Preparation method of favipiravir
A technology of favipiravir and formamide, applied in the field of drug synthesis, to achieve the effects of less waste, low production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
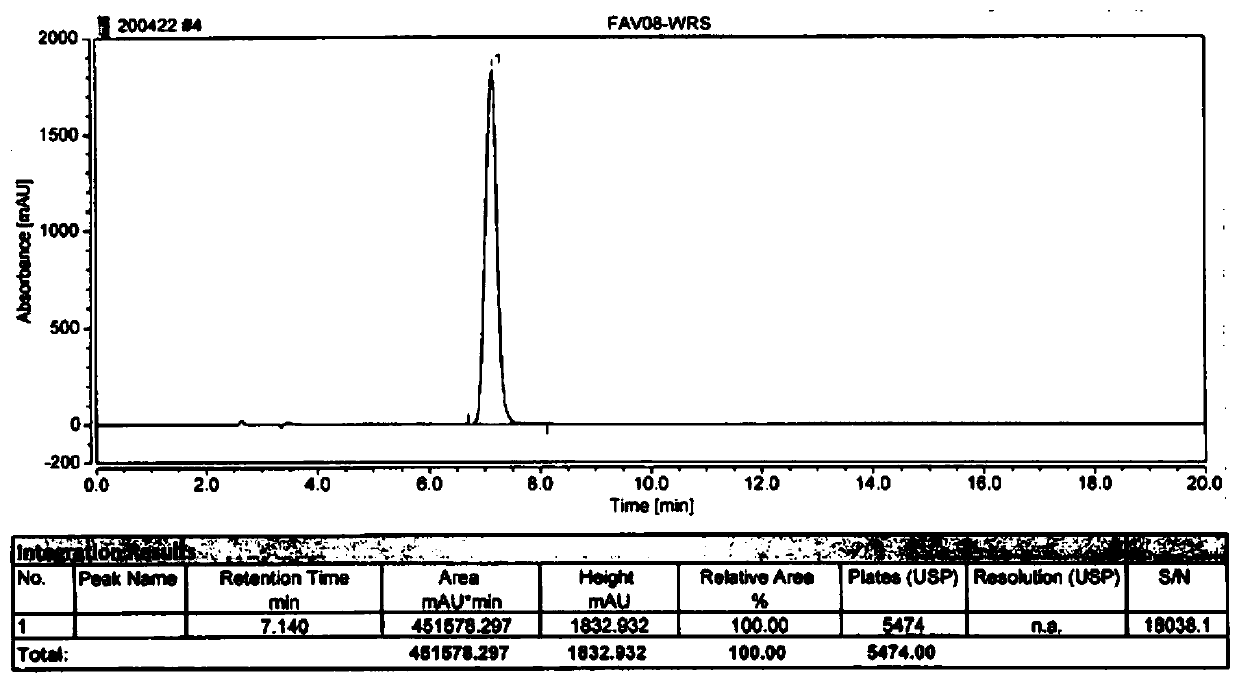
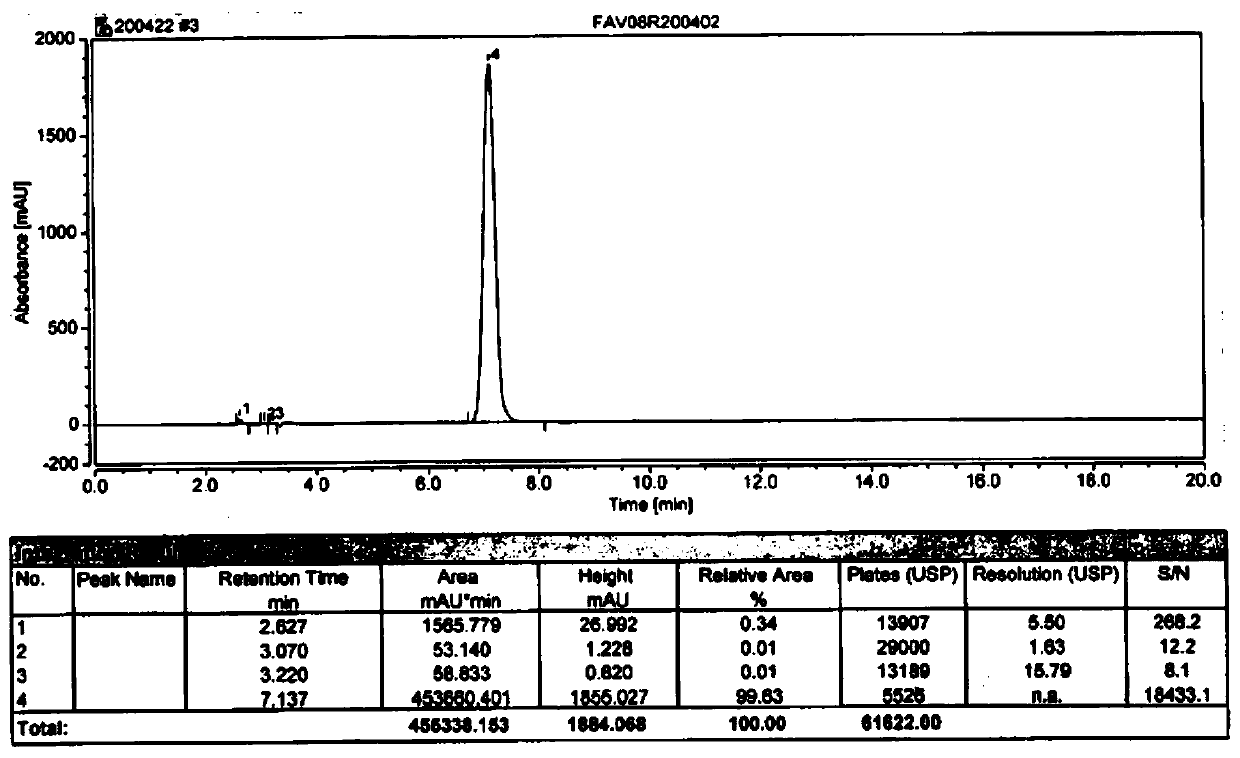
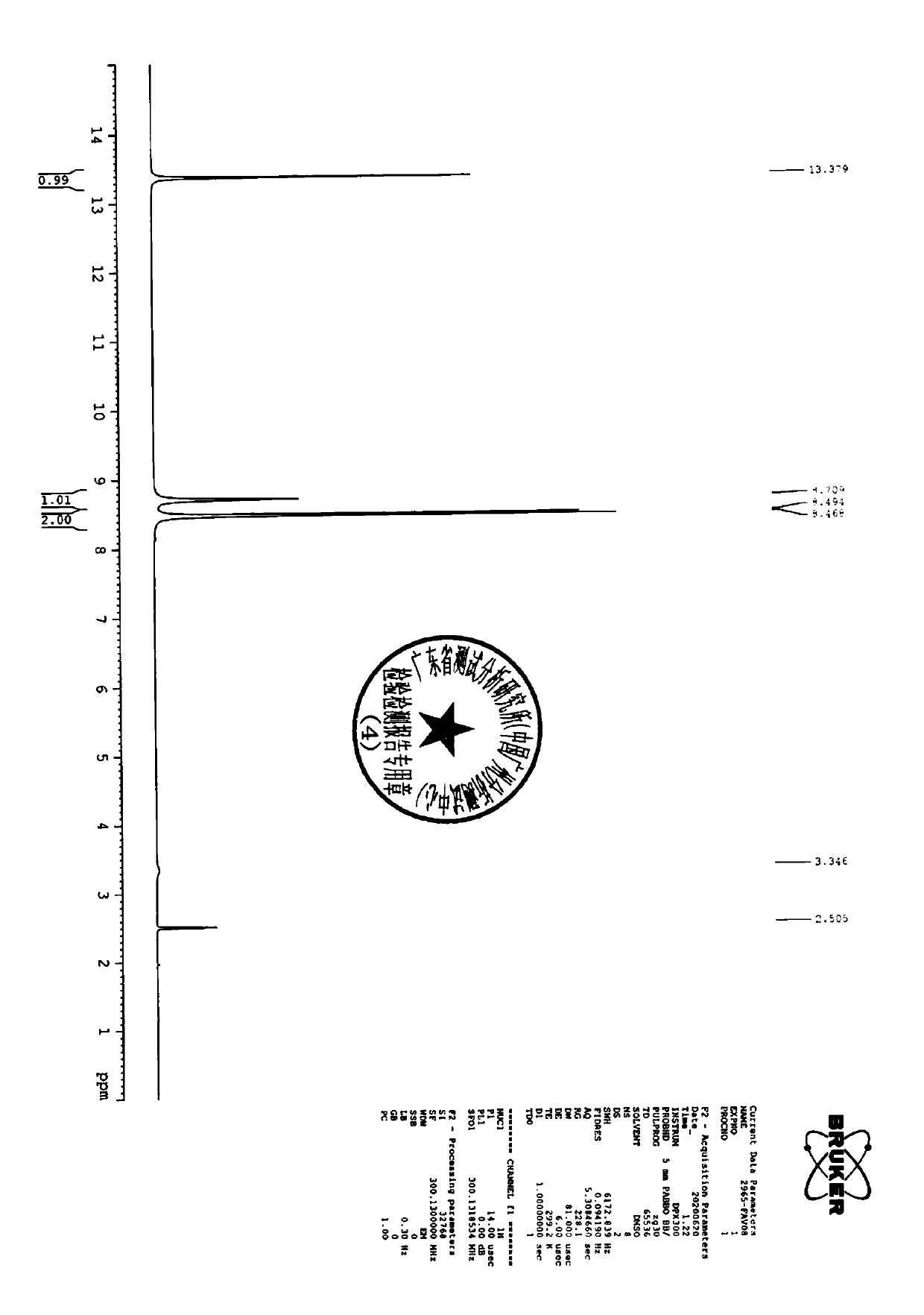
Image
Examples
preparation example Construction
[0049] A preparation method of Favipiravir, comprising the following steps:
[0050] Step 1. Dissolving 6-bromo-3-hydroxypyrazine-2-carboxamide in the first solvent, adding the first fluorinating agent, stirring and reacting to prepare 6-bromo-3-fluoropyrazine-2-carboxamide;
[0051] Step 2. Dissolve the 6-bromo-3-fluoropyrazine-2-carboxamide, the second fluorinating agent and the catalyst in the second solvent, and heat the reaction to prepare 6-fluoro-3-fluoropyrazine-2- Formamide;
[0052] Step 3. Carrying out hydroxyl substitution to the 6-fluoro-3-fluoropyrazine-2-carboxamide to prepare Favipiravir.
[0053] Understandably, the synthetic route of the present invention is shown in the following formula:
[0054]
