Favipiravir synthesis method
A synthesis method and favipiravir technology, applied in organic chemistry and other directions, can solve problems such as difficult industrial production, and achieve the effects of mild conditions, simple raw materials, and good industrial value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] The following clearly and completely describes the technical solutions in the embodiments of the present invention. Obviously, the described embodiments are only some of the embodiments of the present invention, but not all of them. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
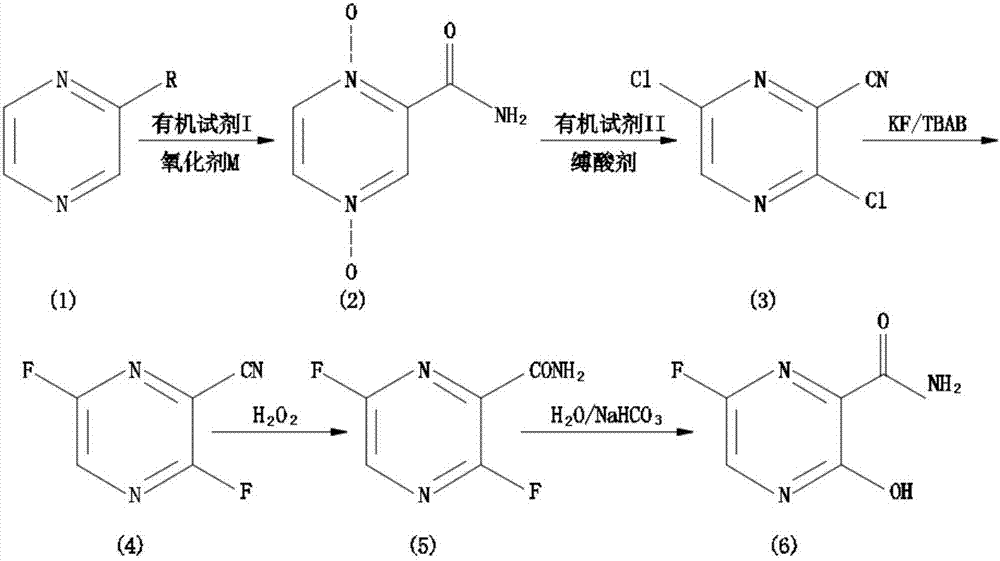
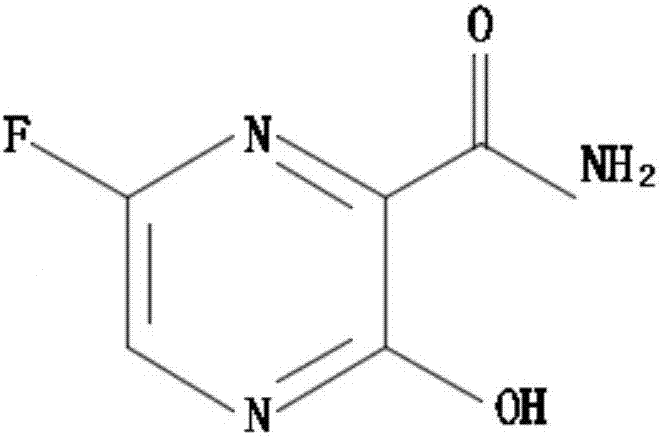
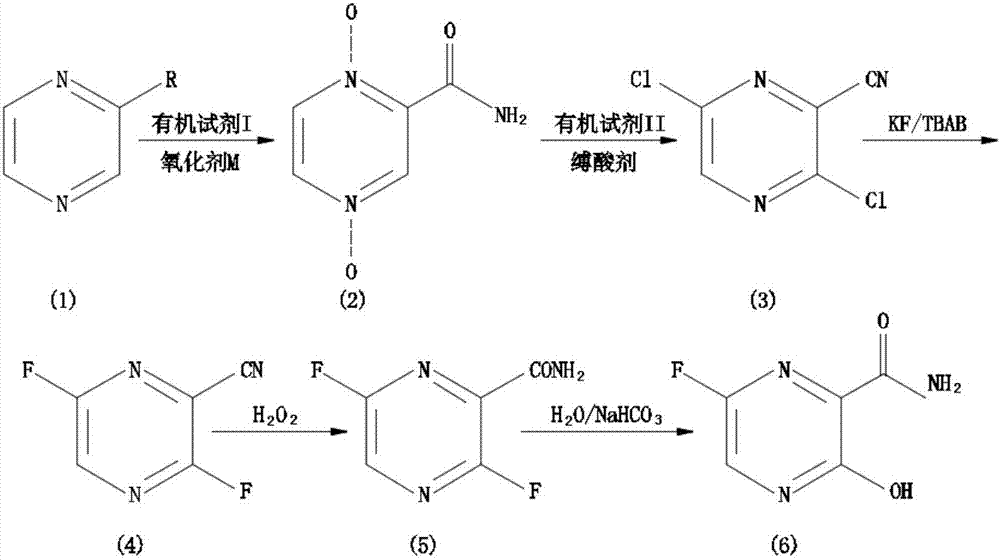
[0037] In the embodiment of the present invention, a synthetic method of Favipiravir:
[0038] 1) Preparation of 1.4-dioxypyrazinamide (2): Control the temperature at -5 to 5°C, mix 5.25 g of 2-cyanopyrazine (1) with 29.95 g of glacial acetic acid and 45.30 g of 30% hydrogen peroxide, Heat up to 95°C, reflux for 22 hours, TLC shows no raw material, 40°C vacuum rotary evaporation to remove the solvent, add 15ml of water, vacuum rotary evaporation, repeat several times to remove glacial acetic acid, add 15ml of water, add hot chlorofo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com