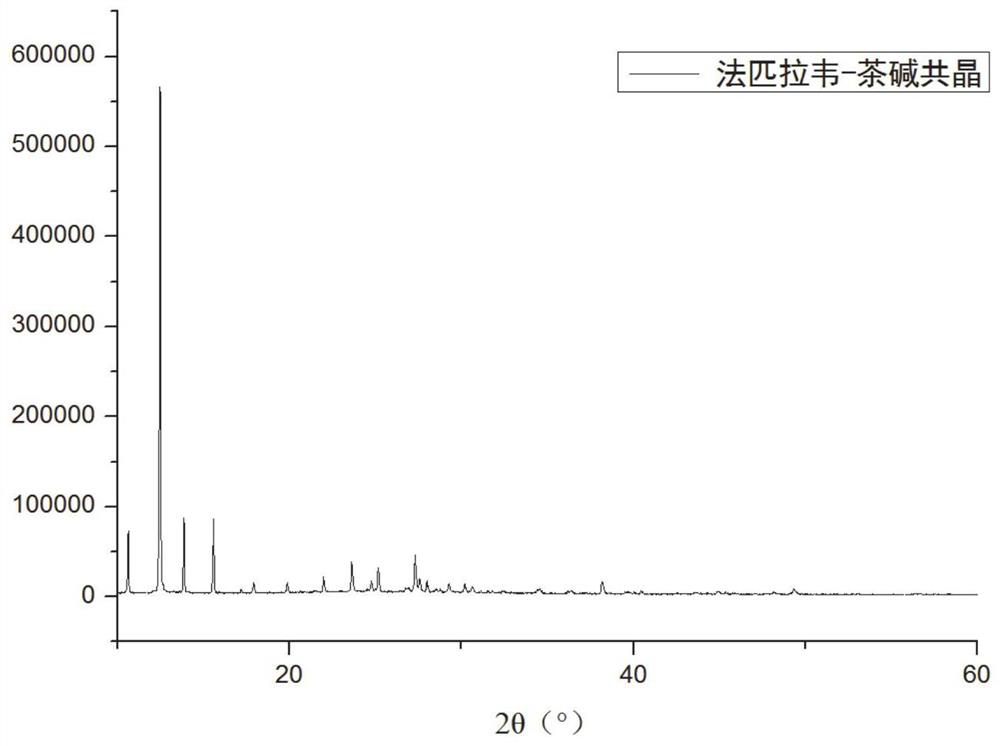
[0068] For the favipiravir-theophylline cocrystal obtained in this embodiment, use an X-ray powder diffractometer, produced by Bruker, Germany, model D8 Advance, Cu-kα, tube voltage 40kV, tube current 40mA, step size 0.08, scan Range 10-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com