[0055] With 6-bromo-3-hydroxypyrazine-2-carboxamide as the starting material, 6-fluoro-3-fluoropyrazine-2-carboxamide was prepared by two fluorination reactions, and then, the 6- Fluoro-3-fluoropyrazine-2-carboxamide undergoes hydroxyl substitution to obtai...
Embodiment 1
[0080] This embodiment provides a kind of Favipiravir and its preparation method, the synthetic route and specific steps are as follows:
[0081] 1. Preparation of compound 2a
[0082]
[0083] Add 300ml of dichloromethane and 20g (0.0917mol) of 6-bromo-3-hydroxypyrazine-2-carboxamide into a 500ml three-necked flask, and cool the temperature of the material in the there-necked flask to 0°C under stirring, and add dropwise 15.5g ( 0.0963mol) DAST reagent, keep the temperature of the reaction solution at 0°C and stir for 5 hours after dripping, TLC detection, the color of the plate layer does not show the raw material point, stop the reaction, add 120ml process water to the reaction solution, stir for 0.5 hours after the addition, The material was transferred into a separatory funnel and left to stand for 1 hour. The organic phase was separated and concentrated to obtain 18.56 g of compound 2a with a molar yield of 92%. The obtained product was directly used in the next react...
Embodiment 2
[0092] This embodiment provides a kind of Favipiravir and its preparation method, which is basically the same as that of Example 1, the only difference is that the stirring reaction temperature is different, and the synthetic route and specific steps are as follows:
[0093] 1. Preparation of compound 2a
[0094]
[0095] Add 300ml of dichloromethane and 20g (0.0917mol) of 6-bromo-3-hydroxypyrazine-2-carboxamide into a 500ml three-necked flask, cool the material temperature in the there-necked flask to 20°C under stirring, and add dropwise 15.5g ( 0.0963mol) DAST reagent, keep the temperature of the reaction solution at 20°C and stir for 5 hours after dropping, TLC detection, the color of the plate layer does not show the raw material point, stop the reaction, add 120ml process water to the reaction solution, stir for 0.5 hours after the addition, The material was transferred into a separatory funnel and left to stand for 1 hour. The organic phase was separated and concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com